Abstract
Primary ramification morphogenesis has a significant influence on the yield of rapeseed. In order to quantify the relationship between rapeseed architecture indices and the organ biomass, a rapeseed primary ramification structural model based on biomass were presented. Intended to explain effects of cultivars and environmental conditions on rapeseed PR morphogenesis. The outdoor experiment with cultivars: Ningyou 18 (V1, conventional), Ningyou 16 (V2, conventional) and Ningza 19 (V3, hybrid), and designed treatment of cultivar-fertilizer, cultivar-fertilizer-density, and cultivar tests in 2011–2012 and 2012–2013. The experimental result showing that the leaf blade length of PR, leaf blade width of PR, leaf blade bowstring length of PR, PR length, and PR diameter from 2011 to 2012 were goodness, and their d a values and RMSE values were −1.900 cm, 5.033 cm (n = 125); −0.055 cm, 3.233 cm (n = 117); 0.274 cm, 2.810 cm (n = 87); −0.720 cm, 3.272 cm (n = 90); 0.374 cm, 0.778 cm (n = 514); 0.137 cm, 1.193 cm (n = 514); 0.806 cm, 8.990 cm (n = 145); and −0.025 cm, 0.102 cm (n = 153), respectively. The correlations between observation and simulation in the morphological indices were significant at P < 0.001, but the d ap values were <5 % for the second leaves length and the third leaves length, leaf blade bowstring length, PR length, and PR diameter, which indicated that the model’s accuracy was high. The models established in this paper had definite mechanism and interpretation, and the impact factors of N, the ratio of the leaf length to leaf dry weight of primary ramification (PRRLW), and the partitioning coefficient of leaf blade dry weight of primary ramification (PRCPLB) were presented, enabled to develop a link between the plant biomass and its morphogenesis. Thus, the rapeseed growth model and the rapeseed morphological model can be combined through organ biomass, which set a reference for the establishment of FSPMs of rapeseed.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Rapeseed is a very good oil crop with high economic and nutritional values. In the ten years from 2004 to 2014, the production and consumption of rapeseed have increased significantly, and the growth rates of the plant area and total production of rapeseed were 35 % and 54 %, respectively [1]. Plant morphological structure simulation and visualization is one of the important content of agro-informatics in nowadays, and its latest trend is to establish Functional-structural Plant Models (FSPMs). Some crop morphological structure models had been developed based on GDD [2–7], which described the main stems, branches, leaves, leaf sheaths, and internodes of the morphological model of the crops, and realized the quantitative simulation of the crop morphogenesis process. With the maturity of the research conditions, crop morphological structure and physiological ecological process will become the new focus. In order to analyze the relationships between the morphological parameters of the organs of crops and biomass, some related studies had been reported [8–11]. Rapeseed morphology directly influences its biomass production. In recent years, studies of the rapeseed morphological structure model had also been proposed [12–14]. A rapeseed leaf geometric parameters model based on biomass presented quantified the relationship between biomass and rapeseed leaf geometric parameters, a leaf curve model based on biomass for rapeseed was established, which described the relationships between the leaf curve and the corresponding leaf biomass for rapeseed on main stem, and a morphological structure model of leaf blade space based on biomass at pre-overwintering stage in rapeseed plant presented revealed the relationships between biomass and rapeseed architecture indices. These studies laid a good foundation for FSPMs of rapeseed. By combining LEAFC3-N with the FSPMs, rapeseed functional structural plant model was established, which could respond to the environmental conditions [15, 16].
However, the rapeseed primary ramification morphological structural model based on biomass has not been reported. Vegetative organs of rapeseed including leaf, stem, ramification, and root [17]. Leaf is the crucial organs of photosynthesis, ramification leaves play gradually an important role in the rapeseed mid and late growth stage, and the ramification becomes a vital source. Therefore, how to accurate quantitative description of the rapeseed morphological variation is vitally important and difficult.
