Abstract
Plasmonic nanoparticles (Au, Ag, Cu) have attracted increasing attentions due to their excellent optical, chemical and physical characters, especially localized surface plasmon resonance (LSPR) property. Plasmonic nanoparticles have been widely applied in the fields of catalysis, energy sources and various sensors. Notably, the development of optical techniques achieved the investigation of single plasmonic nanoparticles including dark-field microscopy, differential interference contrast (DIC) microscopy and surface plasmon resonance imaging, etc. The single-nanoparticle detection eliminates the average effect compared with bulk system and improves detection sensitivity dramatically, even to single molecule level. Thus, in this chapter, we focus on the determination on a single nanoparticle mainly based on plasmon resonance scattering spectroscopy under dark-field microscopy. The points of morphology and composition modulation, inter-particle coupling, plasmon resonance energy transfer and opto-electrochemical detection are highlighted.
Similar content being viewed by others
8.1 Introduction
Localized surface plasmon resonance (LSPR) enables noble metal nanoparticles unique scattering and absorption spectroscopy [1–3]. After the incident light irradiating on nanoparticle whose size is far smaller than the light wavelength, the surface electrons are excited and collectively oscillated with the incident light. When the oscillation frequencies of the incident light and surface electrons come to resonance, LSPR occurs and gives rise to the strong extinction light and catalysis ability owing to the abundant active surface electrons [4, 5]. The scattering light of a single plasmonic nanoparticle was obtained since 2000, via dark-field microscopy [6, 7]. After that, scattering spectra of single nanoparticles have been exploited in ultra-sensitive sensors and cell imaging, etc. [8, 9].
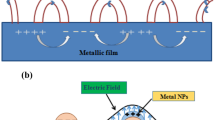
Dark-field microscopy as a side illumination technique has extremely high contrast with distinguished signal to noise ratio. The setup of a dark-field microscopy is illustrated in Fig. 8.1 [10]. Dark-field condenser contains an opaque solid center that a ring-like pathway is formed for the incident light transferring. After the reflection of a prism, the ring-like beam of incident light is irradiated on the sample with an angle. With a transparent substrate, the incident light will keep transferring as the incoming direction and only the scattering light is collected by objective lens, resulting in a dark background. Due to the special functions of dark-field microscopy, the scattering spectra of a single plasmonic nanoparticle is obtained readily with equipment of spectrograph and light collection device.
Schematic of the experimental arrangement for dark-field microscopy studies of metal nanoparticles. Reproduced by permission from [10] of The Royal Society of Chemistry
8.2 Morphology and Composition Modulated Sensors
Size factor plays important roles in the optical properties and sensing abilities of nanoparticles [11, 12]. As single-nanoparticle detection has attracted more and more interest, analyzing the size of single nanoparticles readily and rapidly has become critical issues. Therefore, we proposed a novel method to measure the size of gold nanoparticles (GNPs) in situ and in real-time using the RGB (red, green, and blue) information from dark-field images [13]. After obtaining a dark-field image of single nanoparticles, as depicted in Fig. 8.2a, the RGB values of every pixel in one scattering color spot were calculated. Then RGB values were converted into spectral wavelength and intensity based on the chromaticity diagram. The calculated intensity of pixels with the same wavelength were added together and we considered the wavelength with the highest intensity as scattering spectral peak of nanoparticles. After that, the diameters of nanoparticles were calculated according to Mie theory correlating the relationship between size and scattering wavelength. Owing to the limitations of CCD sensitivity and color recognition, this method is only applicable to the calculation of gold nanospheres at the current stage. However, it opens a new way for the measurement of particle size which is more facile and economical than SEM and TEM characterization. Notably, by optimizing the calculation program, this approach enables measurements of thousands of nanoparticles within several minutes using a laptop computer. In Fig. 8.2c–d, the statistical wavelengths and diameters of 1766 particles in Fig. 8.2b were calculated in 1 min. Furthermore, observation of numerous individual nanoparticles not only eliminates the average effect of the bulk system, but also prevents random events in single nanoparticle detection.
a Calculation process of the RGB-based method. b Dark-field image and c calculated wavelength peaks and d diameter distribution of 1766 GNPs. Reprinted with permission from [13]. Copyright (2012) American Chemical Society
In addition, gold nanoparticles have been widely applied in cell imaging as contrast agents for their excellent biocompatibility and stability. However, it is difficult to recognize the scattering spectra of single gold nanoparticles in living cells for the strong background such as scattering interference of organelles. Thus, we optimized the RGB-based method to obtain the scattering information of single gold nanoparticles in cells with high sensitivity and high throughput [14]. The flow diagram of data calculation process is depicted in Fig. 8.3a. The recorded dark-field image for living cell incubated with GNPs (Fig. 8.3b) is converted into gray scale image (Fig. 8.3c). Figure 8.3d is the binary image converted from the gray scale image through a threshold. In Fig. 8.3d, white pixels reflect to the recognized region of scattering light. It is obvious that the pixels belong to the scattering background of cells have been merged with the pixels of scattering spots for GNPs. GNPs in the binary image are difficult to be distinguished. This result is mainly due to the high intensity of biased field caused by the high background light and it is hard to find an appropriate threshold to segment the scattering area of nanoparticles. Thus, the bias-modified fuzzy C-means (BM-FCM) algorithm was introduced to the program to estimate the biased field in the image. Thus, a modified image was obtained by subtracting the biased field from the gray scale image to remove the strong scattering background in the cell as shown in Fig. 8.3e. Figure 8.3f is the binary image calculated from the modified image through a threshold. Then, the recognized pixels of GNPs were merged with the pixels of cell background through the region growth method. As shown in Fig. 8.3g, a total number of 264 GNPs were recognized and labeled in red rectangle. After this, the scattering spectra peak wavelength and intensity of every GNP were calculated using the above RGB-based method. This work provides a facile and rapid approach for the quantitative or semi-quantitative cell imaging detection.
a Flow diagram of the data process. b Dark-field image of HeLa cell incubated with GNPs and the corresponding bright-field image (insertion). c Gray scale image converted from the original image. d Binary image converted from the gray scale image through a threshold. e Modified image by substrate the biased illumination from the gray scale image. f Binary image converted from the modified image through a threshold. g Result of recognition, scattering spots of GNPs are framed by red rectangle. Scale bars in (b)–(g) are 50 μm. The thresholds are calculated by the Otsu method, respectively. Adapted with permission from [14]. Copyright (2015) Ivyspring International Publisher (Color figure online)
The excellent catalytic activity of gold nanoparticles expands their applications in sensing fields [15–17]. Fan constructed a DNA sensor utilizing the glucose oxidase (GOx)-like activity of gold nanoparticles. Glucose was oxidized into gluconic acid and hydrogen peroxide by GNPs in the presence of oxygen [18]. After the addition of HAuCl4, the catalytic product H2O2 reduced gold ions into gold atoms leading to the particle growth as illustrated in Fig. 8.4. It was proved that when GNP surface was covered with molecules such as soft single strand DNA (ssDNA), the enzyme-like catalytic ability was being confined. After the addition of complementary DNA, the hybridized double strand DNA (dsDNA) had rough structure and high surface charge. The interaction between GNP and dsDNA was very weak resulting in the dsDNA leaving and recovery of the surface activity. Thus, the GNP growth was modulated by the surface DNA structure offering a sensitive method to detect DNA according to the size dependent resonance scattering spectral shift of single GNP. This method achieved the recognition of 1-base mismatched DNA hybridization. Based on the GOx-like catalytic ability, an ultra-sensitive ATP sensor was also fabricated through modulation of GNP surface catalytic activity via ATP/aptamar interaction [19].
