Abstract
Global battery demand for stationary storage is expected to increase up to more than 2500 GWh in the next 10 years. In this scenario, the redox flow batteries (RFBs) and metal–oxygen (air) batteries (MABs) represent a strategic alternative to LIBs.
RFBs and MABs share a unique feature: unlike conventional LIBs and conventional batteries that are made by two solid electrodes, separated by an electrolyte/separator assembly, and that are hermetically sealed, RFBs and MABs can be considered as “open systems.” Besides the specific electrochemical processes that drive RFB and MAB operation and that will be discussed in the next sections, the open architecture of RFBs and MABs provides an inherent advantage vs. the closed batteries in terms of safety. Indeed, dangerous internal pressure and/or temperature rise that accidentally take place in case of battery failure can be mitigated.
In the following, the most recent developments of novel open battery architectures are presented, while promises and challenges of these open systems are discussed.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Redox Flow Batteries
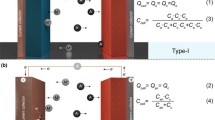
In RFBs, the electroactive species are dissolved in solutions (anolyte and catholyte) that are stored in tanks external to the cell and that are flown in the cell core that is made by the anode and cathode current collectors, intercepting the anolyte and catholyte flows, respectively, and separated by the separator (Fig. 11.1a).
MABs typically consist of a metal anode, a separator soaked in the electrolyte, and an air (oxygen) cathode that has one face exposed to the environment (Fig. 11.1b). To solve kinetics and cyclability issues that are still slowing MAB development, flow MABs (FMABs) have been proposed. FMABs exploit the RFB design, with the anolyte and/or the catholyte (only one half-cell or both) acting as carrier of the anodic metal species or of the oxygen, respectively [1].
The main advantage of RFBs relies on their peculiar architecture that allows for decoupling of power and energy but also enables easy maintenance and scale-up of the system. However, as the active materials are in solution, their solubility severely limits the energy density of the system.
Under this open system concept, many chemistries have been postulated looking at SET plan targets in terms of cost and durability, which are primarily determined by the active materials’ availability and stability [1]. In this context, vanadium-based RFBs have demonstrated their supremacy considering their long lifetime (20 years) and high performance. The scarcity of the raw material is the main reason behind the need to replace vanadium. Thus, systems such as the H2/Br2 battery, which offer high power density and a cell voltage of 1.09 V, are also paving their way to market.
Alternatively, the so-called hybrid redox flow batteries contain at least one solid active material that is plated or stripped within the cell. Zn/Br and Zn/Fe are successful examples of this concept [2], while all-iron (ca. 1.2 V) and all-copper (ca. 0.6 V) RFBs can be listed as sustainable options considering the abundance and the low toxicity of the employed materials when compared with the most used vanadium-based compounds [1]. The problems inherent to the plating process are the main hurdles to be solved. Despite the benefits in terms of energy density of using a solid metal as anode, a direct consequence is that power and energy cannot be independently regulated.
Back to conventional RFBs, flow batteries based on organic active materials have been postulated as real candidates to dare VRFB, and based on the ubiquitous nature of carbon-based materials, they may offer a global solution. Indeed, there is a bloom of start-ups working on organic flow batteries.
1.1 Organic-Based Chemistries
Irruption of organic active materials in RFBs is an open door to a vast chemical space. Thus, beyond the abundance and the potential inexpensive manufacturing at large scale, the high tunability of those compounds is the main reason for the interest aroused by aqueous organic redox flow batteries (AORFBs) [3].
A wise design of active material should allow to determine the redox potential, the solubility, the chemical and electrochemical stability, the reaction kinetics, the gravimetric capacity, and even the crossover rate. In this regard, selection of the functionality of the active site, the inclusion of polar groups, and the definition of the molecular weight and the number of active sites would serve to modulate the properties. The ideal electrolyte is still to come, and a compromise has to be accepted. The most relevant examples on AORFB are either based on quinone-type or on viologen-based anolyte solutions (Table 11.1).
Quinone–Fe System
Quinone-type molecules can undergo a fast and reversible 2e− and 2H+ transfer according to Eq. 11.1:

Among the multiple examples in the literature, anthraquinone–iron couples have shown the highest stability. Recent efforts have been devoted to further push the stability of those compounds to capacity decays as low as 0.0018% day−1 [4]. This has been possible by including highly stable carboxylate (DBEAQ), pivalate (DPivOHAQ) [5], or phosphonate (DPPE) [6] moieties in anthraquinone core structure, which can be coupled with ferrocyanide leading to a cell voltage of 1.0 V and operated in alkaline media with high energy efficiencies (>80% at 100 mA cm−2). The lack of a more competitive catholyte solution is identified as the main limitation, while despite the high solubility of the anthraquinones, the system has only been validated for low-energy-density electrolytes (0.5 M anthraquinone). With a similar reaction mechanism, pyrazines have been employed in alkaline batteries leading to high cell voltages (1.2–1.4 V) and good cell performance when coupled with ferrocyanide [6].