The objectives of this paper were to link primary ramification architectural parameters of rapeseed plant with biomass, by analyzing field experimental data from 2011–2012, and 2012–2013, to develop finally the rapeseed primary ramification morphological structural model, and to lay a foundation for rapeseed morphological structure model and visualization.
2 Experiments and Methods
2.1 Experimental Samples
Three varieties Ningyou 18 (V1) (conventional), Ningyou 16 (V2) (conventional), and Ningyou 19 (V3) (hybrid) were used in experiments, and they all belong to brassica napus. Canopy morphology structure of the three cultivars had following traits: V1 with the overwintering half-vertical cultivars, and medium height, had higher rankof branch, compacter in plant type; V2 with the overwintering half-vertical cultivars, medium height, andcompact plant type; V3 with the overwintering half-vertical cultivars, had broader and thicker leaves, the leaves light green, and edge of leaves with saw teeth.
2.2 Experimental Methods
Experimental Conditions:
Field experiment was conducted at the experimental site (32.03° N, 118.87° E) of JAAS, China during the 2011 to 2013 rapeseed growing period. The soil type is the yellow-brown soil, and its basic nutrient status: organic carbon, 13.8 gkg−1; available Nitrogen, 58.95 mgkg−1; available Phosphorus, 29.25 mgkg−1; available Potassium, 109.05 mgkg−1; and pH 7.84. Three experiments were designed to implement.
Experiment on Cultivar and Fertilizer:
By split-plot design, fertilization levels (N 180 kghm−2, P2O5 120 kghm−2, K2O 180 kghm−2 borax 15 kghm−2 and CK) was assigned to the whole-plot, and cultivars (V1, V2, V3) to the sub-plot. Six treatments were repeated three times, and the 18 subplots were arranged randomly. The area of each plot was 7.0 m × 5.7 m = 39.9 m2, the density design is 30 cm of row spacing and 17–20 cm of distance between plants, respectively. The 13 rows were planted in each subplot, and a blank line were stayed in the inter-plot. The sowing date and the transplanting date was October 15 and November 4 respectively, for both 2011 and 2012. The total amount of N-fertilizer application was 3.26 kg in the fertilization area, and the basal: seedling: winter ratio was 5:3:2 respectively. Basalrate of N + P2O5 + K2O-compound fertilizer was at 16.3 kg (mass fraction ≥ 25 %), rate of calcium superphosphate was at 7.3 kg (mass fraction 12 %), and rate of agricultural potassium sulphate was at 6.4 kg (mass fraction 33 %). the manure for seedling and winter dressing were all urea and its application amount was 1.41 kg, 2.12 kg, respectively (mass fraction of total N ≥ 46.2 %). Other management activities followed local production practice.
Experiment on Cultivar, Fertilizer, and Density:
By split-plot design, fertilization levels (N, P2O5, K2O each of 180 kghm−2, 90kghm−2 kghm−2 and CK) was assigned to the whole-plot and cultivars (V1, V3) and density levels (D1 (6 × 104 planthm−2), D2 (1.2 × 105 planthm−2) and D3 (1.8 × 105 planthm−2)) to the sub-plot. Eighteen treatments were repeated three times, and the 54 plots were arranged randomly. The area of each plot was 3.99 m × 3.5 m = 13.97 m2, the density design is 42 cm of row spacing and the distance between plants was calculated by density. The 9 rows were planted in each subplot, and a blank line was stayed in the inter-plot. The sowing date and the transplanting date was October 8 and November 9 respectively, for both 2011 and 2012.
The basal: seedling: winter ratio of fertilizer application was 5:3:2 respectively. Folia application of borax was at a rate of 15 kghm−2 after bolting of rapeseed. Other management activities followed local production practice.