Illustration of the GOx-like catalytic activity of AuNPs regulated by DNA hybridization, which can be either amplified by HRP-cascaded color or chemiluminescence variations (path a) or lead to nanoplasmonic changes owing to size enlargement (path b). Orange strand = target, green strand = adsorption probe. Reprinted with permission from [18]. Copyright 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim (Color figure online)
Except size factor, the shape of plasmonic nanoparticles also has crucial influence on the LSPR band. Particles with various shapes have been synthesized for different applications [20–22]. Exploring the particle producing process is important to the preparation method for nanomaterials [23]. Recently, we monitored the controllable morphological changing process of a single gold nanorod using dark-field microscopy and resonance scattering spectroscopy [24]. Gold nanoparticles were corroded under the condition of HAuCl4 and cetyltrimethylammonium bromide (CTAB). To determine the dissolving mechanism, nanorods were modified with cysteine which selectively conjugated with the ends of nanoparticles. Figure 8.5 illustrates the modification process of nanoparticles. GNR was firstly capped with CTAB, and then assembled via cysteine through Au–S bonding on nanorods’ ends. After the addition of HAuCl4 and CTAB micellar solution, the nanorods showed distinct spectral red shift, as well as the color change in dark-field image as shown in Fig. 8.5c. In TEM images, the nanorod exhibited a dumbbell shape that the side parts of GNR were etched. This result was attributed to the change of GNR curvature that the gold [110] surface was changed into [111] surface under the CTAB micellar solution. CTAB (0.8–9.4 mM) as spherical micelle is more easily reacted with gold atoms and tends to react on low curvature structures. In addition, modified glutathione (GSH) also induced the transverse etching, yet mercaptoethylamine and 11-mercaptoundecanoic acid did not exhibit selective etching positions indicating that only zwitterionic molecules with both carboxyl and amino group affect the reaction. Thus, using the scattering spectra of nanoparticles to monitor the morphology change process in real-time offers a new way to reveal the particle synthesis mechanism and control the particle growth trends.
Schematic illustration of transverse etching reaction on the single particle level. a Diagram of GNRs before reaction. b–c Diagram, TEM image, dark-field image and scattering spectra of GNRs in the solution of 0.1 mm cysteine before (b) and after (c) adding micellar solution of CTAB and HAuCl4. Reproduced by permission from [24] of The Royal Society of Chemistry
In addition, the resonance scattering spectra of nanoparticles are also dependent on their compositions. Gold, silver and copper nanoparticles have various resonance bands, respectively. Zhang utilized single plasmonic nanoparticle as sensitive probe to map the distribution of NADH in living cells according to the composition change of nanostructures [25]. In the presence of NADH and copper ions, gold nanoparticles promote the reduction of copper ions into copper atoms by NADH. The produced copper atoms would adsorb on the surface of gold nanoparticles to form the Au–Cu core-shell structure, resulting in the distinct scattering spectral wavelength red shift of nanoparticles. As shown in Fig. 8.6e, gold nanospheres of 50 nm with excellent water solubility and compatibility exhibited green color after incubation in HeLa cells. With treatment of copper ions for 3 h, it was obvious that the color of nanoparticles changed into orange and red along with the spectral red shift (Fig. 8.6l) indicating the presence of NADH in cells. To confirm the function of NADH in this process, an anti-cancer drug, taxol, was added into the cells to confine the production of NADH. In Fig. 8.6i, the nanoparticles did not show clear color change owing to the suppressive effect of taxol. This cell imaging method provides an efficient means for the investigation of metabolism process, and also the screening of cancer drugs.
a Bright-field images of HeLa cell. b DFM images of corresponding HeLa cell in (a). c The detail view of HeLa cell DFM images (b). d Bright-field images of HeLa cell after 24 h incubation with AuNPs. e DFM images of corresponding HeLa cell in (d). f The detail view of HeLa cell containing AuNPs DFM images (e). g Bright-field images of HeLa cell containing AuNPs with treatment by taxol (10 μm) and then incubation in TBS containing 50 μm CuCl2 for 3 h. h DFM images of corresponding HeLa cell in (g). i The detailed view of HeLa cell DFM images (h), i-1 to i-4: Corresponding scattering spectra of different AuNPs in living HeLa cell. j Bright-field images of HeLa cell containing AuNPs without treatment by taxol and then incubation in TBS containing 50 μm CuCl2 for 3 h. k DFM images of corresponding HeLa cell in (j). l The detailed view of HeLa cell DFM images (k), l-1 to l-4: Corresponding scattering spectra of different Au@Cu core–shell NPs in living HeLa cell (the color bar in the scattering spectra indicate the wavelength of the maximum scattering intensity, and reflect the resulting color). Reprinted with permission from [25]. Copyright 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
In the past decades, nanoparticles with core-shell structure have been widely used for their multi-functions of different compositions [26]. The scattering spectra of core-shell nanoparticles have a wide range from visible to infrared region. In addition, the water-solubility, toxicity and catalytic ability could also be optimized by modifying nanoparticles with functional shells such as silicon, magnetic materials and plasmonics. Yan He and co-workers proposed an elegant method to detect hydrogen sulphide in living cells based on the unique LSPR property of Au–Ag core-shell nanostructures [27]. It has been reported that silver atoms react with sulphide ions in the presence of oxygen, and Ag2S is generated on the particle surface inducing the plasmon resonance peak red shift along with color change from orange to red. The authors attributed the spectral shift to the refractive index change surrounding gold cores, the refractive index of Ag2S is 2.2 much more than Ag atoms (0.17). When the Au/Ag core-shell nanoparticles were incubated into cells, the spectral peak wavelength change of nanoparticles revealed the presence of H2S as shown in Fig. 8.7. Due to the homogenous dispersion of nanoparticles in cells, this method enabled the mapping of H2S in a single cell that the various spectral shift of particles indicated the concentration of H2S at different positions. In addition, the silver-sulphide reaction showed high selectivity towards hydrogen sulphide compared with other sulfocompounds. In this work, gold core provides a plasmon resonance substrate which enhanced the observed signals, and silver shell behaved as the active reaction sites. This di-functional nanomaterial has promising potentials in biosensing and cell imaging.
