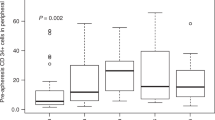
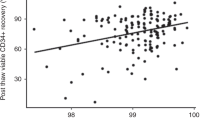
Abstract
Peripheral blood stem cells have largely replaced harvested bone marrow stem cells both in the autologous and allogeneic settings. Advantages of peripherally harvested cells include higher stem cell dose, more rapid engraftment, reduced donor/patient discomfort, and better graft-versus-leukemia effect in the allogeneic setting. Within the apheresis machine, whole blood is separated into its components by centrifugation, and the red cell-depleted, stem cell-rich buffy coat is extracted for use as a stem cell product, simultaneously returning the other blood components back to the donor. Prediction of procedure length is based on the required cell dose target but still remains challenging. Moreover, the number of apheresis procedures needed should be as few as possible in order to reduce costs and patient/donor discomfort and to increase safety. The volume of blood which needs to be processed in order to collect an adequate number of stem cells depends on several factors such as method of stem cell mobilization, vascular access, and collection efficiency.
Another issue to take into consideration is cell storage: according to GITMO’s study (Perseghin et al. 2014) in most Italian centers, even up to 83.4% correspond to useless storage and only the remaining 16.6% to useful storage. Therefore, SIdEM and GITMO proposed a policy for autologous HPC disposal that fulfills clinical, ethical, and economic criteria.
JACIE standards on peripheral blood stem cell (PBSC) collection by apheresis require that collection, manipulation, and clinical use of peripheral blood stem cells must be validated and monitored rigorously. The validation procedure consists of systematic review of all apheresis procedures performed at the collection facility of a transplant program.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Stem cell source
- Peripheral stem cell mobilization
- SC mobilization and apheresis
- Vascular access
- Quality and apheresis
5.1 Cell Source: Where Do We Get the Cells From?
Hematopoiesis refers to the production of all types of blood cells including formation, development, and differentiation of blood cells. In adults, hematopoiesis primarily occurs in bone marrow contained in the pelvis, sternum, vertebral column, and skull. All blood cells are derived from progenitor stem cells – pluripotent stem cells. These cells have the capacity for unlimited self-renewal and the ability to differentiate into all types of mature blood cells starting from the common myeloid or the common lymphoid progenitor (Fig. 5.1). This process occurs continually in order to maintain adequate concentrations of circulating components necessary for normal immune system function and hemostasis.
Stem cell maturation cascade (Adapted from EBMT NG (2009)) (Epo erythroprotein, G-CSF granulocyte colony-stimulating factor, GM-CSF granulocyte-macrophage colony-stimulating factor, IL interleukin, M-CSF macrophage colony-stimulating factor, SCF Stem cell factor, Tpo thrombopoietin)
Cells in the myeloid lineage, such as red blood cells, platelets, and white blood cells, are responsible for hemopoiesis (tissue nourishment, oxygenation, coagulation) and immune function such as innate and adaptive immunity. The lymphoid lineage components, namely, T cells and B cells, provide the foundation for the adaptive immune system.
Hematopoietic stem cell (HSC) products for autologous or allogeneic transplantation are available from bone marrow, peripheral blood, and umbilical cord blood (UCB) sources. Bone marrow was the original source of cells for transplantation because of the easier process of collection. UCB has been found and established to have an important role in treatment due to relative immunologic naiveté of the donor with the feasibility of multiple antigen-mismatched transplantations where there is a lack of related or unrelated volunteer donor. UCB had limited use in adult transplantation because of the small cell dose collected which may result in greater risk of posttransplant opportunistic infection due to a longer time to hematologic recovery and a higher risk of primary engraftment failure. However, the use of “double cords” has to a certain extent ameliorated some of the difficulties by improving engraftment times. Nevertheless, late infections remain a concern, and lack of available donor lymphocytes means that other products are often favored over UCB in some settings.
Peripheral blood stem cells (PBSC) have largely replaced the bone marrow in both autologous and allogeneic transplantation setting. The rapid engraftment kinetics of PBSC compared to the bone marrow is widely recognized. Median times to achieve an absolute neutrophil count greater than 500/ul after autologous PBSC transplantation are approximately 11–14 days in autologous setting (Klaus 2007).
In allogeneic settings the choice of HSC product source for transplantation may depend on donor availability. The appropriate donor selection, quality control, and risk assessment are paramount not only for patient and donor safety but also impact on transplant outcome. In the absence of a sibling HLA-identical donor, the search and identification of an unrelated donor may take up to 5 months. Depending on recipients underlying disease and “urgency” for HSCT, haploidentical donors or cord blood unit(s) might be selected (Ruggeri et al. 2015).
Nurses who assist patients during the donation procedure should be particularly aware of factors that influence risk for donation which include:
-
Age
-
Gender
-
Low weight
-
Duration of procedure
-
Type of anesthesia (BM donation)
-
CD34+ cell dose requested
-
Vascular access (PBSC)
5.1.1 Cell Collection
-
Bone Marrow Collection
The bone marrow is harvested from the posterior iliac crest region under general or regional anesthesia in a hospital operating room. For the healthy donor, the risk of serious complication from either general or local anesthesia is similar (Hoffman et al. 2008). With adequate fluid and sometimes blood replacement, overnight hospitalization is often not required. Multiple aspirations are performed with collection of approximately 5 ml of liquid marrow blood from each puncture site. The usual volume harvested from healthy donors is approximately 10–15 ml of marrow per kilogram of recipient body weight in order to achieve the desired CD34+ cell doses. This results in an average blood loss of 800–1000 ml for an adult donor. Some patients and donors receive pre-donated blood transfusions to alleviate symptoms of volume depletion usually with preharvested autologous blood, but salt or colloid solutions are acceptable replacements too. Anesthesia and blood loss present the greatest risk of serious complications associated with bone marrow harvesting, and therefore patients and marrow donors must undergo a detailed medical examination including questionnaire of health history. After confirming they wish to proceed to be a donor, they are asked to sign the written consent to undergo donation.