Experiment on Cultivar Experiment:
The experiments were a randomized complete block design, with three cultivars (V1, V2, V3), under the same fertilization level, N90(N, P2O5, K2O each of 90 kghm−2) and density level, D2 (1.2 × 105 planthm−2), 3 replications, and the 9 subplots. The area of each plot was 7.98 m × 3.5 m = 27.93 m2, the density design is 42 cm of row spacing. Other treatments with the Cultivar, fertilizer and density experiment.
Measurements:
We selected 3 plants with similar growth status in each treatment, and determined the blade length, blade width, and blade bowstring length at various leaf ranks on primary ramification more than 2.5 cm, and the length and the diameter of the primary ramification. Then, leaves were separated from the ramification, and into the paper bags, then put in oven, the temperature of green removing in 105 °C for 30 min, in 80 °C until reaching a stable weight.
Blade:
Leaf blade length: measuring the length of the blade straight state from the leaf tip to the leaf base; Leaf blade width: measuring the maximum length of the leaf width value (in the middle of the blade), average value was gained by multiple measurements. Leaf blade bowstring length: as the elongation of the blade, due to gravity and other effects, the leaf is deformed, and bends into an arc downwardly. So the leaf blade bowstring length is from the leaf base to the leaf tip of the linear distance of space, average value was gained by multiple measurements.
Primary Ramification (PR):
Length of PR: the length of the straight state which is the distance from the basal of the PR to the top of the PR; Diameter of PR: measuring the base, middle and top of PR several times using the vernier caliper and average value was gained by multiple measurements.
Data Analysis:
We used the MS EXCEL 2007 and SPSS version 19 to analyzed the experiment data. Part of the data of different cultivars and fertilizer levels were for the modeling and parameter determination in 2011–2012. The remaining independent data were for model testing and inspection.
Model Validation:
We used the root mean squared errors (RMSE), the correlation (r), the average absolute difference (d a ), the ratio of d a to the average observation (d ap ), and 1:1 chart of measured values and simulated values properties to validate the models developed in this paper (Cao et al. 2012). The smaller RMSE, d a , and d ap values, the better consistency of simulated and observed values, and the deviation will be small, the simulation results of the model proved to be accurate and reliable. So the d a , and d ap are defined as:
With i = sample number, X oi = observed values, X si = simulated values, n = total number of measurements.
3 Results and Discussion
3.1 Model Description
Leaf is an important organ of photosynthesis, and the blades of PR are the main organ of photosynthesis in the mid and late period of rapeseed growth stages, which directly determines the photosynthetic capacity and the final yield. The morphology of the rapeseed leaves have characteristics with complexity, variability, and difficult to obtain, so it is an important part of the rapeseed plant model. According to the status of the petiole, the leaf for brassica napus can be divided into long-petiole, short-petiole, and sessile leaf on the main stem, and the leaves of PR are similar to the sessile leaf [18].
3.2 Leaf Blade Length Model
The production of the effective PR usually occurs in the axillary buds above the tenth leaf in the upper on the main stem. According to observation in 2011 to 2012 experiment, the leaf blade length with the leaf dry weight was close to proportional increasing trend (Fig. 1). Hereby, the models can be expressed as follows:
where, PRLL jk (i) is the kth leaf blade length of the jth primary ramification (cm), DWPRLB jk (i) is the kth leaf dry weight of the jth primary ramification (cm), PRRLW jk (i) is the ratio of the kth leaf length of the primary ramification to leaf biomass (cm g−1), PRCPLB jk (i) is the ratio of the kth leaf biomass of the primary ramification to the biomass of upper plant part (g g−1), MDW SP (i) is the mean dry weight of per plant (g plant−1), DW SP (i) is the dry weight of per plant (gplant−1), DW CP (i) is the biomass in canopy per unit area (g m−2), SDW SP (i) is the standard error of dry weight of per plant (g plant−1) (determined by experiment), and DES is the plant number unit area (plant m−2).