Local variations of intracellular sulphide levels can be determined in real time. a–c Representative images showing the gradual colour changes of two individual PNPs after adding 0.1 μM Na2S to the cell culture medium for (a) 2 min, (b) 26 min and (c) 42 min. Scale bar, 10 μm. The red and green square inserts are enlarged images of the two circled PNPs. d Observed (hollow dots) and fitted (lines) time-dependent λmax shifts of the two particles. e Calculated time-dependent change in local sulphide concentrations surrounding the two particles according to the fitted results in (d). Reprinted by permission from Macmillan Publishers Ltd: [Nature communications] [27]. Copyright (2013)
Another sensor using the Au/Ag core-shell nanoparticles was fabricated for the detection of entire autophagy process at single cell level. Superoxide radicals (O ·−2 ) as main regulator was commonly utilized in the detection of autophagy process [28]. In the presence of O ·−2 , Ag atoms were oxidized into Ag+ ions inducing the etching of particles. This reaction also had high selectivity to O ·−2 compared with other superoxide radicals. Thus, obvious plasmon resonance scattering spectral red shift and intensity decreasing of particles were observed due to the composition and morphology change (Fig. 8.8). After modification with polyethylene glycol (PEG) and Arg-Gly-Asp-Cys (RGD) peptides, the nanoprobes were captured by cells through the specific binding interactions between RGD peptides and integrins (ανβ3 or ανβ5) on the membrane. The spectral shift was well correlated with the concentration of O ·−2 on cell membrane and made it possible to monitor the O ·−2 level in situ and in real-time. Due to the long-term autophagy process of cell, it was difficult to monitor the entire autophagy process via one single nanoparticle. Therefore, Zhu and co-workers introduced the “relay” concept in the cell imaging detection. 8 groups of nanoprobes were incubated in cells and statistic data of the time-dependent spectral shift were obtained to analyze the detailed cytometaplasia process. The “relay” concept offered an effective method to investigate continuous cell activities.
a Illustration of the core-shell structure Au@AgNRs. b The model controlling PRS spectrum of Au@AgNRs, where the prolate spheroidal dielectric (ε1) core, with semiaxes a1 < c1, is coated with another confocal spheroidal dielectric (ε2) shell with semiaxes a2 < c2. εm is the dielectric functions of adjacent medium. c PRS spectra of a single probe etched by O ·−2 for 0, 30 and 60 min. Insets showed scattering images of the probe in each status. Scale bar was 500 nm. d HRTEM micrograph illustrated the transitions of nanoprobes during the etching process. Scale bar was 20 nm. Reprinted with permission from [28]. Copyright (2015) American Chemical Society
8.3 Inter-particle Coupling
When two or more plasmonic nanoparticles get close to each other, their plasmon resonance band will be coupled giving rise to significant scattering spectral peak wavelength red shift and intensity increasing [29]. The coupling of nanoparticles offers a valuable method in highly sensitive detection, even at single-molecule level [30, 31]. It is possible to monitor the coupling on an individual nanoparticle surface that per inter-particle binding event could be observed.
Li and coworkers developed a new method for imaging latent fingerprints (LFPs) based on the inter-particle coupling of plasomonic nanoparticles [32]. Cocaine as a common drug was selected as the representative analyte. In this work, 50 nm GNPs were used as both imaging and recognition probes modified with cocaine-specific aptamers. To enhance the signal to noise ratio, the cocaine-specific aptamers were divided into two flexible ssDNA pieces and were modified on the surface of different particles via Au-S bonding (Fig. 8.9). When normal sebaceous fingerprint was obtained on the glass slide surface, the added GNPs modified with DNA were monodispersed showing green color. On the contrary, if the fingerprint contained cocaine, the nanoparticles with different DNA would be conjugated leading to the inter-particle coupling. The color of aggregated GNPs displayed obvious change from green to red as illustrated in Fig. 8.9. This color-coded fingerprint detection method is fast and low-cost, and has promising application prospect in the detection of drugs, explosives and other substances.
Principle of nanoplasmonic imaging of LFPs and identification of cocaine in LFPs by dark-field microscopy. Reprinted with permission from [32]. Copyright 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Owing to the ultra-sensitive response, the inter-particle coupling was applied to monitor chemical reactions at single nanoparticle level. For instance, click reaction as a powerful and widely-applied chemistry is crucial in chemical synthesis. Particularly,Cu+-catalyzed azide-alkyne 1,3-dipolar cycloaddition has attracted considerable attentions. Using dark-field microscopy, the click reaction process was investigated based on plasmon resonance scattering light [33]. Azide with thiol group was modified on the surface of 60 nm gold nanoparticles which were dispersed on glass slide. Then, alkyne capped 14 nm gold nanoparticles were added in the system. From Fig. 8.10, after treatment with sodium ascorbate and copper ions, the generated Cu+ catalyzed the addition reaction between azide and alkyne inducing the coupling of nanoparticles. The inter-particle cross-linking obviously altered the scattering light of nanoparticles. The color in dark-field images was changed from green to red with the scattering spectral red shift. The dark-field images in Fig. 8.11 confirmed the observed reaction is universal without randomness and the reaction occurs to the major in nanoparticles showing color changes. The scattering spectral peak wavelengths were calculated via the previous home-made program based on RGB information. The statistic diagrams indicated the averaged peak wavelength of GNPs shifted from 565 to 585 nm proving the reliability and repeatability. Notably, this method makes it possible to investigate the chemical and biological reaction even at single molecule level with optimization of surface modification and reaction conditions.
a The detection of Cu2+ using click chemistry between two types of GNPs modified with terminal azide-functionalized and alkyne-functionalized thiols, respectively; Detailed experimental configuration; b Typical dark-field image of GNP modified on a microscopy slide before (I) and after (II) the addition of Cu2+ and sodium ascorbate; c Scattering spectra of single GNP before (I) and red-shift after (II) the click reaction. Reprinted with permission from [33]. Copyright 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Dark-field images and calculated wavelength of the GNPs (60 nm) before (a and b, respectively) and after (c and d, respectively) the addition of Cu2+ (1 μm) and excess sodium ascorbate. Reprinted with permission from [33]. Copyright 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
8.4 Plasmon Resonance Energy Transfer
In 2007, Lee and co-workers discovered the phenomenon of “plasmon resonance energy transfer” (PRET) [34]. PRET occurs on the plasmonic nanoparticle surface with modification of chromophores. When the absorption band of chromophore molecules overlaps with the scattering resonance band of nanoparticles, the energy transfers from particles to the surface molecules resulting in the quenching of scattering light. The quenching position of scattering spectra is corresponding to the absorption band of chromophores. PRET mechanism has not been fully confirmed, one hypothesis proposed that the energy transfers from nanoparticles to chromophores through dipole-dipole interactions, which is similar to the fluorescence resonance energy transfer (FRET) mechanism. This discovery enhanced the sensitivity of absorption spectroscopy with several orders of magnitude, down to hundreds of molecules adsorbed on single nanoparticle surface under dark-field microscopy as shown in Fig. 8.12.
Schematic diagrams of quantized plasmon quenching dips nanospectroscopy via PRET. a Experimental system configuration. b Typical Rayleigh scattering spectrum of bare gold nanoparticles. c Typical absorption spectra of biomolecule bulk solution. d Typical quantized plasmon quenching dips in the Rayleigh scattering spectrum of biomolecule-conjugated gold nanoparticles. Spectra were drawn based on representative data. Reprinted by permission from Macmillan Publishers Ltd: [Nature Methods] [34]. Copyright (2007)
For instance, cytochrome c (Cyt. C) molecules have absorption band between 520 and 550 nm. When Cyt. C molecules were conjugated to gold nanoparticles, distinct quenching dips were observed in the scattering spectra which were matched with the absorption peaks of Cyt. C. Nevertheless, when the scattering band of GNPs was not overlapped with the absorption band of chromophores, no obvious quenching was observed. Owing to the good biocompatibility and water solubility of GNPs, PRET was applied in the real-time detection of Cyt. C in living cells [35]. After stimuli with ethanol which promoted the generation of Cyt. C, clear quenching dips were obtained in the scattering spectra of single GNPs. In addition, the real-time results of GNPs at different positions exhibited various spectral changes indicating the dynamic mapping of Cyt. C production process.