-
Peripheral Blood Stem Cells (PBSC)
The concentrations of hematopoietic stem cells (HSC) in the peripheral blood are normally very low compared to 10–100 times greater concentration in the bone marrow (EBMT NG 2009). Therefore it is necessary to increase the circulating concentrations of HSC for adequate PBSC collections. HSC mobilization into the peripheral blood can be stimulated by different disease-related and relatively predictable mobilization regimens. Rapid and widespread adoption of PBSC as a source of HSC for transplantation is due to rapidly available results of CD34+ count by the laboratory. Before commencing a leukapheresis, we count the number of CD34+ cells in peripheral blood, and if the number is adequate, the collection procedure may be performed.
-
Cord Blood Collection
UCB is collected from the placental vein after infant delivery and transection of the cord. UCB can be collected either by the obstetrician before delivery of the placenta or by laboratory personnel after delivery of the placenta. The timing of cord clamping after delivery of the infant is associated with the volume of cord blood collected with earlier clamping relating to greater collection volumes. Cell dose is an important predictor of outcome after UCB transplantation, and many cord blood units are discarded because of small cell doses. Greater cell quantities are found for infants with greater birth weight, independent of gender and gestational age. Many cord blood banks reduce the volume of the product by depleting red cells and plasma in order to minimize storage space and reduce possible infusion-related toxicities from mature blood cells contained in unfractioned cord blood units. UCB will maintain viability for period of at least 15 years if appropriately cryopreserved (Hoffman et al. 2008; Schoemans et al. 2006). Allogeneic transplant access can be severely limited for patients of racial and ethnic minorities without suitable sibling donors. Whether umbilical cord blood (UCB) transplantation can extend transplant access because of the reduced stringency of required HLA match is not proven.
Patients had highly diverse ancestries including 35% non-Europeans. In Barker’s article from 2010 (Barker n.d.), of 525 patients undergoing combined searches, 10/10 HLA-matched URDs were identified in 53% of those with European ancestry but only 21% of patients with non-European origins. However, the majority of both groups had 5–6/6 UCB units. Availability of UCB significantly extends allotransplant access, especially in non-European patients, and has the greatest potential to provide a suitable stem cell source regardless of race or ethnicity. Minority patients in need of allografts, but without suitable matched sibling donors, should be referred for combined URD and CB searches to optimize transplant access.
5.2 Mobilization of Stem Cells and Apheresis
Administration of hematopoietic cytokines, such as granulocyte colony-stimulating factor (G-CSF), causes a transient increase (mobilization) of HSC in the peripheral bloodstream and enables the collection of adequate numbers of PBSC for transplantation.
Pluripotent stem cells express the cell surface marker antigen CD34 (CD34+ cells). Cytokine- mobilized PBSC components contain much greater numbers of cells expressing the CD34 antigen which then acts as a surrogate marker for the engraftment capacity of the stem cell component.
5.2.1 Cytokines
Several cytokines have been identified to play an important role in hematopoiesis. When progenitor cells are exposed to these cytokines, the maturation cascade producing committed mature blood cell components can occur. Examples of important cytokines are listed in Fig. 5.1. These cytokines are externally administered to patients in an effort to enhance the yield of stem cells within a short time period. An example of such a cytokine is glycosylated granulocyte colony-stimulating factor (G-CSF).
Chemokines are a subset of cytokines and can induce activation and migration of specific types of white cell and assist the process of stem cells homing to the marrow compartment. Stem cell CXCR4 is responsible for anchoring stem cells to the marrow matrix through interactions with adhesion molecules such as stem cell-derived factor-1 alpha (SDF-1α). Blockade of this receptor with a receptor antagonist such as plerixafor has produced elevations of circulating hematopoietic progenitor cells, which has aided stem cell collections in patients with multiple myeloma and lymphoma “poor mobilizers,” those not able to reach the sufficient number of CD34+ cells for commencing a leukapheresis.
Because of its efficacy compared to other cytokines and its low toxicity profile, G-CSF is the cytokine most commonly used to increase the level of myeloid progenitor cells in the blood.
Recombinant methionyl human G-CSF (filgrastim) and recombinant human G-CSF (lenograstim) are the two forms of this cytokine available for clinical use.
In clinical practice, for autologous PBSC, the most frequent mobilization procedure is the administration of filgrastim in combination with chemotherapy. Nowadays we are observing increased use of mobilization regimens without chemotherapy (chemo-free mobilization regimens) in patients affected by multiple myeloma (Afifi et al. 2016).