The data in the 2011 to 2012 and 2012 to 2013 experiment showed that the number of the ramification leaf has a definite relationship with ramification rank. Generally effective ramification was on the middle and upper part of the main stem, all can reach four leaves, and some ramifications was up to six or more but relatively less. Therefore, the first four leaves were studied in this study, and the other blades were not measured as it is difficult to determine the relationship with biomass.
The data in the 2011 to 2012 experiment showed that the values of PRRLW jk (i) with the leaf rank on primary ramification were close to quadratic function. The significant R = 0.768 (n = 74, R (72,0.001) = 0.375, P < 0.001) and R 2 = 0.589 for the first leaf; R = 0.584 (n = 77, R (75,0.001) = 0.367, P < 0.001) and R 2 = 0.341 for the second leaf; R = 0.489 (n = 32, R (30,0.01) = 0.452, P < 0.01) and R 2 = 0.240 for the third leaf; R = 0.557(n = 36, R (34,0.001) = 0.525, P < 0.001) and R 2 = 0.310 (Fig. 2) for the fourth leaf (Eq. (5), Table 1).
The F-values, t-values, c 1, d 1, e 1, c 2, d 2, e 2, c 4, and e 4 all were significant at P<0.001, apart from c3, d 3, d 4, and e3 (Table 1).
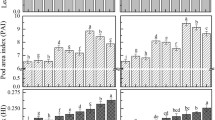
The data in the 2011 to 2012 experiment showed that PRCPLB jk (i) and PRRLB jk (i) with primary ramification rank were linear function model and exponential function model, respectively. R19 was the inflexion under different treatment levels, and the fitting precision for PRCPLB jk (i) in various treatments were: fertilizer with cultivars significant R = 0.603 (n = 56, P < 0.001, R (54,0.001) = 0.428), R 2 = 0.363 and R = 0.568 (n = 34, P < 0.001, R (32,0.001) = 0.539), R 2 = 0.323; no fertilizer with cultivars significant R = 0.567 (n = 39, P < 0.001, R (37,0.001) = 0.507), R 2 = 0.321 and R = 0.599(n = 32, P < 0.001, R (30,0.001) = 0.554), R 2 = 0.359 (Fig. 3). The fitting precision for PRRLB jk (i) in various treatments were: fertilizer with cultivars significant R = 0.592 (P < 0.001, n = 159, R (157,0.001) = 0.264), R 2 = 0.351; no fertilizer with cultivars significant R = 0.466 (n = 102, P < 0.001, R (100,0.001) = 0.321), R 2 = 0.217(Fig. 4, Eqs. (6)– (8), Table 2).
The F-values, t-values, all model parameters apart from B 5 were at P < 0.001 (Table 2).
3.3 Maximum Leaf Blade Width Model
The experiment on cultivar and fertilizer in the 2011 to 2012 showed that the PRLW jk(i) with the leaf length were described by a growth function (Eq. (9)). R = 0.914, n = 262, P < 0.001, R (260, 0.001) = 0.206, R 2 = 0.835 (Fig. 5).
The F-values, t-values, c 5, d 5 all at P < 0.001 (Table 1).
3.4 Leaf Blade Bowstring Length Model
Apparently, the maximum PRBL jk (i) = PRLL jk (i). The experiment on cultivar and fertilizer in the 2011 to 2012 showed that variation of leaf bowstring length with the leaf length could be represented by the linear function. R = 0.846, P < 0.001, n = 262, R (260, 0.001) = 0.206, R 2 = 0.715 (Fig. 6).
The F-value, t-values, c 6, d 6 all were t at P < 0.001 (Table 1).
3.5 Stem Length Model of Primary Ramification
From the experimental data in the 2011 to 2012 we can see that the jth stem length of primary ramification (cm), PRSL ji , changes with the dry weight could be described by a power function. R = 0.776, n = 106, P < 0.001, R (104, 0.001) = 0.314, R 2 = 0.603 (Fig. 7).
The F-value, t-values, c 7, d 7 all were at P < 0.001 (Table 1).