Due to the necessity of the matching between scattering band of nanoparticles and absorption band of conjugated molecules, PRET provided a new method for the highly selective detection at single nanoparticle level [36]. PRET mechanism was applied in the detection of metal ions based on the metal-ligand complex [37]. Common optical methods for the detection of heavy metal ions including fluorescence and absorption spectroscopy were generally based on organic compounds which suffered from low water solubility. However, PRET method based on single nanoparticles enabled the detection in aqueous solutions with high sensitivity.
It was reported that copper ions had high affinity with amine group to form copper-amine complex which had new absorption bands at visible range. For example, ethylenediamine could selectively bind with copper ions and the absorption band of this amine-copper complex was at ca. 550 nm. Then, 50 nm gold nanoparticles were selected as sensing probe whose scattering spectra were near 550 nm meeting the PRET conditions. The ethylenediamine molecules were firstly modified on GNPs, after binding to copper ions, the scattering spectra intensity decreased gradually indicating the PRET process. The scattering intensity decreasing showed excellent linear relationship to the concentration of copper ions and the detection limit was as low as 1 nM. Furthermore, for other ligands which may bind with several kinds of metal ions, PRET method could enhance the selectivity by modulating the scattering band of nanoparticles to match different metal-ligands. Especially, gold nanorods have greatly potential applications in PRET detections due to their broad scattering band from 600 nm to infrared (IR) region [10]. The broad resonance bands of GNRs expanded the sensing areas significantly by constructing functional probe chromophores.
Previous PRET reports were all based on the “on to off” type that the chromophores quenched the intensity of scattering light. However, compared with “on to off” type, “off to on” detection had more sensitivity because of the low background. Therefore, Long and co-workers proposed a scattering recovered plasmon resonance energy transfer (SR-PRET) method for the “off to on” sensing [38]. In this work, the chromophore molecules were conjugated with plasmonic nanoparticle to generate the PRET process. Then, the target compound was introduced to unlock the conjugation of nanoparticles and chromophore to brighten the nanoparticles. The increasing light signals were more readily to be recognized compared with the quenching type. In Fig. 8.13, a thiol terminated compound Rhodamine B (RdBS) containing a Si–O bond with absorption band of 560 nm was modified on 60 nm GNPs whose scattering band was near 560 nm, either. The scattering intensity deceasing induced by RdBS provided a low detection background. F− had high affinity to silicon leading to the cleavage of Si–O bond to release the rhodamine group and recovered the scattering light of GNPs. This SR-PRET sensor exhibited high sensitivity to F− with detection limit of 0.1 nM and provided a new method for the living cell imaging to observe the dynamic interactions of biomolecules.
a Schematic representation of the process of SR-PRET. b The color images of a typical GNP before (I) and after (II) adding F− ions, demonstrating the recovery of the scattering intensity. c Scattering spectra intensity before (I) and after (II) the addition of F− ions, the remarkable resonance enhancement on the Rayleigh scattering spectrum is clearly observed. Reprinted by permission from Macmillan Publishers Ltd: [Scientific Reports] [38]. Copyright (2015) (Color figure online)
8.5 Spectroelectrochemistry
Nobel metal nanoparticles exhibit unique surface plasmon resonance property owing to their abundant free surface electrons. The oscillation of electrons and holes makes nanoparticles good electron acceptors and donors to be widely exploited in photovoltaic and catalysis [39]. Also, the excellent conductivity of nanoparticles enables their application in electrochemistry detection as conductor wires to promote the redox reaction on electrode surface [40, 41]. Nanoparticles’ surface becomes the most active reaction area during the electrochemistry process. Thus, in recent years, investigating electrochemical reaction on single nanoparticles have attracted increasing attentions [42]. For instance, Tao used surface plasmon resonance imaging to determine the electrochemical reaction on single Pt nanoparticles based on the plasmonic-induced electrochemical current microscopy (P-ECM) signals [43, 44]. This method achieved the mapping of electrochemical reaction process on single nanoparticles with high time and spatial resolution.
In addition, combination of dark-field microscopy and electrochemical workstation provided a novel approach for the observation of electrochemical process on single plasmonic nanoparticle. In previous reports, the plasmon resonance bands of nanoparticles are dependent on their surface electron density as depicted in (8.1). The resonance peak wavelength is proportional to the electron density [45, 46]. Here, Δλmax is the wavelength peak shift, N is the electron density of GNPs, λ is the wavelength of incident light, ε is the dielectric constant of the GNP, εm is the dielectric constant of the surrounding environment, and L is the shape factor. Mulvaney investigated the relationship between the electron density and scattering peak wavelength using dark-field microscopy [47, 48]. Gold nanorods modified ITO glass slide was acted as working electrode. After applying negative potentials, the electrons were injected into nanorods inducing the scattering spectra blue shift indicating the increasing of resonance energy. From (8.1), it was also found that the scattering peak shift of nanoparticles was morphology dependent. Under negative potentials, nanoparticles with lower geometric factor were more sensitive to the surface electron density change and exhibited more spectral shift. It was confirmed that nanorods had more obvious blue shift compared with nanospheres and nanotriangles.
Both the electron transfer and refractive index change resulted in the plasmonic scattering wavelength and intensity alternation. Based on this, the integrated spectroelectrochemical instrument was applied to determine the oxidation process of single gold nanorod during cyclic voltammetry (CV) scanning [49]. The potential was applied from −1.0 to 2.3 V under double-electrode system, two ITO slides were acted as working electrode and counter electrode, respectively. From Fig. 8.14, we could see that when the potential was less than 0.3 V, the scattering spectra of nanoparticles showed slight energy loss due to the double-layer charging on the particles’ surface. At the range of −0.3 to 1.0 V, the energy decreased rapidly because of the adsorption of water molecules and ions in the electrolyte which was reversible under the potential cycle. When the potential was larger than 1.0 V, the oxidation of gold atoms was the dominant reaction on the surface which induced the obvious scattering peak red shift. Studying the voltage-induced adsorbate damping on a single nanorod improved our understanding of electrochemical process and reaction mechanism.
Plasmon resonance peak energy (a) and damping ℏ (ΓS + ΓC (U)) (b) versus applied potential on the full potential range from −1 to +2.3 V. c Sketch of the positive jellium background in the AuNR (solid black line) and the sp-electron density (dotted line). Three different regions can be associated: (i) Charging of double layer capacitance leads to a spectral shift but no additional damping. An interlayer of thickness d is formed where the electron spill-out repels both the solvent molecules and dissolved ions (left sketch in c). No chemical damping takes place via the solvent or dissolved ions (left sketch in d). In region (ii), potentials positive of the point of zero charge (PZC) cause a rapid nonlinear red shift (anodic scan in (a)) and substantial additional damping (region (ii) in panel b). The sp-electron spill-out retracts (reducing d) and solvent molecules and anions adsorb (center sketch in c). Chemical surface damping of the NPPR becomes possible by the excitation of either sp-electrons or adsorbate electrons into empty adsorbate states (dotted arrows in the center sketch of d). (iii) At potentials above 1.1 V, Au oxidation leads to no additional damping (b) but some further spectral red shift (a) due to the trapping of sp-electrons at the oxide (right sketches in c, d). Reprinted with permission from [49]. Copyright (2012) American Chemical Society
A novel method was developed to real-time monitor the deposition of single silver nanoparticles based on dark-field microscopy [50]. Under the potential of 0.1 to −0.2 V, silver ions in solutions were reduced into silver atoms depositing on the ITO substrate. In a 100 μm × 100 μm area, the total measured scattering intensity during the potential scanning matched well with the total electrochemical current. Thus, it was possible to detect the ultra-low electrochemical current generated by a single silver nanoparticle via the scattered optical signals. Referring the SEM characterization, the size of single particles, which were corresponding to the silver atoms, were calculated on the basis of the correlation between scattering spectra intensity and size according to Mie theory. Therefore, the generated electric quantity and reduction current of a single silver nanoparticle were obtained relating to the calculated silver atoms. This work opened a new way for the observation of electrochemical curves of both entire and single nanoparticles that was extremely difficult for traditional electrochemical technique.