5.2.2 The Role of CD34+
CD34+ is the indicator most frequently used in clinical practice to determine the extent and efficiency of peripheral blood stem cell collections (Brando et al. 2000). Once specific cell dose targets are achieved, cell collections are completed and stored for future use. Standard target levels can vary among treating centers, and a patient’s specific goal is based on the underlying disease, the source of stem cells, and the type of transplant to be performed. In general, a target level of 2 × 106 CD34+ cells/kg recipient body weight is considered the minimum for transplant with optimal levels being > 5 × 106 CD34+ cells/kg for a single transplant and > 6 × 106 CD34+ cells/kg for a tandem transplant (Pierelli et al. 2012).
Peripheral blood stem cell is considered the preferred cell source due to patient convenience, decreased morbidity, and faster engraftment of white blood cells and platelets (Table 5.1).
Poor stem cell yields after mobilization might occur. The most important risk factor for inadequate mobilization is the amount of myelosuppressive chemotherapy a patient has received prior to their autologous collection. Agents which are toxic to stem cells such as cyclophosphamide (doses > 7.5 g), melphalan, carmustine, procarbazine, fludarabine, nitrogen mustard, and chlorambucil are particularly detrimental to stem cell collection yields. Other risk factors associated with low CD34+ cell collections include advanced age (> 60 years), previous radiation therapy, short time interval between chemotherapy and mobilization, extensive disease burden, and tumor infiltration of the bone marrow (Olivieri et al. 2012).
5.2.3 Chemo-mobilization
Chemo-mobilization is a combination of myelosuppressive chemotherapy with filgrastim which appears to work synergistically to mobilize HSC, although the exact mechanism for this observation has not been fully elucidated. Filgrastim is thought to stimulate HSC mobilization by decreasing SDF-1 gene expression and protein levels while increasing proteases that can cleave interactions between HSC and the bone marrow environment. These growth factors are typically given as subcutaneous injections at a total daily dose of 3–24 mcg/kg/day.
5.2.4 Alternative Mobilization Strategies
Despite different mobilization regimen attempts, some patients are not able to achieve enough CD34+ cells to start apheresis. Those groups of patients are defined “poor mobilizers.” In this case the use of plerixafor, CXCR4 antagonist that reversibly inhibits the interaction between CXCR4 and SDF-1, is needed. The use of plerixafor in combination with G-CSF has been shown to improve CD34+ cell collections in lymphoma and multiple myeloma patients (Olivieri et al. 2012).
Because no single chemotherapy mobilization regimen has demonstrated superiority, some clinicians may elect to mobilize patients during a cycle of a disease-directed chemotherapy regimen. With this approach, examples of regimens utilized have included cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and ifosfamide, carboplatin, and etoposide (ICE).
A comparison between mobilization methods is listed on Tables 5.2, 5.3, and 5.4.
5.2.5 PBSC Collection by Apheresis
The quantity of CD34+ cells in a PBSC component varies greatly, and its achievement by apheresis procedure depends on:
-
Mobilization protocol
-
Patient condition
-
Timing of the PBSC collection
-
Equipment
-
Operator-dependent technique
-
Volume of blood processed
The timing of PBSC collection has a critical role, and based on the mobilization regimen, we are able to appropriately schedule the apheresis procedures. For growth factor mobilization alone, the first collection procedure is calculated on days 4–5 when the peak of CD34+ cell count is expected to be achieved. After mobilization with chemotherapy regimens and growth factor, the expected day can vary between days 12 and 15 (Pierelli et al. 2012).
The first day collection procedure is determined by WBC count and CD34+ cell count. Established thresholds for apheresis initiation may vary across centers but typically range from 10 to 20 CD34+ cells/mL. Once mobilization has reached an optimal level according to WBC and CD34+ levels, a patient can attend their scheduled sessions in the apheresis area. Optimized scheduling will avoid unnecessary collection procedures and unnecessary PBSC processing and will save freezing space.
The apheresis objective is to collect a product with requested PBSC counts with low cross cellular contamination in the smallest possible collect volume (ideally 80–90 ml/bag) and in as few procedures as possible. This will ensure cost optimization and enhance patient comfort and safety.
Clinical nurses working in the apheresis unit are responsible for educating the patient about the stem cell collection process and monitoring patients for any adverse reactions. The apheresis nurse challenges are:
-
To understand how and when to administer agents used in the mobilization process
-
To schedule mobilization regimen which occurs with or without chemotherapy
-
To explain what medications the patient should and should not take during mobilization
-
To expect adverse events and to be aware of their management for all agents used in mobilization
-
To manage venous access catheter used for apheresis
-
To be aware of the importance of laboratory monitoring and how to manage electrolyte imbalances
-
To be aware of stem cell collection target level and options for patients who mobilize poorly or fail to mobilize
Patients are connected to the apheresis machine by their centrally or peripherally inserted venous catheters. One lumen is used to withdraw blood out of the patient and into the machine. Here the blood is centrifuged in a bowl housed within the cell separator machine. The desired stem cells are then siphoned off before the remaining blood components are returned to the patient through the second lumen of their catheter. This second lumen can be used to administer intravenous fluids, electrolyte supplements, and medications to the patient if necessary. Each apheresis session lasts approximately 2–4 h during which an average of 7–10 l of blood, or twice the average total human blood volume, is exchanged or processed. Collections can occur on a daily basis until target CD34+ levels are achieved, which can last for up to 3–4 days depending on patient characteristics and mobilization regimen used (Figs. 5.2 and 5.3).
