Abstract
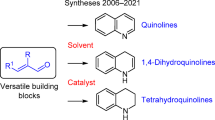
Also known as the Friedländer condensation, it combines an α-amino aldehyde or ketone with another aldehyde or ketone with at least one methylene α adjacent to the carbonyl to furnish a substituted quinoline. The reaction can be promoted by either acid, base, or heat.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Friedländer, P. Ber. 1882, 15, 2572–2575. Paul Friedländer (1857–1923), born in Königsberg, Prussia, apprenticed under Carl Graebe and Adolf von Baeyer. He was interested in music and was an accomplished pianist.
Elderfield, R. C. In Heterocyclic Compounds, Elderfield, R. C., ed.; Wiley: New York, 1952, 4, Quinoline, Isoquinoline and Their Benzo Derivatives, 45–47. (Review).
Jones, G. In Heterocyclic Compounds, Quinolines, vol. 32, 1977; Wiley: New York, pp 181–191. (Review).
Cheng, C.-C.; Yan, S.-J. Org. React. 1982, 28, 37–201. (Review).
Shiozawa, A.; Ichikawa, Y.-I.; Komuro, C.; Kurashige, S.; Miyazaki, H.; Yamanaka, H.; Sakamoto, T. Chem. Pharm. Bull. 1984, 32, 2522–2529.
Gladiali, S.; Chelucci, G.; Mudadu, M. S.; Gastaut, M.-A.; Thummel, R. P. J. Org. Chem. 2001, 66, 400–405.
Henegar, K. E.; Baughman, T. A. J. Heterocycl. Chem. 2003, 40, 601–605.
Dormer, P. G.; Eng, K. K.; Farr, R. N.; Humphrey, G. R.; McWilliams, J. C.; Reider, P. J.; Sager, J. W.; and Volante, R. P. J. Org. Chem. 2003, 68, 467–477.
Pflum, D. A. Friedländer Quinoline Synthesis. In Name Reactions in Heterocyclic Chemistry; Li, J. J., Ed.; Wiley: Hoboken, NJ, 2005, 411–415. (Review).
Vander Mierde, H.; Van Der Voot, P.; De Vos, D.; Verpoort, F. Eur. J. Org. Chem. 2008, 1625–1631.
Augustine, J. K.; Bombrun, A.; Venkatachaliah, S. Tetrahedron Lett. 2011, 52, 6814–6818.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Li, J.J. (2014). Friedländer quinoline synthesis. In: Name Reactions. Springer, Cham. https://doi.org/10.1007/978-3-319-03979-4_110
Download citation
DOI: https://doi.org/10.1007/978-3-319-03979-4_110
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-03978-7
Online ISBN: 978-3-319-03979-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)