On the other hand, Tao used the plasmonic-induced electrochemical current microscopy to image both the electrochemical reaction process and the size of nanoparticles during the silver oxidation [51]. When the silver nanoparticles collided on electrode, the applied potential oxidized silver atoms into silver ions leading to the size and resonance light decrease. Due to the correlation between the imaging intensity and size of particles, the real-time electrochemical current of single silver nanoparticles were calculated revealing the kinetics of the reaction process. It provided a promising approach for the electrochemical activity observation of particles with different size.
The dark-field microcopy based spectroelectrochemical method was then applied in the monitoring of electrochemical catalytic reaction on a single gold nanorod [52]. Nanorods were adsorbed on ITO glass slide and two Pt wires were acted as reference and counter electrodes, respectively. During CV scanning, the scattering spectra of single GNR were recorded simultaneously. After applying scanning potential from −0.1 to 1 V in KNO3 solution, GNRs modified ITO electrode exhibit obvious oxidation current peak at about 0.78 V in the presence of H2O2. As control, bare ITO electrode showed no current peaks indicating the catalytic ability of GNRs. Figure 8.15 displayed two circles of scattering spectra changes of one single GNR under the CV scanning at scan rate of 5 mV/s in the absence (a) and presence (b) of H2O2. It was found that GNR showed distinct reversible scattering changes without H2O2 due to the surface oxidation as mentioned above. The scattering spectra first exhibited red shift then blue shift to the initial position. On the contrary, GNR did not show obvious spectral shift in the presence of H2O2. In most of the published reports, GNP’s catalytic ability in electrochemical reactions was attributed to their good conductivity and active surface electrons. However, these results revealed that GNP was acted as a reactant in the catalysis process. Obviously, under high potential scanning, GNR was oxidized into hydroxide/oxide leading to the spectral red shift. After treatment with H2O2, the gold hydroxide/oxide was reduced into gold atoms inducing the spectral blue shift, meanwhile, H2O2 was oxidized into oxygen. Under positive potentials with and without H2O2, both the scattering peak intensity decreased due to the lower resonance energy. There may be three reasons for the energy loss: (1) the oxidation of GNR surface reduced the amount of resonant gold atoms; (2) the generated oxide and adsorbed molecules on particle surface increased the surrounding refractive index; (3) the positive potential reduced the electron density of particle surface.
Furthermore, in the presence of chloride, the catalytic reaction showed extremely different results. In 0.1 M KCl solution, after applying 1 V potential, the scattering light of GNR decreased rapidly and then disappeared in the view indicating the dissolution of GNRs. In 0.02 M KCl solution, GNR showed irreversible red shift after one circle CV scanning. After the addition of H2O2, the spectra showed no clear difference indicating that GNR seemed no catalytic ability. This is because of the strong interaction between gold atoms and chloride ions. After applying oxidation potential, the formed gold-chloride complex was first adsorbed on GNR surface causing spectral red shift, and then was dissolved into solution inducing the irreversible morphology change. However, H2O2 could not reduce the stable gold-chloride complex into gold atoms, that is, the catalytic ability of gold nanoparticles was passivated. Thus, electrochemical reaction on electrodes containing gold should avoid the influence of chloride ions. Notably, the investigation of electrochemical reaction on single gold nanorod confirmed the heterogeneity of every individual particles. Nanoparticles showed different catalysis activity and potential response under same conditions [53].
As GNPs are sensitive to the surface electron density, the spectroelectrochemical technique was applied in the detection of Faradaic reaction on single GNR [54]. The electrochemical oxidation of NADH was selected as a typical reaction in this system. As illustrated in Fig. 8.16b, GNRs were modified on ITO slide covered with graphene layer. Graphene has excellent electronic and catalytic property to catalyze the oxidation of NADH in non-biological system. When NADH was oxidized into NAD+, one Faradaic electron was released and transferred to GNRs through graphene owing to the good electron acceptor ability of GNRs. In the absence of NADH, during the CV scanning from 0 to 0.7 V versus Ag/AgCl electrode, the scattering spectra of single GNR showed reversible red shift due to the surface electron loss induced by anodic charging. After addition of NADH, an obvious oxidation current peak was observed at about 0.55 V and the GNR exhibited a blue shift which was opposite with the condition without NADH. This indicated that a competing effect has existed for the anodic charging and Faradaic charging as depicted in Fig. 8.16a. The scattering peak shift without NADH in the CV scanning process was considered as the background. After subtracting from the spectral shift in the presence of NADH, a distinct blue shift was obtained at a positive potential. This spectral blue shift of a GNR was exclusively resulted from electron injection in Faradaic reaction.
a The scattering spectra for individual gold nanorods charged by synchronized Faradaic reaction and non-Faradaic double layer anodic charging current. The black and blue solid lines show the scattering spectra of gold nanorods at open circuit and an applied positive potential, while blue and red dashed lines indicate the contribution of Faradaic reaction and non-Faradaic charging, respectively. b The charged gold nanorods in the presence (left) and absence (right) of Faradaic reaction in the electrochemical system. Reproduced by permission from [54] of The Royal Society of Chemistry (Color figure online)
According to the relationship between the scattering spectra shift and electron density, the electron injection amount on a single GNR was calculated as 8.43 × 104 inducing 6.5 nm peak wavelength shift. Furthermore, the rate of Faradaic reaction was investigated via altering the concentration of electroreactant. Interestingly, the scattering spectra showed a linear increasing blue shift along with the NADH concentration increasing. In addition, the surface property effect was observed on electrochemical reduced graphene oxide (erGO). The results indicated that the erGO showed better electron transfer ability leading to more spectral blue shift of GNRs in the presence of NADH. This work demonstrated the electron accumulation based on Faradaic reaction at different reaction conditions on a single GNR surface, which may improve the understanding of electroplasmonic effect and offer an effective approach for the observation of electrochemical reaction at single molecule level.
Except monitoring electrochemical reactions at single nanoparticle surface, this spectroelectrochemistry method was applied in the controllable fabrication of plasmonic nanoparticles including Ag, Au, and Cu NPs and real-time monitoring of the growth process [55]. Hybrid bilayer membranes (HBMs) were modified on ITO slide functioned as substrate. A ring-like stable protein 1 (SP1) was used as the template which had an inner pore of 2–3 nm and a width of 4–5 nm as shown in Fig. 8.17a. SP1 was proposed as a channel protein which could form a nanochannel on phospholipid membrane. Because of the non-conductivity of HBMs, the electrochemical reactions could only take place in the SP1 nanochannels where were conductive areas. After applying reduction potential, metal ions in the solution were reduced into metal atoms deposited in the nanochannels. Utilizing dark-field microscopy, the nanoparticles growth process in SP1 channels was monitored in situ and in real-time. Notably, it is possible to modulate the particle size and morphology through the electrochemical conditions. The visualized growth of a single plasmonic nanoparticle provided more detailed information regarding to the growth mechanism and routes. In addition, the density of nanoparticles could be modulated via the concentration of SP1 proteins. Interestingly, the growth of copper particles underwent different procedure [56]. Under reduction potentials, copper particles became larger and larger showing constantly scattering spectral red shift. However, after stopping the applied potential, the generated copper particles with various colors faded gradually with scattering intensity decrease, and the color of copper particles changed into dark red. This phenomenon was attributed to the oxidation of copper atoms for that the scattering color was recovered after adding acetic acid to dissolve the copper oxide. Thus, investigation of the synthesis process of a single plasmonic nanoparticle is important in the development of nanomaterials synthesis.