5.3 Vascular Access
Appropriate catheter selection and placement is scheduled prior to the first stem cell collection (Toro et al. 2007). Catheters used for apheresis procedures must be able to tolerate large fluctuations in circulating blood volume on more than one occasion. Peripheral catheters are preferred where possible and placed in the cubital vein for drawing. If available, a port-a-cath or other previously inserted central catheters can be used for blood return.
In the absence of adequate peripheral veins, a large-bore, double lumen device can be inserted. These can be used temporarily during cell collections only, or they can be placed permanently and used throughout the transplant process. As with most centrally placed catheters, when placed in the area of the upper extremities, patients should be monitored for signs and symptoms of hypotension, shortness of breath, and decreased breath sounds as these can be indicative of venous wall perforation and require urgent attention. Hemothorax and pneumothorax, which are rare but serious complications, can occur and also require immediate attention. In some cases, catheters for apheresis may be placed in a femoral vein. In case of catheter positioning in the jugular vein, radiographic evaluation is used to verify catheter placement prior to clearance for its use. Instructions on caring for the catheter to prevent infection and maintain patency should be extensively reviewed with the patient and/or caregivers. Internal SOPs need to be observed.
5.4 Adverse Reactions
Apheresis procedures are relatively safe for the patient. While the procedure-related mortality rates are low at an estimated 3 deaths per 10,000 procedures, apheresis-associated morbidity is more frequently reported (Halter et al. 2009).
5.4.1 Citrate Toxicity
One of the most common adverse effects seen during the cell collection process is citrate toxicity, frequently manifested by hypocalcemia. Sodium citrate is used during apheresis to prevent blood from clotting while it is being processed by the machine. Citrate is known to bind to ionized serum calcium leading to hypocalcemia. Signs and symptoms of this complication can include:
-
Burning
-
Numbness and tingling in the extremities and/or the area around the mouth
-
Muscle twitching
-
Abdominal cramps
-
Shivering
5.4.2 Treatment
Citrate toxicity complication can be managed by slowing the apheresis flow rate and providing patients with oral calcium supplements. In severe cases, intravenous supplementation of calcium may be given to the patient in order to prevent severe reactions such as tetany, seizure, and cardiac arrhythmia. Serum monitoring of calcium levels prior to each apheresis session is often helpful in decreasing the likelihood of hypocalcemia.
Other effects stemming from citrate toxicity include hypomagnesemia, hypokalemia, and metabolic alkalosis. Magnesium, like calcium, is a bivalent ion that is bound by citrate. Declines in serum magnesium levels often are more pronounced and take longer to normalize compared to aberrations in calcium levels. Signs and symptoms of hypomagnesemia are muscle weakness or spasm, decreased vascular tone, and abnormal cardiac contractility. Oral and intravenous supplementation with magnesium and potassium is often effective.
5.4.3 Hypovolemia
Because large fluctuations in blood volume can be experienced throughout each collection, patients are at risk of hypovolemia. Prior to starting the collection, baseline pulse and blood pressure should be measured and continually rechecked at designated intervals of approximately 1 h. It is also recommended that hemoglobin and hematocrit be monitored after the procedure as well.
5.4.4 Risk Factors
Patients at risk for developing hypovolemia include those with anemia, a previous history of cardiovascular compromise, and children or adults with a small frame.
5.4.5 Preventative Measures
Preventative measures are aimed at minimizing the extracorporeal volume shift by priming the apheresis machine with red blood cells and fresh frozen plasma in place of normal saline.
5.4.6 Clinical Manifestations
Clinical manifestations of hypovolemia can include dizziness, light-headedness, tachycardia, hypotension, and diaphoresis. Most concerning is the development of a cardiac dysrhythmia which can be life-threatening.
5.4.7 Treatment
Sessions should be interrupted and symptoms should subside before proceeding with collections. Hypovolemia may also be managed with providing intravenous fluid boluses and slowing the rate of flow on the apheresis machine.
5.4.8 Thrombocytopenia
Thrombocytopenia is another complication encountered during stem cell collections. When the patient’s blood is in the cell separator machine, platelets can stick to the bowl used during the centrifuge process. Decreases in platelet concentrations can be precipitous, and obtaining platelet counts prior to each collection is essential.
5.4.9 Treatment
If platelet levels are low, patients may receive a platelet transfusion prior to the procedure. Also helpful, but not necessarily therapeutic, in managing thrombocytopenia is returning the collected platelet-rich plasma to the patient at the conclusion of the collection session.
5.5 Patient Assessment and Preparation
Prior to initiation of the stem cell collection process, patient candidates for autologous transplantation procedure must be thoroughly evaluated and determined fit to be able to tolerate all involved procedures.
5.5.1 Medical Assessment
The medical evaluation is the first step that a patient must complete when undergoing treatment. This involves the patient’s primary medical oncologist making a referral to a transplant center or service. The physician provides the transplant team with information which often includes specifics relating to the care of the patient up to this time point including past medical history, cancer status, summary of cancer treatments and response, and complications experienced during therapy. Accompanying this information are any available radiographic information and laboratory testing results. After review of the patient’s medical information, the transplant team will initiate their own procedure-specific tests and evaluations to assess a patient’s eligibility to proceed with stem cell collection and transplant. This involves restaging the patient to verify or establish their current disease status, ascertaining the function of various organ systems (e.g., renal, hepatic, pulmonary, etc.), documenting the absence of certain comorbid conditions and diseases (e.g., the presence of human immunodeficiency virus), and evaluating the overall performance status and psychosocial condition of the patient.