3.6 Stem Diameter Model of Primary Ramification
The experimental data in the 2011 to 2012 showed that the jth stem diameter of primary ramification, PRSD ji , changed with the dry weight could be described by a linear function with significant R = 0.501, n = 96, P < 0.001, R (94, 0.001) = 0.331, R 2 = 0.251 (Fig. 8).
The F-value, t-values, c 8, d 8 all were at P < 0.001 (Table 1).
3.7 Validation
We used the independent experimental data to validate the biomass-based rapeseed plant primary ramification morphological structure model proposed in this study. The RMSE, and the d a in rapeseed primary ramification morphological parameters, leaf blade length, the maximum leaf blade width, the leaf blade bowstring length, stem length of PR, and stem diameter of PR were 5.033 cm, −1.900 cm (n = 125); 3.233 cm, −0.055 cm (n = 117); 2.810 cm, 0.274 cm (n = 87); 3.272 cm, −0.720 cm (n = 90); 0.778 cm, 0.374 cm (n = 514); 1.193 cm, 0.137 cm (n = 514); 8.990 cm, 0.806 cm (n = 145); and 0.102 cm, −0.025 cm (n = 153), respectively. The r values in rapeseed primary ramification morphological properties all at P < 0.001 or P < 0.01, but the ratio of d a to the average observation (d ap ) values were less than 5 % for the second leaves length, the third leaves length, leaf blade bowstring length, the PR length, the PR diameter, PRRLB values for V2 and V3, which indicated these model’s accuracy is high (Table 3). The 1:1 line in rapeseed primary ramification were represented in Fig. 9.
The r values in rapeseed PRCPLB all at P < 0.001 or P < 0.01, but the ratio of d a to the average observation (d ap ) values were between 5 %−10 % for PRCPLB with fertilizer (j > 19) and PRCPLB no fertilizer (11 ≤ j ≤ 19), which indicated that these model’s accuracy is good. The dap values of more than 10 % which indicated that the model had a lower accuracy, but the d a values and the RMSE of PRCPLB and PRRLB were small (Table 3). The 1:1 chart of measured values and simulated values in PRCPLB and PRRLB are represented in Fig. 9.
Notably, we had seen that the first leaf blade length and PRRLB value(no fertilizer) models had obvious errors from Table 3, and Fig. 9, which showed that the two models still needed to be improved and perfected in the further.
3.8 Discussion
The study on FSPMs of rapeseed has important theoretical and application value for selection ideal plant type and regulation of plant type. One of the most important methods to establish the Function-Structural Model of rapeseed is to combine the rapeseed growth model with the morphological structure model by biomass. This paper established the relationships between morphological parameters of rapeseed and organ biomass, realized the organic combination of rapeseed growth model and rapeseed morphological model. It lays the basis for the establishment of FSPMs of rapeseed.
Studies on crop morphological structural models, such as rice, wheat, cotton, corn have been many reported. Chang et al. [19] constructed the simulation model of leaf elongation process in rice, analyzed the variation of leaf blade geometric morphology indices of rice with the growth process and environment conditions, and provided facilitate to digital and visualization of rice. Zhang et al. [20] established a process-based model with the methods of system analysis and dynamic modeling techniques. Fournier et al. [21] by using L-systems proposed a detailed description of the relationship between leaf functions and chlorophyll content of leaf. The concept of the relative leaf area index (LAI) and the relative accumulated temperature were put forward, and fitted parameters of the model by using MATLAB, the dynamic simulation model of leaf area index of corn was established [22]. By the computer model based on combining crop physiological and ecological process with visualization, a digital and visualization techniques of cotton growing system was presented [23].
Through there were some studies on rapeseed growth models and morphological models [24, 25], there are many research on growth-development law and structural characteristics of rapeseed [15, 26]. However, the combination of rapeseed growth model and morphological model is lack of further study. Yue [6] conducted a rapeseed ramification morphological structure model based on GDD, but the combined with the growth model was not mentioned.