Electrodeposition in the SP1 generated nanochannels, and Ag, Au, and Cu NPs deposited on the SP1-HBM/ITO template using a developing solution of AgClO4, HAuCl4, and CuSO4 with the standard deposition potentials of −0.05, −0.05, and −0.6 V versus Ag/AgCl, respectively. a Structure of SP1. b SP1-HBM/ITO template. c The images of typical color changes of a single Ag NP changing from blue to red, as the electrodeposition time is increased, indicating the in situ and real time monitoring of the growth process of single NPs on the SP1-HBM/ITO template. Reprinted with permission from [55]. Copyright 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Monitoring the electrochemical reaction and electron transfer process on single plasmonic nanoparticle is significant in the development of photovoltaic conversion and opto-electro catalysis. The obtained reaction kinetics and thermodynamics at single nanoparticle/molecule level would reveal the reaction essence such as electron transfer route and temporal mediates.
8.6 Conclusions and Future Prospects
In conclusion, we have reviewed the developed sensors based on single plasmonic nanoparticles according to the morphology, composition and energy modulation. The strong and stable scattering light makes plasmonic nanoparticles excellent detection probes in the fields of biology, chemistry and catalysis [57–59]. Benefitting from their biocompatibility, water-solubility and low-toxicity, nanoplasmonics attracted considerable concerns in the fields of biosensors, cell imaging, and thermal therapy [60–62]. On the other hand, the numerous free electrons on the particle surface offer active reaction areas which promote the catalytic reactions efficiency. Also, the plasmon enhanced Raman, fluorescence and IR spectroscopy have developed rapidly in biological detection, environmental monitoring and life science [63–65].
To expand the advantages of plasmonics, one effective way is fabricating nanomaterials possessing functional morphology and compositions with multi-dimensions and multi-functions. Nanorods, stars, triangles, chains, wires and alloys, etc. exhibit absolutely different properties and have specific utilizations. The wave-guide ability of nanochains and wires, showing potential functions in the communications [66]. Additionally, plasmonics could alter the electron transfer and charge separation of semi-conductors including TiO2, Cu2O, etc. [67, 68]. The metal nanoparticles are potential materials for the enhancement of photovoltaic conversion and photocatalysis, promisingly to be exploited in new type of solar cells and other optical energy resources [69, 70]. Moreover, under conjugating with other advanced technique, plasmonics exhibit more excellent and intelligent performance [71]. For instance, combination plasmonic spectroscopy with electrochemistry enables observing electrochemical process on a single plasmonic nanoparticle and provides dynamic reaction information [72]. The integration of plasmonics with electric nanopore technique promotes the detection at single-molecule level on the basis of opto-electro signals [73, 74]. Utilizing single plasmonics in Raman detection provides accurate reaction positions in cell imaging which improved spatial resolution significantly [75]. Furthermore, plasmonics play important roles in near-field spectroscopy to reveal fantastic quantum phenomena at single molecule level, even at atom scale [76, 77]. We believe that plasmonics will have great exploitations in the development of new energy resources, life science and environments in the future.
References
H. Chen, L. Shao, Q. Li, J. Wang, Gold nanorods and their plasmonic properties. Chem. Soc. Rev. 42, 2679–2724 (2013)
Y. Li, C. Jing, L. Zhang, Y.-T. Long, Resonance scattering particles as biological nanosensors in vitro and in vivo. Chem. Soc. Rev. 41, 632–642 (2012)
C. Jing, Y.-T. Long, Localized Surface Plasmon Resonance Based Nanobiosensors (Springer, London, 2014)
B. Sepúlveda, P.C. Angelomé, L.M. Lechuga, L.M. Liz-Marzán, LSPR-based nanobiosensors. Nano Today 4, 244–251 (2009)
S. Eustis, M.A. El-Sayed, Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 35, 209–217 (2006)
S. Schultz, D.R. Smith, J.J. Mock, D.A. Schultz, Single-target molecule detection with nonbleaching multicolor optical immunolabels. Proc. Natl. Acad. Sci. U.S.A. 97, 996–1001 (2000)
C. Sonnichsen, S. Geier, N.E. Hecker, G. von Plessen, J. Feldmann, H. Ditlbacher, B. Lamprecht, J.R. Krenn, F.R. Aussenegg, V.Z.H. Chan, J.P. Spatz, M. Moller, Spectroscopy of single metallic nanoparticles using total internal reflection microscopy. Appl. Phys. Lett. 77, 2949–2951 (2000)
H.D. Song, I. Choi, Y.I. Yang, S. Hong, S. Lee, T. Kang, J. Yi, Picomolar selective detection of mercuric ion (Hg2+) using a functionalized single plasmonic gold nanoparticle. Nanotechnology 21, 145501 (2010)
A. Wax, K. Sokolov, Molecular imaging and darkfield microspectroscopy of live cells using gold plasmonic nanoparticles. Laser Photon. Rev. 3, 146–158 (2009)
C. Jing, L. Shi, X.Y. Liu, Y.T. Long, A single gold nanorod as a plasmon resonance energy transfer based nanosensor for high-sensitivity Cu(II) detection. Analyst 139, 6435–6439 (2014)
W. Haiss, N.T.K. Thanh, J. Aveyard, D.G. Fernig, Determination of size and concentration of gold nanoparticles from UV–vis spectra. Anal. Chem. 79, 4215–4221 (2007)
W. Jiang, J.T. Rutka, W.C. Chan, Nanoparticle-mediated cellular response is size-dependent. Nat. Nanotechnol. 3, 145–150 (2008)
C. Jing, Z. Gu, Y.L. Ying, D.W. Li, L. Zhang, Y.T. Long, Chrominance to dimension: a real-time method for measuring the size of single gold nanoparticles. Anal. Chem. 84, 4284–4291 (2012)
Z. Gu, C. Jing, Y.L. Ying, P.G. He, Y.T. Long, In situ high throughput scattering light analysis of single plasmonic nanoparticles in living cells. Theranostics 5, 188–195 (2015)
C.T. Campbell, PHYSICS: the active site in nanoparticle gold catalysis. Science 306, 234–235 (2004)