5.5.2 Patient Education
Education of the patient and their respective family and/or caregivers is integral to preparatory phase. Often a member of the nursing profession (clinic nurse, nurse educator, or nurse coordinator) will coordinate the education process for the patient and carers.
5.5.3 Donor Assessment and Preparation
Allogeneic related or non-related donors are submitted initially to interview and medical history questionnaire evaluation, including vaccination and travel history, blood transfusion, and risk for transmission of communicable disease together with physical and psychological exams that involves multidisciplinary teamwork and coordination as well.
The risks of donation shall be evaluated, discussed, and documented as a possible need for central venous access, the risk of hemoglobinopathy prior to administration of the mobilization therapy for collection of HPC, apheresis, and vascular access or anesthesia for collection of HPC by bone marrow harvesting.
Allogeneic donor eligibility, as defined by applicable laws and regulations, shall be determined by a physician after history, exam, medical record review, and testing. The donor eligibility determination shall be documented clearly in the recipient’s medical record before the recipient’s preparative regimen is initiated and before the allogeneic donor begins the mobilization regimen (EBMT NG 2009; Pierelli et al. 2012; JACIE n.d.).
The collection procedure shall be explained in terms the donor can understand the risks and benefits of the procedure, tests and procedures performed, and the rights according to applicable laws and regulations. Alternative collection methods need to be explained, and confidentiality must be guaranteed (EBMT NG 2009; JACIE n.d.). The donor shall have the right to refuse to donate but shall be informed of the potential consequences to the recipient of such refusal. Informed consent from the allogeneic donor shall be obtained by a licensed health-care professional who is not the primary health-care professional overseeing care of the recipient.
5.6 Quality in Apheresis
FACT-JACIE International Standards Accreditation (sixth edition) (JACIE n.d.) requires that the clinical program has access to personnel who are formally trained, experienced, and competent in the management of patients receiving cellular therapy. The Apheresis Collection Facility shall be licensed, registered, or accredited as required by the appropriate governmental authorities for the activities performed. The quality management plan should include, or summarize and reference, policies and standard operating procedures addressing personnel requirements for each key position in the Apheresis Collection Facility.
5.6.1 Training and Competencies
Core competencies are specified within the JACIE standards, and evidence of training in these competencies must be documented. This may be achieved by evidence of in-service training, attendance at conferences, etc. While initial supervised training is easily documented, annual competency maintenance can be difficult to demonstrate. Ongoing training for clinical personnel should reflect their experience, individual competencies and proficiencies, orientation for new staff, and necessary training. Training also needs to be undertaken in a timely manner to demonstrate annual updating (Babic et al. 2015).
5.7 Cell Source and Apheresis in the Pediatric Population
Abstract
Hematopoietic stem cell transplantation has become a well-established treatment for many malignant and nonmalignant disorders in children. Small body weight, venous access, and ethical dilemmas in minors represent a challenge in the pediatric population.
Keywords
Apheresis • Cell source • Children • Pediatric population
5.7.1 Introduction
During the past three decades, bone marrow transplantation and transplantation of peripheral blood stem cells have become a well-established treatment for many malignant and nonmalignant disorders in children and in adult patients (Bader et al. 2005).
Research has shown a continued and constant increase in the annual numbers of hematopoietic stem cell transplant (HSCT) and transplant rates (number of HSCT/10 million inhabitants) for both allogeneic and autologous HSCT (Passweg et al. 2013).
Indications for pediatric HSCT have changed considerably during the last 7 years. These changes provide tools for decision-making in health-care planning and counseling (Miano et al. 2007).
There is accumulating evidence of the role of hematopoietic SCT in non-hematological disorders such as autoimmune diseases (Sureda et al. 2015).
Some common nonmalignant diseases treated with hematopoietic stem cell transplant are (Nuss et al. 2011):
-
Hematologic (severe aplastic anemia, Fanconi anemia, thalassemia, sickle-cell disease, Diamond-Blackfan anemia, Chédiak-Higashi syndrome, chronic granulomatous disease, congenital neutropenia)
-
Solid tumors (Ewing’s sarcoma, soft tissue sarcoma, neuroblastoma, and Wilms’ tumor, where there is high risk or 4CR1, osteogenic sarcoma, and brain tumors)
-
Immunodeficiency (severe combined immunodeficiency disease, Wiskott-Aldrich syndrome, functional T-cell deficiency)
-
Genetic (adrenoleukodystrophy, metachromatic leukodystrophy, Hurler syndrome, Hunter disease, Gaucher syndrome)
-
Cell Source in Pediatric Population
-
There are three commonly used sources of hematopoietic stem cells:
-
BM
-
Cytokine-mobilized PB progenitor cells (PBSC)
-
UCB cells
The proportion of autologous to allogeneic HSCT is different in pediatric population (29% autologous) compared with adults (62% autologous) and is mainly used for treating solid tumors (Passweg et al. 2013).
Stem cells may be collected from the bone marrow (BM), peripheral blood, or umbilical cord blood (UCB). Each of these sources has several advantages and disadvantages.
Despite the increased use of peripheral blood and umbilical cord blood, BM is the primary graft source in pediatric patients.