Viologen-Based Systems with Neutral pH
Bipyridinium salts can undergo 1e− or 2e− reduction processes (2); the first reduction is considered as fully reversible, while the capacity retention is generally lower if the second process is involved. No protons are involved in the redox equilibrium of viologens neither in the case of the materials selected as catholyte counterparts, i.e., iron complexes or TEMPO derivatives mainly. Those batteries are operated under mild conditions, close to neutral pH values. This may elongate the durability of the battery’s components, but it also entails a challenge in terms of conductivity due to the low proton and hydroxyl concentration in the electrolyte media. Thus, the efficiency of neutral pH systems ranges between 40% and 80% energy efficiency (EE) for current densities between 50 and 100 mA·cm−2. The low cell voltage (ca. 0.8 V) [7, 8] of the viologen–iron complex systems is their main weakness, while the most stable viologen–TEMPO systems employ low-energy-density electrolytes (0.5 M) [9]. Molecular engineering works are still in progress, and stable 2e− storage extended bipyridinium compounds have been developed [10] boosting potential energy density of viologen systems.

The stability of organic materials is still under debate, and comparison of different systems is not straightforward even if the capacity fade over time has been taken as a more reliable parameter [11]. Cycling conditions, capacity utilization, and concentration of active materials among other factors may impact the electrolyte stability.
In a lower extent, polymer redox active materials have been employed as a measure to mitigate crossover [12], and organics have also been combined with a metal anode to boost energy density [13]. However, those trends remain as a secondary option after weighing pros and cons.
The use of nonaqueous organic redox flow batteries (NARFBs) has been also explored. This full organic electrolyte could theoretically mitigate the energy density problems of RFB as the cell voltage can be increased over 3 V and the solubility of the active materials is significantly higher in organic solvents. However, this ideal scenario is hurdled by the poor conductivity of the electrolytes, the high viscosity of high concentrated solutions, and the low chemical compatibility of the membranes and the organic solvents. Thus, high concentrations >2 M cannot be exceeded without compromising diffusion properties [14]. NARFBs are generally operated at low current densities (< 40 mA·cm−2) [15] or at low concentrations [16].
2 Metal–Air Batteries
2.1 Steady Metal–Air Batteries
Metal–air (oxygen) batteries (MABs) have the advantage of using the lightest cathode material available in nature: oxygen. Furthermore, O2 is not stored inside the cell, but it is continuously supplied from air or tanks outside the cell. Therefore, cell capacity is not limited by cathode active material depleting. In addition, in conventional (not-flow) MABs, the anode is a thin metal foil with an extremely high density. Combining a metal anode and an O2 cathode enables to use most of the system volume for the anode material, and this results in a battery with an extremely high energy density.
Several MAB chemistries have been proposed, including those based on alkali, transition, and multivalent metals [17,18,19]. Table 11.2 summarizes the cell reactions, the metal and discharge product densities, the nominal cell voltage, and the theoretical specific capacity and specific energy and energy density of different MABs.
Li, Na, K, Mg, and Al-MABs feature cell voltages higher than 2 V and therefore require the use of nonaqueous electrolytes. In the case of Li and Na, which are not stable in water, organic/inorganic hybrid electrolytes have been proposed. In these systems, the metal is in contact with an organic electrolyte, and the cathode operates with an aqueous electrolyte. Anode and cathode are alienated by a solid ionic conductor separator. Zn- and Fe-MABs featuring cell voltages lower than 2 V can operate with aqueous electrolytes, and they typically make use of alkaline solutions.
The specific capacity evaluated based on both metal and oxygen contents depends on the number of electrons exchanged for mole of reactants and mainly on the atomic mass of the metal. It spans from 0.46 Ah kg−1 of the Fe–O2 cells (four-electron process) to 1.17 Ah kg−1of the Li–O2 (two-electron process), which features the lightest metal, i.e., lithium.