In this paper, the leaf length models can be linked with the rapeseed dry matter production models through biomass, and the models also can be linked with the rapeseed growth models by biomass. The maximum leaf blade width model and the leaf blade bowstring length model are represented by a linear function with leaf blade length well. Stem length model and stem diameter model of primary ramification are represented by a power function and a linear function, respectively. One of the most important factors is the angle between primary ramification and main stem, and we will complete it in the coming.
The research on morphological structure of rapeseed primary ramification hasa certain complexity, and influenced by the external environment and difficult to obtain morphological indices accurately, tends to appear some experimental error. We will improve measurement methods, such as digital camera, and 3D laser scanner etc., in order to get the whole morphology of plant leaves and ramifications, exploration of the way to enhance the model accuracy.
4 Conclusions
In this paper, we developed a rapeseed PR structural model, which indented to explain the response mechanism of the PR morphogenesis to environmental conditions and varieties. Validation of the model with the independent experiment data indicated a good fitness between the simulated and observed in rapeseed.
The PRRLW was first put forward, and the relationships between rapeseed plant primary ramification morphological structure and the organ biomass to be established by PRRLW. It is a morphological structural parameter of rapeseed with a biological significance, and enhanced mechanism of this study.
Thus, the rapeseed plant primary ramification morphological structure model in this paper is feasible. We expect the proposed to be useful for other morphological indices of rapeseed in the further.
References
Foreign Agricultural Service. Oilseeds: World Markets and Trade (2014)
Xinyu, G., Xuyang, D., Wengang, Z., et al.: Study on the 3-D visualization of leaf morphological formation in corn. J. Maize Sci. 12(special column), 27–30 (2004). (in Chinese with English abstract)
Zihui, T.: Study on simulation model of morphological development in wheat plant. Master degree dissertation of Nanjing Agricultural University (2006) (in Chinese)
Chunlin, S.: Study on morphological model and visual growth in rice. Ph.D. dissertation of Nanjing Agricultural University (2006) (in Chinese)
Juan, Z., Shuang, J., Binglin, C., et al.: Study on morphological model of stem, branch and leaf in cotton. Cotton Sci. 21(3), 206–211 (2009). (in Chinese with English abstract)
Yanbin, Y.: The morphological structural model and visualization of rapeseed (Brassica napus L.) plant. Master degree dissertation of Nanjing Agricultural University (2010) (in Chinese)
Aizhen, S., Huojiao, H., Hongyun, Y., et al.: A visualization simulation of leaf shape elongation process in rice based on accumulated temperature change. Acta Agricuturae Universitatis Jiangxiensis 34(5), 1058–1063 (2012). (in Chinese with English abstract)
Youhong, S., Yan, G., Baoguo, L., et al.: Virtual maize modelII. Plant morphological constructing based on organ biomass accumulation. Acta Ecolegica Sinica 23(12), 2579–2586 (2003). (in Chinese with English abstract)
Mengzhen, K., Evers, J.B., Vos, J., et al.: The derivation of sink functions of wheat organs using the Greenlab model. Ann. Bot. 101(8), 1099–1108 (2008)
Liu Yan, L., Jianfei, C.H., et al.: Main geometrical parameter models of rice blade based on biomass. Scientia Agricultural Sinica 42(11), 4093–4099 (2009). (in Chinese with English abstract)
Hongxin, C., Yan, L., Yongxia, L., et al.: Biomass-based rice (Oryza sativa L.) above ground architectural parameter models. J. Integr. Agric. 11(10), 101–108 (2012)