For children and adolescents aged 8–20, allogeneic transplantation of HLA antigen-identical sibling peripheral blood stem cells is associated with higher mortality than the bone marrow (Eapen, et al JCO 2004). In contrast, previous work showed that peripheral blood was superior to the bone marrow as a stem cell source for adult and adolescent (aged 12–55) recipients of matched related donor grafts (Bensinger et al. N Engl J Med. 2001). More pediatric patients receive BM than PBSC, irrespective of donor type. This is explained by the higher incidence of nonmalignant conditions and the higher risk for chronic GvHD with peripheral blood as a stem cell source (Passweg et al. 2013).
The bone marrow remains the most common graft source for unrelated donors accounting for 49% in 2013. The use of umbilical cord blood is declining steadily, after peak in 2009, from 46% to 32% of all unrelated donor transplants performed in this age group (Sureda et al. 2015).
However, the recently completed NMDP/CIBMTR randomized study of unrelated marrow versus PBSC, which included pediatric patients, found that there were no significant differences in mortality between recipients of PBSC compared to the bone marrow.
The donor’s preferences must also be taken into account as there are differences in the side effects experienced by the donors from a BM or PBSC harvest (Sureda et al. 2015; Pasquini and Zhu 2016).
From 2012 onward, there was an increase in the numbers of transplants from other relatives, which is likely due to rapid adoption of the strategy of posttransplant cyclophosphamide in the haploidentical setting. In 2014, the numbers of transplants using other relatives surpassed the total numbers of umbilical cord transplant performed in the USA, accounting for 11% of all allogeneic transplants performed in the USA (Pasquini and Zhu 2016).
Annual numbers for allogeneic transplants in pediatric population demonstrate increase in the overall utilization of alternative donors.
Box: Bone marrow stem cell source in nonmalignant diseases in pediatric population
-
The BM remained the preferred source of stem cells for allogeneic transplants for nonmalignant disorders (62%). For diseases that do not require graft-versus-tumor effect, such as aplastic anemia, sickle-cell disease, or metabolic disorders, many consider it advantageous to use the bone marrow. Disadvantages include the need for general or epidural anesthesia and the risk of infection, bleeding, and bone damage. Also this population, although a minority, is still a significant minority that includes a number of unique diagnoses such as the hemoglobinopathies, immune deficiencies, immune dysregulation, and metabolic diseases, all of which are not common or present at all in the adult HSCT population (Passweg et al. 2013).
From 2003 to 2011, there was a steady increase in the numbers of transplants using umbilical cord blood as a result of several published studies demonstrating its benefit in both children and adults.
Umbilical cord blood (UCB) characteristically differs from the marrow in a number of ways. The median doses of total nucleated cells (TNC), CD34+ cells, and CD3+ cells in UCB unit are approximately ten times lower than that of a bone marrow graft (Moscardo et al. 2004; Barker and Wagner 2003).
The indications for the use of UCB as a source for stem cells in children are identical to the indications for matched unrelated donor transplants (Sureda et al. 2015).
5.7.2 Apheresis in Pediatric Population
Experience with pediatric stem cell apheresis collections is limited. Challenges of apheresis in small children (<20 kg) include:
-
Small total blood volume
-
Vascular access issues
-
Concerns about tolerable anticoagulant doses
-
Limitations in product volumes that can be safely collected
Therefore, in many countries worldwide, children under the age of 18 years are not allowed to donate hematopoietic stem cells (HSCs) for unrelated recipients (Sörensen et al. 2013).
Adequate peripheral vascular access is difficult to find in young children, so we often need to consider a central apheresis catheter placement with its attendant risks.
Placement of central venous catheter (5–7 Fr with double offset lumens) is widely used for apheresis procedure; therefore, some PBSC donors receive a general anesthesia, and others have conscious sedation.
The apheresis team must consider the size and type of catheter that will yield the highest flow rate during apheresis, as well as patient or donor comfort. Often the catheter used for apheresis may be used for venous access during the high-dose therapy, reinfusion of stem cells, and recovery phases. We should try to avoid adverse events such as pain, hemorrhage, and inadequate sedation. A trained team in pediatric apheresis is mandatory as well as central line placement by ultrasound technique.
5.7.3 Key Differences: Pediatric vs Adult
Apheresis procedure in children differs from adult in device (machine set) priming.
Priming of the apheresis circuit with heterologous packed red blood cells is widely undertaken where donors weigh less than 20 kg. We should consider the risk of heterologous blood product administration in healthy donors – such as transfusion reaction and the risk of system overload if primed blood for some reason is reinfused. Usually we prime with RBC cross-matched, irradiated and leukocyte-depleted in patient and prime with 4% albumin solution in donors.
In the pediatric setting, the most common adverse event is pain, observed after central venous catheter (CVC) placement for PBSC collection. Pain at the site of puncture occurs more frequently in donors with a central (58%) than peripheral vein access (38%) (Hequet, 2015).
Often reported side effects after pediatric apheresis are:
-
Hematoma formation
-
Hypotension and cyanosis
-
Allergic reaction to red blood cells
-
Thrombocytopenia
Rare side effects reported are:
-
Low-grade fever during the mobilization
-
Hypovolemic signs: tachycardia >120 (most cases), hypotension, systolic blood pressure < 80 mmHg, pallor, and diaphoresis
-
Nausea related to citrate effects during the apheresis procedure
In the absence of consensus and in order to prevent signs and symptoms of hypocalcemia, some centers administrate orally calcium gluconate (adult patients), and some centers routinely replace calcium i.v. during the entire apheresis procedure (mostly in pediatric apheresis). Nurses who perform pediatric procedures need to achieve competence in machine settings to allow the proper anticoagulation of the patient during the procedure (ACD-A or heparin or combination anticoagulation). The nurse must be competent in blood priming and in use of diluted or undiluted packed red blood cells, in prevention of hypocalcaemia and fluid balance, etc.