All the MABs listed in Table 11.2 feature high theoretical specific energy densities that are about two- to tenfold higher than that of today’s lithium-ion batteries. Among the nonaqueous MABs, the Li–O2 cells exhibit the highest value that in theory can be as high as 3.5 kW kg−1. Among the aqueous MABs, Zn–O2 holds the best promises with 1.1 kW kg−1.
In MABs, at the anode, metal stripping/deposition occurs. At the cathode, the sluggish kinetics of the oxygen reduction and evolution reactions (ORR/OER) are promoted by catalysts (or mediators) that are supported on the electrode surface, typically a porous carbon with high surface area. Like in fuel cells, in static MABs, the optimization of the cathode three-phase boundary (catalyst–electrolyte–gas) is of paramount importance to achieve a high conversion efficiency and fast cell response under high-current regimes. Today, typical discharge currents are lower than 0.5 mA·cm−2 for nonaqueous Li-MABs and 500 mA·cm−2 for aqueous Zn-MABs. In air-breathing cells, the slow natural diffusion of O2 to the cathode is one of the processes that cause not negligible cell overvoltages during discharge at high currents. Furthermore, during the discharge, insoluble by-products are deposited on the surface of the metal anode and air cathode, therefore passivating the electrodes, clogging cathode pores, and further limiting the diffusion of oxygen. These passivating products limit the discharge capacity of MABs to values that can be 50% lower than the theoretical ones. They also cause high recharge overvoltages and, consequently, low recharge energy efficiency that is typically lower than 70%.
As it concerns the metal anode, stripping/deposition inherently induces changes in the metal surface morphology, dendrite growth, and metal fragmentation into particles with subsequent loss of electric contact and of material. Here, the electrolyte can play a role by forming a suitable solid electrolyte interphase that protects the anode and controls the uniform metal deposition.
2.2 Flow Metal–Air Batteries
Despite such promising theoretical performance, still many challenging problems need to be solved to let MABs become a consolidated technology. Combining MAB chemistries with a flow cell design in flow metal–air batteries (FMABs) can be an answer. FMAB cell commonly consists of a metal anode (tin foil or a flowable anolyte), a separator soaked in the electrolyte, and a flowable air (oxygen) cathode. At the anode, the metal stripping/deposition occurs. At the cathode side, an electrolyte enriched with oxygen is flowed across a porous current collector where the oxygen reduction and evolution reactions (ORR/OER) occur. As in their static counterparts, to overcome the sluggish kinetics of the ORR/OER, the surface of the porous electrodes is decorated with catalysts or mediators.
The exploitation of a flowable cathode (catholyte) is a smart strategy to overcome some of MAB’s intrinsic challenges, such as the slow oxygen diffusion at the cathode and the passivation of electrodes by deposition of insoluble by-products [1]. The convective transport of the catholyte allows for overcoming the mass transport limitations due to the oxygen diffusion [20, 21]. To reduce the current collector passivation, driven by the deposition of the insoluble discharge products (such as metal oxides), a valuable strategy is the exploitation of slurries rather than solutions, in which the suspended particles act as nucleation centers [1, 22]. Moreover, to alleviate the dendrite formation, while increasing the current density at the anode, a possible solution is the exploitation of anolyte slurries [23]. The main drawback is the nontrivial design of the flow frame, which strongly depends on the cell chemistry and the rheological properties of the electrolyte.
Nowadays, zinc, aluminum, and lithium are the main metallic anodes on which the research activity in MAFBs has been focused. According to the metal reactivity, both aqueous and nonaqueous electrolyte media have been explored [24, 25].
Zn-MAFBs are the most mature technology. Indeed, the company “Zinc 8” is currently manufacturing 100 kW, targeting 1 MW installation, forecasting a price below 100 € kW−1. Li-MAFBs, for their exceptionally high theoretical energy density, are holding great promises for energy storage.
By using the abundant, readily available seawater as catholyte, the seawater battery (SWB) arises as an attractive option for low-cost, large-scale energy storage [26,27,28]. During its charge, at the cathode, the electrolysis (oxidation) of seawater occurs, contemporary with the reduction of Na+ ions, extracted from seawater on the anode side. Indeed, seawater features a salinity of ≈3.5% (35 g L−1) in which Na+ and Cl− ions account for most of the dissolved salts. The metallic sodium requires an anhydrous anolyte, aprotic solvent solutions with sodium-based organic salts, e.g., 1 M sodium trifluoromethanesulfonate (NaCF3SO3) in tetraethylene glycol dimethyl ether (TEGDME) or 0.1 M sodium bis(fluorosulfonyl)imide (NaFSI) in ionic liquid solutions [27]. The anolyte chamber must be physically separated from the aqueous catholyte while being in ionic contact. Therefore, Na-ion conducting, solid electrolytes (e.g., NASICON) that separate the anhydrous anodic chamber and the aqueous cathodic chamber are adopted [26,27,28]. To improve the kinetics of the ORR and OER, Pt/C- and Ir/Ru-based catalysts could be exploited [29]. However, in SWB, the presence of Cl− in the catholyte requires the use of a proper current collector to control its oxidation reactions during charge, and this represents an additional problem.