I.L. Buurmans, B.M. Weckhuysen, Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy. Nat. Chem. 4, 873–886 (2012)
Y. Wu, P. Jiang, M. Jiang, T.-W. Wang, C.-F. Guo, S.-S. Xie, Z.-L. Wang, The shape evolution of gold seeds and gold@silver core–shell nanostructures. Nanotechnology 20, 305602 (2009)
X. Zheng, Q. Liu, C. Jing, Y. Li, D. Li, W. Luo, Y. Wen, Y. He, Q. Huang, Y.-T. Long, C. Fan, Catalytic gold nanoparticles for nanoplasmonic detection of DNA hybridization. Angew. Chem. Int. Ed. 50, 11994–11998 (2011)
Q. Liu, C. Jing, X. Zheng, Z. Gu, D. Li, D.-W. Li, Q. Huang, Y.-T. Long, C. Fan, Nanoplasmonic detection of adenosine triphosphate by aptamer regulated self-catalytic growth of single gold nanoparticles. Chem. Commun. 48, 9574–9576 (2012)
J. Becker, A. Trügler, A. Jakab, U. Hohenester, C. Sönnichsen, The optimal aspect ratio of gold nanorods for plasmonic bio-sensing. Plasmonics 5, 161–167 (2010)
U. Hohenester, J. Krenn, Surface plasmon resonances of single and coupled metallic nanoparticles: a boundary integral method approach. Phys. Rev. B 72, 195429 (2005)
E. Ringe, J. Zhang, M.R. Langille, C.A. Mirkin, L.D. Marks, R.P. Van Duyne, Correlating the structure and localized surface plasmon resonance of single silver right bipyramids. Nanotechnology 23, 444005 (2012)
R. Jin, Photoinduced conversion of silver nanospheres to nanoprisms. Science 294, 1901–1903 (2001)
T. Xie, C. Jing, W. Ma, Z. Ding, A.J. Gross, Y.T. Long, Real-time monitoring for the morphological variations of single gold nanorods. Nanoscale 7, 511–517 (2015)
L. Zhang, Y. Li, D.-W. Li, C. Jing, X. Chen, M. Lv, Q. Huang, Y.-T. Long, I. Willner, Single gold nanoparticles as real-time optical probes for the detection of NADH-dependent intracellular metabolic enzymatic pathways. Angew. Chem. Int. Ed. 50, 6789–6792 (2011)
M.S. Shore, J. Wang, A.C. Johnston-Peck, A.L. Oldenburg, J.B. Tracy, Synthesis of Au(Core)/Ag(Shell) nanoparticles and their conversion to AuAg alloy nanoparticles. Small 7, 230–234 (2011)
B. Xiong, R. Zhou, J. Hao, Y. Jia, Y. He, E.S. Yeung, Highly sensitive sulphide mapping in live cells by kinetic spectral analysis of single Au–Ag core-shell nanoparticles. Nat. Commun. 4, 1708 (2013)
Z. Chen, J. Li, X. Chen, J. Cao, J. Zhang, Q. Min, J.J. Zhu, Single gold@silver nanoprobes for real-time tracing the entire autophagy process at single-cell level. J. Am. Chem. Soc. 137, 1903–1908 (2015)
C. Sönnichsen, B.M. Reinhard, J. Liphardt, A.P. Alivisatos, A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotechnol. 23, 741–745 (2005)
D. Aili, R. Selegård, L. Baltzer, K. Enander, B. Liedberg, Colorimetric protein sensing by controlled assembly of gold nanoparticles functionalized with synthetic receptors. Small 5, 2445–2452 (2009)
Y.W. Jun, S. Sheikholeslami, D.R. Hostetter, C. Tajon, C.S. Craik, A.P. Alivisatos, Continuous imaging of plasmon rulers in live cells reveals early-stage caspase-3 activation at the single-molecule level. Proc. Natl. Acad. Sci. U.S.A. 106, 17735–17740 (2009)
K. Li, W. Qin, F. Li, X. Zhao, B. Jiang, K. Wang, S. Deng, C. Fan, D. Li, Nanoplasmonic imaging of latent fingerprints and identification of cocaine. Angew. Chem. Int. Ed. 52, 11542–11545 (2013)
L. Shi, C. Jing, W. Ma, D.W. Li, J.E. Halls, F. Marken, Y.T. Long, Plasmon resonance scattering spectroscopy at the single-nanoparticle level: real-time monitoring of a click reaction. Angew. Chem. Int. Ed. 52, 6011–6014 (2013)
G.L. Liu, Y.-T. Long, Y. Choi, T. Kang, L.P. Lee, Quantized plasmon quenching dips nanospectroscopy via plasmon resonance energy transfer. Nat. Methods 4, 1015–1017 (2007)
Y. Choi, T. Kang, L.P. Lee, Plasmon resonance energy transfer (PRET)-based molecular imaging of cytochrome c in living cells. Nano Lett. 9, 85–90 (2008)
W.G. Qu, B. Deng, S.L. Zhong, H.Y. Shi, S.S. Wang, A.W. Xu, Plasmonic resonance energy transfer-based nanospectroscopy for sensitive and selective detection of 2,4,6-trinitrotoluene (TNT). Chem. Commun. 47, 1237–1239 (2011)
Y. Choi, Y. Park, T. Kang, L.P. Lee, Selective and sensitive detection of metal ions by plasmonic resonance energy transfer-based nanospectroscopy. Nat. Nanotechnol. 4, 742–746 (2009)
L. Shi, C. Jing, Z. Gu, Y.-T. Long, Brightening gold nanoparticles: new sensing approach based on plasmon resonance energy transfer. Sci. Rep. 5, 10142 (2015)
M. Turner, V.B. Golovko, O.P.H. Vaughan, P. Abdulkin, A. Berenguer-Murcia, M.S. Tikhov, B.F.G. Johnson, R.M. Lambert, Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nature 454, 981–983 (2008)
A. de la Escosura-Muñiz, A. Ambrosi, A. Merkoçi, Electrochemical analysis with nanoparticle-based biosystems. TrAC-Trend Anal. Chem. 27, 568–584 (2008)
J.M. Pingarrón, P. Yáñez-Sedeño, A. González-Cortés, Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 53, 5848–5866 (2008)
M. Brust, G.J. Gordillo, Electrocatalytic hydrogen redox chemistry on gold nanoparticles. J. Am. Chem. Soc. 134, 3318–3321 (2012)
X. Shan, I. Díez-Pérez, L. Wang, P. Wiktor, Y. Gu, L. Zhang, W. Wang, J. Lu, S. Wang, Q. Gong, J. Li, N. Tao, Imaging the electrocatalytic activity of single nanoparticles. Nat. Nanotechnol. 7, 668–672 (2012)
X. Shan, U. Patel, S. Wang, R. Iglesias, N. Tao, Imaging local electrochemical current via surface plasmon resonance. Science 327, 1363–1366 (2010)
C. Novo, A.M. Funston, P. Mulvaney, Direct observation of chemical reactions on single gold nanocrystals using surface plasmon spectroscopy. Nat. Nanotechnol. 3, 598–602 (2008)
C. Novo, A.M. Funston, A.K. Gooding, P. Mulvaney, Electrochemical charging of single gold nanorods. J. Am. Chem. Soc. 131, 14664–14666 (2009)
P. Mulvaney, J. Pérez-Juste, M. Giersig, L.M. Liz-Marzán, C. Pecharromán, Drastic surface plasmon mode shifts in gold nanorods due to electron charging. Plasmonics 1, 61–66 (2006)
C. Novo, P. Mulvaney, Charge-induced Rayleigh instabilities in small gold rods. Nano Lett. 7, 520–524 (2007)