5.7.4 Ethical Dilemmas
The approach to minor donors is different in many countries.
Styczynski and colleagues compared donor and recipient children’s age, both being small where donors were with smaller body weight than recipient and at higher risk for requiring a blood transfusion, additional apheresis procedures, pain, and cardiovascular complications after anesthesia. Most pediatric physicians who perform transplants believe it is acceptable to expose minors to the risks of a stem cell donation when donation offers a substantial prospect of benefit to a close family member and when proper consent is obtained (often from parents of both, donor and recipient).
The key issue that must be addressed with childhood HSC donors is their initial inability to understand and to voluntarily consent the procedure. Understanding increases as they age into an ability to assent and then finally to legally consent. Because HSC donation can benefit their sibling more than any other tissue sources and because the procedure can be performed with limited risk, sibling donation under parental consent has been considered appropriate to date (Bitan et al. 2016).
Summarizing:
-
Advocacy and medical review of donors by individual(s) without dual responsibility to the recipient are recommended.
-
Regarding the relationship between a pediatric donor and a family recipient, it is recommended to focus on avoiding psychological harm rather than predicting whether donation will result in a psychological benefit to the donor.
-
Pediatric donors may be considered for research that carries minimal risk above the standard procedure or studies aimed at improving the safety and efficacy of the donation process.
-
Donors with medical conditions that may increase the risk of complications associated with donation are not ever to be considered fit for donation.
-
Centers should always avoid performing human leukocyte antigen typing on potential donors with medical/psychological reasons not to donate (Bitan et al. 2016).
5.7.5 Psychosocial Risks and Benefits
The primary benefit to the donor is the psychosocial value of helping a sibling or other close family members. This benefit may accrue even if the transplant is unsuccessful, because the donor and family can at least be reassured that the stem cell transplant was tried. There is a small but growing literature on the psychosocial risks and harms caused by hematopoietic stem cell donation by children. Data show that many children experience distress related to their role as a donor. Many pediatric donors believe that they did not have a choice about whether to serve as a marrow donor, report being poorly prepared for the procedures, and describe feeling responsible for the recipient’s course after transplantation (Weisz 1996).
References
Afifi S, Adel NG, Devlin S, et al. Upfront plerixafor plus G-CSF versus cyclophosphamide plus G-CSF for stem cell mobilization in multiple myeloma: efficacy and cost analysis study. Bone Marrow Transplant. 2016;51:546–52.
Babic A, Wannesson L, Trobia M. Transplant unit personnel competency maintenance: online testing by sharepoint application. Lazzaro – EBMT 2015, oral presentation N003.
Bader P, Niethammer D, Willasch A, Kreyenberg H, Klingebiel T. How and when should we monitor chimerism after allogeneic stem cell transplantation? Bone Marrow Transplant. 2005;35(2):107–19. Review. PubMed PMID: 15502849.
Barker JN. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. n.d.. http://www.sciencedirect.com/science/article/pii/S1083879110003502.
Barker JN, Wagner WJ. Umbilical-cord blood transplantation for the treatment of cancer (review). Nat Rev Cancer. 2003;3(7):526–32.
Bitan M, van Walraven SM, Worel N, Ball LM, Styczynski J, Torrabadella M, Witt V, Shaw BE, Seber A, Yabe H, Greinix HT, Peters C, Gluckman E, Rocha V, Halter J, Pulsipher MA. Determination of eligibility in related pediatric hematopoietic cell donors: ethical and clinical considerations. Recommendations from a Working Group of the Worldwide Network for Blood and Marrow Transplantation Association. Biol Blood Marrow Transplant. 2016;22(1):96–103.
Brando B, Barnett D, Janossy G, et al. Cytofluorimetric methods for assessing absolute numbers of cell subsets in blood. European Working Group on Clinical Cell Analysis. Cytometry. 2000;42(6):327–46.
Klaus J. Effect of CD34. cell dose on hematopoietic reconstitution and outcome in 508 patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Eur J Haematol. 2007;78(1):21–8.
Haematopoietic stem cell mobilisation and apheresis: a practical guide for nurses and other allied health care professionals (EBMT NG), 2009.
Halter J, Kodera Y, Ispizua AU, et al. Severe events in donors after allogeneic hematopoietic stem cell donation. Haematologica. 2009;94(1):94–101.
Hequet OJ. Hematopoietic stem and progenitor cell harvesting: technical advances and clinical utility. J Blood Med. 2015;6:55–67.
Hoffman R, et al. Hematology, Basic principles and practice. In: Practical aspects of stem cell collection, vol. 2: Churchill Livingstone-Elsevier; 2008. p. 1695–7. ISBN: 978-0-443-06715-0.
International standards for hematopoietic cellular therapy product collection, processing, and administration – Foundation for the Accreditation of Cellular Therapy (FACT) and Joint Accreditation Committee -ISCT and EBMT (JACIE). n.d..