SWB features a theoretically high cell voltage ≈3.48 V, with reported practical voltage of 2.2 V. Although extremely promising, today, this technology is still in R&D phase, and efforts are required to decrease the cost of the components to efficiently scale up [26].
3 Conclusions
The open batteries might change the paradigm of storing, using, and distributing energy. Besides their inherently higher safety, especially when compared to LIBs, they feature great flexibility, and a variety of materials and cell design are under exploitation. RFBs and MABs are interesting open systems that may play an important role in stationary energy storage application. In RFBs, the electrolyte is identified as the most critical component of those batteries, and there is an ongoing search for the most stable, cost-effective, safe, and abundant active materials. Thus, systems relying on safe aqueous electrolytes comprising organic active materials are gradually closing the gap with vanadium and have the potential to compete or coexist with lithium to fulfil the global demand.
MABs, even based on abundant metals, hold the promise of extremely high volumetric energy density because light and multivalent metals can be exploited. However, in MABs, low cycling stability and power are still the main limitations that could be overcome by exploiting the RFB architecture in the emerging flow and semisolid flow MABs.
References
Sanchez-Díez E, Ventosa E, Guarnieri M, Trovò A, Flox C, Marcilla R, Soavi F, Mazur P, Aranzabe E, Ferret R (2021) Redox flow batteries: status and perspective towards sustainable stationary energy storage. J Power Sources 481:228804. https://doi.org/10.1016/j.jpowsour.2020.228804
Arenas LF, Loh A, Trudgeon DP, Li X, Ponce de León C, Walsh FC (2018) The characteristics and performance of hybrid redox flow batteries with zinc negative electrodes for energy storage. Renew Sust Energ Rev 90:992–1016. https://doi.org/10.1016/j.rser.2018.03.016
(a) Chen Q, Lv Y, Yuan Z, Li X, Yu G, Yang Z, Xu T (2022) Organic electrolytes for pH-neutral aqueous organic redox flow batteries. Adv Funct Mater 32:2108777. https://doi.org/10.1002/adfm.202108777; (b) Liu Y, Chen Q, Sun P, Li Y, Yang Z, Xu T (2021) Organic electrolytes for aqueous organic flow batteries. Materials Today Energy 20:100634. https://doi.org/10.1016/j.mtener.2020.100634
Wu M, Jing Y, Wong AA, Fell EM, Jin S, Tang Z, Gordon RG, Aziz MJ (2020) Extremely stable Anthraquinone Negolytes synthesized from common precursors. Chem 6:1432–1442. https://doi.org/10.1016/j.chempr.2020.03.021
Ji Y, Goulet MA, Pollack DA, Kwabi DG, Jin S, De Porcellinis D, Ker EF, Gordon RG, Aziz MJ (2019) A phosphonate-functionalized Quinone redox flow battery at near-neutral pH with record capacity retention rate. Adv Energy Mater 9:1900039. https://doi.org/10.1002/aenm.201900039
Hollas A, Wei X, Murugesan V, Nie Z, Li B, Reed D, Liu J, Sprenkle V, Wang W (2018) A biomimetic high-capacity phenazine-based anolyte for aqueous organic redox flow batteries. Nat Energy 3:508–514. https://doi.org/10.1038/s41560-018-0167-3
Luo J, Hu B, Debruler C, Bi Y, Zhao Y, Yuan B, Hu M, Wu W, Liu TL (2019) Unprecedented capacity and stability of ammonium Ferrocyanide Catholyte in pH neutral aqueous redox flow batteries. Joule 3:149–163. https://doi.org/10.1016/j.joule.2018.10.010
Lv XL, Sullivan P, Fu HC, Hu XX, Liu H, Jin S, Li W, Feng D (2022) Dextrosil-Viologen: a robust and sustainable Anolyte for aqueous organic redox flow batteries. ACS Energy Lett 7:2428–2434. https://doi.org/10.1021/acsenergylett.2c01198