Hongxin, C., Wenyu, Z., Weixin, Z. et al.: Biomass-based rapeseed (Brassica napus L.) leaf geometric parameter model. In: Proceedings of the 7th International Conference on Functional-Structural Plant Models, Saariselkä, Finland, 9–14 June 2013 (2013) http://www.metal.fi/fspm2013/proceedings
Wenyu, Z., Weixin, Z., Daokuo, G., et al.: A biomass-based leaf curve model for rapeseed (Brassica napus L.). Jiangsu Agric. Sci. 30(6), 1259–1266 (2014). (in Chinese with English abstract)
Weixin, Z., Hongxin, C., Yan, Z., et al.: Morphological structure model of leaf space based on biomass at pre-overwintering stage in rapeseed (Brassica napus L.) plant. Acta Agronomica Sinica 41(2), 318–328 (2015). (in Chinese with English abstract)
Groer, C., Kniemeyer, O., Hemmerling, R. et al.: A dynamic 3D model of rape (Brassica napus L.) computing yield components under variable nitrogen fertilization regimes (2007). http://algorithmicbotany.org/FSPM07/proceedings.html
Müller, J., Braune, H., Wernecke, P. et al.: Towards universality and modularity: a generic photosynthesis and transpiration module for functional structural plant models (2007). http://algorithmicbotany.org/FSPM07/proceedings.html
Oil crops research institute, Chinese academy of agricultural sciences. Chinese rape cultivation. Agricultural Press, Beijing (1987) (in Chinese)
Houli, L.: Practical rapeseed cultivation. Shanghai Science and Technology Press, Shanghai (1987). (in Chinese)
Chang Liying, G., Dongxiang, Z.W., et al.: A simulation model of leaf elongation process in rice. Acta Agronomica Sinica 34(2), 311–317 (2008). (in Chinese with English abstract)
Wenyu, Z., Liang, T., Xiangcheng, Z., et al.: Dynamic simulation of wheat stem-sheath angle based on process. Chin. J. Appl. Ecol. 22(7), 1765–1770 (2011). (in Chinese with English abstract)
Fournier, C., Andrieu, B.: A 3D architectural and process-based model of maize development. Ann. Bot. 81, 233–250 (1998)
Xinlan, L., Xianglan, C., Yunsheng, Y., et al.: Study on dynamic simulation model of leaf area index of corn in Northeast. Jiangsu Agric. Sci. 40(1), 91–94 (2012). (in Chinese)
Xuebiao, P., Lizhen, Z., Chao, C., et al.: Digital and visualization techniques of cotton growing system. China Sci. Technol. Achievements 10, 24 (2011). (in Chinese)
Liangzhi, G.: The basis of agricultural model. Tianma book co., LTD, Hong Kong (2004). (in Chinese)
Weixing, C., Weihong, L.: Crop System Simulation and Intelligent Management, pp. 149–166. Higher Education Press, Beijing (2003). (in Chinese)
Hongxin, C., Chunlei, Z., Guangming, L., et al.: Researches of simulation models of rape (Brassica napus L.) growth and development. Acta Agronomica Sinica 32(10), 1530–1536 (2006). (in Chinese with English Abstract)
Acknowledgment
This work was supported by NFSC (31171455, 31201127, and 31471415), Jiangsu Province Agricultural Scientific Technology Innovation Fund [CX(12)5060], National High-Tech Research and Development Program of China (2013AA102305-1), and JAAS Basic Scientific Research Work Special Fund [ZX(15)4001, ZX(15)2008].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 IFIP International Federation for Information Processing
About this paper
Cite this paper
Zhang, W. et al. (2016). Rapeseed (Brassica napus L.) Primary Ramification Morphological Structural Model Based on Biomass. In: Li, D., Li, Z. (eds) Computer and Computing Technologies in Agriculture IX. CCTA 2015. IFIP Advances in Information and Communication Technology, vol 478. Springer, Cham. https://doi.org/10.1007/978-3-319-48357-3_47
Download citation
DOI: https://doi.org/10.1007/978-3-319-48357-3_47
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-48356-6
Online ISBN: 978-3-319-48357-3
eBook Packages: Computer ScienceComputer Science (R0)