Miano M, Labopin M, Hartmann O, Angelucci E, Cornish J, Gluckman E, Locatelli F, Fischer A, Egeler RM, Or R, Peters C, Ortega J, Veys P, Bordigoni P, Ior AP, Niethammer D, Rocha V, Dini G. Haematopoietic stem cell transplantation trends in children over the last three decades: a survey by the paediatric diseases working party of the European Group for Blood and Marrow Transplantation for the Paediatric Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2007;39:89–99. https://doi.org/10.1038/sj.bmt.1705550.
Moscardo F, Sanz GF, Sanz MA. Unrelated-donor cord blood transplantation for adult haematological malignancies (review). Leuk Lymphoma. 2004;45(1):11.
Nuss S, Barnes Y, Fisher V, Olson E, Skeens M. In: Hematopoietic cell transplantation. In: Baggott C, Fochtman D, Foley GV, Kelly KP, editors. Nursing care of children and adolescent with cancer and blood disorders. 4th ed: APHON Association of Pediatric Hematology/Oncology Nurses; 4th edition 2011. p. 405–66.
Olivieri A, Marchetti M, Lemoli R, et al. Proposed definition of “poor mobilizer” in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo Italiano trapianto di Midollo Osseo. Bone Marrow Transplant. 2012;47(3):342–51.
Pasquini MC, Zhu X. CIBMTR.Summary slides – HCT trends and survival data current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR summary slides. Available at: http://www.cibmtr.org. Accessed 05 Sept 2016.
Passweg JR, Baldomero H, Bregni M, Cesaro S, Dreger P, Duarte RF, Falkenburg JH, Kröger N, Farge-Bancel D, Gaspar HB, Marsh J, Mohty M, Peters C, Sureda A, Velardi A, Ruiz de Elvira C, Madrigal A. European Group for Blood and Marrow Transplantation. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant. 2013;48(9):1161–7. https://doi.org/10.1038/bmt.2013.51. PubMed PMID: 23584439; PubMed Central PMCID: PMC3763517.
Perseghin P, et al. A policy for the disposal of autologous hematopoietic progenitor cells: report from an Italian consensus panel. Transfusion. 2014;54(9):2353–60. https://doi.org/10.1111/trf.12619. Epub 2014 Mar 24
Pierelli L, Perseghin P, Marchetti M, et al. Best practice for peripheral blood progenitor cell mobilization and collection in adults and children: results of a Società Italiana Di Emaferesi e Manipolazione Cellulare (SIDEM) and Gruppo Italiano Trapianto Midollo Osseo (GITMO) consensus process. Transfusion. 2012;52(4):893–905.
Pulsipher MA, Levine JE, Hayashi RJ, Chan KW, Anderson P, Duerst R, Osunkwo I, Fisher V, Horn B. Safety and efficacy of allogeneic PBSC collection in normal pediatric donors: the pediatric blood and marrow transplant consortium experience (PBMTC) 1996–2003. Bone Marrow Transplant. 2005;35(4):361–7. PubMed PMID: 15608659
Ruggeri A, Labopin M, Sanz G, et al. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29(9):1891–900. Epub 2015 Apr 17
Schoemans H, Theunissen K, Maertens J, et al. Adult umbilical cord blood transplantation: a comprehensive review. Bone Marrow Transplant. 2006;38:83–93.
Sörensen J, Jarisch A, Smorta C, Köhl U, Bader P, Seifried E, Bönig H. Pediatric apheresis with a novel apheresis device with electronic interface control. Transfusion. 2013;53(4):761–5.
Styczynski J, Balduzzi A, Gil L, Labopin M, Hamladji R, Marktel S, Akif Yesilipek M, Fagioli F, Ehlert K, Matulova M, Dalle J-H, Wachowiak J, Miano M, Messina C, Diaz MA, Vermylen C, Eyrich M, Badell I, Dreger P, Gozdzik J, Hutt D, Rascon J, Dini G, Peters C. Risk of complications during hematopoietic stem cell collection in pediatric sibling donors: a prospective European Group for Blood and Marrow Transplantation Pediatric Diseases Working Party study. Blood. 2012;119(12):2935–42.
Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, Dufour C, Falkenburg JH, Farge-Bancel D, Gennery A, Kröger N, Lanza F, Marsh JC, Nagler A, Peters C, Velardi A, Mohty M, Madrigal A. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 2015;50(8):1037–56. Epub 2015 Mar 23
Toro JJ, Morales M, Loberiza F, Ochoa-Bayona JL, Freytes CO. Patterns of use of vascular access devices in patients undergoing hematopoietic stem cell transplantation: results of an international survey. Support Care Cancer. 2007;15:1375–83.
Weisz VR. Risks and benefits of pediatric bone marrow donation: a critical need for research. Behav Sci Law. 1996;14(4):375–91. https://www.ncbi.nlm.nih.gov/pubmed/9156419
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the work's Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work's Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2018 EBMT and the Author(s)
About this chapter
Cite this chapter
Babic, A., Trigoso, E. (2018). Cell Source and Apheresis. In: Kenyon, M., Babic, A. (eds) The European Blood and Marrow Transplantation Textbook for Nurses. Springer, Cham. https://doi.org/10.1007/978-3-319-50026-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-50026-3_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-50025-6
Online ISBN: 978-3-319-50026-3
eBook Packages: MedicineMedicine (R0)