S.K. Dondapati, M. Ludemann, R. Muller, S. Schwieger, A. Schwemer, B. Handel, D. Kwiatkowski, M. Djiango, E. Runge, T.A. Klar, Voltage-induced adsorbate damping of single gold nanorod plasmons in aqueous solution. Nano Lett. 12, 1247–1252 (2012)
C.M. Hill, S.L. Pan, A dark-field scattering spectroelectrochemical technique for tracking the electrodeposition of single silver nanoparticles. J. Am. Chem. Soc. 135, 17250–17253 (2013)
Y. Fang, W. Wang, X. Wo, Y. Luo, S. Yin, Y. Wang, X. Shan, N. Tao, Plasmonic imaging of electrochemical oxidation of single nanoparticles. J. Am. Chem. Soc. 136, 12584–12587 (2014)
C. Jing, F.J. Rawson, H. Zhou, X. Shi, W.H. Li, D.W. Li, Y.T. Long, New insights into electrocatalysis based on plasmon resonance for the real-time monitoring of catalytic events on single gold nanorods. Anal. Chem. 86, 5513–5518 (2014)
C.P. Byers, B.S. Hoener, W.S. Chang, M. Yorulmaz, S. Link, C.F. Landes, Single-particle spectroscopy reveals heterogeneity in electrochemical tuning of the localized surface plasmon. J. Phys. Chem. B 118, 14047–14055 (2014)
H. Zhou, Q. Liu, F.J. Rawson, W. Ma, D.W. Li, D. Li, Y.T. Long, Optical monitoring of faradaic reaction using single plasmon-resonant nanorods functionalized with graphene. Chem. Commun. 51, 3223–3226 (2015)
L.-X. Qin, Y. Li, D.-W. Li, C. Jing, B.-Q. Chen, W. Ma, A. Heyman, O. Shoseyov, I. Willner, H. Tian, Y.-T. Long, Electrodeposition of single-metal nanoparticles on stable protein 1 membranes: application of plasmonic sensing by single nanoparticles. Angew. Chem. Int. Ed. 51, 140–144 (2012)
L.-X. Qin, C. Jing, Y. Li, D.-W. Li, Y.-T. Long, Real-time monitoring of the aging of single plasmonic copper nanoparticles. Chem. Commun. 48, 1511–1513 (2012)
Y. Ge, B. Kang, Surface plasmon resonance scattering and absorption of biofunctionalized gold nanoparticles for targeted cancer imaging and laser therapy. Sci. China Technol. SC. 54, 2358–2362 (2011)
L. Cognet, C. Tardin, D. Boyer, D. Choquet, P. Tamarat, B. Lounis, Single metallic nanoparticle imaging for protein detection in cells. Proc. Natl. Acad. Sci. U.S.A. 100, 11350–11355 (2003)
K.A. Willets, R.P. Van Duyne, Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 58, 267–297 (2007)
E.E. Connor, J. Mwamuka, A. Gole, C.J. Murphy, M.D. Wyatt, Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 1, 325–327 (2005)
P.K. Jain, X. Huang, I.H. El-Sayed, M.A. El-Sayed, Review of some interesting surface plasmon resonance-enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics 2, 107–118 (2007)
J.N. Anker, W.P. Hall, O. Lyandres, N.C. Shah, J. Zhao, R.P. Van Duyne, Biosensing with plasmonics nanosensors. Nat. Mater. 7, 442–453 (2008)
E. Cohen-Hoshen, G.W. Bryant, I. Pinkas, J. Sperling, I. Bar-Joseph, Exciton-plasmon interactions in quantum dot-gold nanoparticle structures. Nano Lett. 12, 4260–4264 (2012)
K.V. Kong, Z. Lam, W.D. Goh, W.K. Leong, M. Olivo, Metal carbonyl-gold nanoparticle conjugates for live-cell SERS imaging. Angew. Chem. Int. Ed. 51, 9796–9799 (2012)
L.V. Brown, X. Yang, K. Zhao, B.Y. Zheng, P. Nordlander, N.J. Halas, Fan-shaped gold nanoantennas above reflective substrates for surface-enhanced infrared absorption (SEIRA). Nano Lett. 15, 1272–1280 (2015)
M. Février, P. Gogol, A. Aassime, R. Mégy, C. Delacour, A. Chelnokov, A. Apuzzo, S. Blaize, J.-M. Lourtioz, B. Dagens, Giant coupling effect between metal nanoparticle chain and optical waveguide. Nano Lett. 12, 1032–1037 (2012)
M.A. Mahmoud, W. Qian, M.A. El-Sayed, Following charge separation on the nanoscale in Cu2O–Au nanoframe hollow nanoparticles. Nano Lett. 11, 3285–3289 (2011)
W.-W. Zhao, C.-Y. Tian, J.-J. Xu, H.-Y. Chen, The coupling of localized surface plasmon resonance-based photoelectrochemistry and nanoparticle size effect: towards novel plasmonic photoelectrochemical biosensing. Chem. Commun. 48, 895–897 (2012)
K.R. Catchpole, A. Polman, Plasmonic solar cells. Opt. Express 16, 21793–21800 (2008)
B. Sahoo, M.N. Hopkinson, F. Glorius, Combining gold and photoredox catalysis: visible light-mediated oxy- and aminoarylation of alkenes. J. Am. Chem. Soc. 135, 5505–5508 (2013)
C. Rosman, S. Pierrat, A. Henkel, M. Tarantola, D. Schneider, E. Sunnick, A. Janshoff, C. Sönnichsen, A new approach to assess gold nanoparticle uptake by mammalian cells: combining optical dark-field and transmission electron microscopy. Small 8, 3683–3690 (2012)
Y. Li, J.T. Cox, B. Zhang, Electrochemical responses and electrocatalysis at single Au nanoparticles. J. Am. Chem. Soc. 132, 3047–3054 (2010)
Y.L. Ying, J.J. Zhang, R. Gao, Y.T. Long, Nanopore-based sequencing and detection of nucleic acids. Angew. Chem. Int. Ed. 52, 13154–13161 (2013)
M.P. Jonsson, C. Dekker, Plasmonic nanopore for electrical profiling of optical intensity landscapes. Nano Lett. 13, 1029–1033 (2013)
L.A. Austin, B. Kang, M.A. El-Sayed, A new nanotechnology technique for determining drug efficacy using targeted plasmonically enhanced single cell imaging spectroscopy. J. Am. Chem. Soc. 135, 4688–4691 (2013)
A.E. Klein, N. Janunts, M. Steinert, A. Tunnermann, T. Pertsch, Polarization-resolved near-field mapping of plasmonic aperture emission by a dual-SNOM system. Nano Lett. 14, 5010–5015 (2014)
M. Wagner, Z. Fei, A.S. McLeod, A.S. Rodin, W.Z. Bao, E.G. Iwinski, Z. Zhao, M. Goldflam, M.K. Liu, G. Dominguez, M. Thiemens, M.M. Fogler, A.H.C. Neto, C.N. Lau, S. Amarie, F. Keilmann, D.N. Basov, Ultrafast and nanoscale plasmonic phenomena in exfoliated graphene revealed by infrared pump-probe nanoscopy. Nano Lett. 14, 894–900 (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Jing, C., Long, YT. (2016). Sensing on Single Plasmonics. In: Serpe, M.J., Kang, Y., Zhang, Q.M. (eds) Photonic Materials for Sensing, Biosensing and Display Devices. Springer Series in Materials Science, vol 229. Springer, Cham. https://doi.org/10.1007/978-3-319-24990-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-24990-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24988-9
Online ISBN: 978-3-319-24990-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)