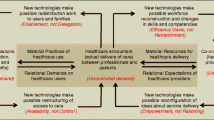
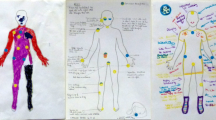
Abstract
Well-designed medical devices that embrace the socio-psychological needs of patients lead to increased customer acceptance, sustained use, improved safety and cost-effectiveness for both the professional and lay users. This paper proposes a new iterative design-led research approach for collecting and evaluating socio-psychological contextual user experience of patients and care providers in the telehealth development process. This approach, which has been applied to a multi-country development of a medical device, is based around the usage of a telehealth prototype from early stages of the design process. This allows for ‘mini’ elements of all design stages to be addressed in each individual stage to ensure the capture of contextual data from users about usage patterns, feelings and impact on the patient-clinician care relationship.
Chapter PDF
Similar content being viewed by others
Keywords
References
World Health Organization (WHO): Medical Devices: Managing the Mismatch: An Outcome of the Priority Medical Devices Project. World Health Organization Report (2010)
International Organization for Standardization (ISO) 14971: Medical Devices: Application of Risk Management to Medical Devices, Geneva, Switzerland (2007)
Tice, J.A., Helfand, M., Feldman, M.D.: Clinical Evidence for Medical Devices: Regulatory Processes Focusing on Europe and the United States of America. The World Health Organization Report, Geneva (2010)
International Organization for Standardization (ISO) 62366: Medical Devices: Application of Usability Engineering to Medical Devices, Geneva, Switzerland (2008)
US Food and Drug Administration (FDA): Medical Devices: Home Use Devices, US Food and Drug Administration (2012)
World Health Organization (WHO): Primary Health Care (Now More Than Ever). The WHO Report (2009)
Lang, A.R., Martin, J.L., Sharples, S., Crowe, J.A.: The effect of design on the usability and real world effectiveness of medical devices: A case study with adolescent users. Applied Ergonomics 44, 799–810 (2013)
Curfman, G.D., Redberg, R.F.: Medical devices: Balancing regulation and innovation. The New England Journal of Medicine 365, 975–977 (2011)
Medical Devices Directive: Council Directive 93/42/EEC of 14 June 1993 concerning medical devices. European Commission (1993)
US Food and Drug Administration (FDA) 21CFR 820.30: Quality System Regulation: Design Controls (2013)
European Commission: Proposal for a Regulation of the European Parliament and of the Council on Medical Devices, and Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 (2012)
US Food and Drug Administration (FDA): Understanding Barriers to Medical Device Quality. FDA (2011)
Kramer, D.B., Baker, M., Ransford, B., Molina-Markham, A., Stewart, Q., Fu, K., Reynolds, M.R.: Security and Privacy Qualities of Medical Devices: An Analysis of FDA Postmarket Surveillance. PLoS ONE 7, e40200 (2012)
US Food and Drug Administration (FDA): “23andMe, Inc. 11/22/13” (2012), http://www.fda.gov/iceci/enforcementactions/warningletters/2013/ucm376296.htm
Gurses, A.P., Ozok, A.A., Pronovost, P.J.: Time to accelerate integration of human factors and ergonomics in patient safety. BMJ Quality and Satefy 21, 347–351 (2012)
US Food and Drug Administration (FDA): General Human Factors Information and Resources: What is Human Factors/Usability Engineering? (2013)
NHS National Patient Safety Agency: Design for patient safety: User testing in the development of medical devices (2010) ISBN: 978-1-906624-11-8
US Food and Drug Administration (FDA): Applying Human Factors and Usability Engineering to Optimise Medical Device Design. US Department of Health and Human Services (2011)
Bitterman, N.: Design of medical devices: A home perspective. European Journal of Internal Medicine 22, 39–42 (2011)
May-Russell, S.: Medical devices designed with patients in mind. European Industrial Pharmacy 14, 13–15 (2012)
Martin, J.L., Norris, B.J., Murphy, E., Crowe, J.A.: Medical device development: The challenge for ergonomics. Applied Ergonomics 39, 271–283 (2008)
Waterson, P.E., Anderson, J.: Bridging the Research Practice Gap in Healthcare Human Factors and Ergonomics. In: Ergonomics & Human Factors Conference, Cambridge, UK, April 15-18, pp. 15–18 (2013)
Demirbilek, O., Sener, B.: Product design, semantics and emotional response. Ergonomics 46 (2003)
Deci, E.L., Ryan, R.M.: Self-determination theory: A macrotheory of human motivation, development, and health. Canadian Psychology-Psychologie Canadienne 49, 182–185 (2008)
Bandura, A.: Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review 84, 191–215 (1977)
Vallerand, R.J., Reid, G.: On the causal effects of perceived competence on intrinsic motivation: A test of cognitive evaluation theory. Journal of Sport Psychology 6, 94–102 (1984)
The Marmot Review: Fair Society Healthy Lives. Strategic Review of Health Inequalities in England post-2010 (2010)
Langer, E.J., Rodin, J.: The effects of choice and enhanced personal responsibility for the aged: A field experiment in an institutional setting. Journal of Personality and Social Psychology 34, 191–198 (1976)
Ogden, J., Daniells, E., Barnett, J.: When is choice a good thing? An experimental study of the impact of choice on patient outcomes. Psychology, Health & Medicine 14, 34–47 (2009)
Huang, Y.H., Lei, W., Junqi, S.: When do objects become more attractive? The individual and interactive effects of choice and ownership on object evaluation. Personality and Social Psychology Bulletin 35, 713–722 (2009)
Csikszentmihályi, M.: Flow: The Psychology of Optimal Experience. Harper Perennial, New York (1991)
Durik, A.M., Harackiewicz, J.M.: Achievement goals and intrinsic motivation: Coherence, concordance, and achievement orientation. Journal of Experimental Social Psychology 39, 378–385 (2003)
Ajzen, I.: The theory of planned behavior. Organizational Behavior and Human Decision Processes 50, 179–211 (1991)
Thomson, R., Martin, J.L., Sharples, S.: The psychosocial impact of home use medical devices on the lives of older people: A qualitative study. BMC Health Services Research 13, 1–8 (2013)
Multidisciplinary Assessment of Technology Centre for Healthcare (MATCH) (2014), http://www.match.ac.uk/
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this paper
Cite this paper
Mieczakowski, A., King, J., Fehnert, B. (2014). A Design-led Research Approach to Contextual Evaluation of Socio-psychological Factors in the Development of Telehealth Devices. In: Stephanidis, C., Antona, M. (eds) Universal Access in Human-Computer Interaction. Aging and Assistive Environments. UAHCI 2014. Lecture Notes in Computer Science, vol 8515. Springer, Cham. https://doi.org/10.1007/978-3-319-07446-7_31
Download citation
DOI: https://doi.org/10.1007/978-3-319-07446-7_31
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-07445-0
Online ISBN: 978-3-319-07446-7
eBook Packages: Computer ScienceComputer Science (R0)