Hu B, Hu M, Luo J, Liu TL (2022) A stable, low permeable TEMPO Catholyte for aqueous Total organic redox flow batteries. Adv Energy Mater 12:2102577. https://doi.org/10.1002/aenm.202102577
Huang M, Hu S, Yuan X, Huang J, Li W, Xiang Z, Fu Z, Liang Z (2022) Five-membered-heterocycle bridged Viologen with high voltage and superior stability for flow battery. Adv Funct Mater 32:2111744. https://doi.org/10.1002/adfm.202111744
Brushett FR, Aziz MJ, Rodby KE (2020) On lifetime and cost of redox-active organics for aqueous flow batteries. ACS Energy Lett 5:879–884. https://doi.org/10.1021/acsenergylett.0c00140
Fu H, Zhang C, Wang H, Du B, Nie J, Xu J, Chen L (2022) Stable aqueous redox flow battery assembled in air atmosphere employing an anionic terpolymer as active cathode material. J Power Sources 545:231905. https://doi.org/10.1016/j.jpowsour.2022.231905
Winsberg J, Stolze C, Schwenke A, Muench S, Hager MD, Schubert US (2017) Aqueous 2,2,6,6-Tetramethylpiperidine-N-oxyl Catholytes for a high-capacity and high current density oxygen-insensitive hybrid flow battery. ACS Energy Lett 2:411–416. https://doi.org/10.1021/acsenergylett.6b00655
Shkrob IA, Robertson LA, Yu Z, Assary RS, Cheng L, Zhang L, Sarnello E, Liu X, Li T, Kaur AP, Suduwella TM, Odom SA, Wang Y, Ewoldy RH, Farag HM, Z Y (2021) Crowded electrolytes containing redoxmers in different states of charge: solution structure, properties, and fundamental limits on energy density. J Mol Liq 334:116533. https://doi.org/10.1016/j.molliq.2021.116533
Rhodes Z, Cabrera-Pardo JR, Li M, Minteer SD (2021) Electrochemical advances in non-aqueous redox flow batteries. Isr J Chem 61:101–112. https://doi.org/10.1002/ijch.202000049
Xu D, Zhang C, Zhen Y, Zhao Y, Li Y (2021) A high-rate nonaqueous organic redox flow battery. J Power Sources 495:229819. https://doi.org/10.1016/j.jpowsour.2021.229819
Zhang X, Wang XG, Xie Z, Zhou Z (2016) Recent progress in rechargeable alkali metal-air batteries. Green Energy Environ 1:4–17. https://doi.org/10.1016/j.gee.2016.04.004
Li CS, Sun Y, Gebert F, Chou SL (2017) Current progress on rechargeable magnesium–air battery. Adv Energy Mater 7:1700869. https://doi.org/10.1002/aenm.201700869
McKerracher RD, Ponce de Leόn C, Wills RGA, Shah AA, Walsh FC (2015) A review of the iron-air secondary battery for energy storage. ChemPlusChem 80:323–335. https://doi.org/10.1002/cplu.201402238
Monaco S, Soavi F, Mastragostino M (2013) Role of oxygen mass transport in rechargeable Li/O2 batteries operating with ionic liquids. J Phys Chem Lett 4:1379–1382. https://doi.org/10.1021/jz4006256
Poli F, Ghadikolaei LK, Soavi F (2019) Semi-empirical modeling of the power balance of flow lithium/oxygen batteries. Appl Energy 248:383–389. https://doi.org/10.1016/j.apenergy.2019.04.133
Ruggeri I, Arbizzani C, Soavi F (2018) Carbonaceous catholyte for high energy density semi-solid Li/O2 flow battery. Carbon 130:749–757. https://doi.org/10.1016/j.carbon.2018.01.056
Pei P, Ma Z, Wang K, Wang X, Song M, Xu H (2014) High performance zinc air fuel cell stack. J Power Sources 249:13–20. https://doi.org/10.1016/j.jpowsour.2013.10.073
Han X, Li X, White J, Zhong C, Deng Y, Hu W, Ma T (2018) Metal–air batteries: from static to flow system. Adv Energy Mater 8:1801396. https://doi.org/10.1002/aenm.201801396
Yu W, Shang W, Tan P, Chen B, Wu Z, Xu H, Shao Z, Liu M, Ni M (2019) Toward a new generation of low cost, efficient, and durable metal–air flow batteries. J Mater Chem A 7:26744–26768. https://doi.org/10.1039/C9TA10658H
Hwang SM, Park JS, Kim Y, Go W, Han J, Kim Y, Kim Y (2019) Rechargeable seawater batteries—from concept to applications. Adv Mater 31:1804936. https://doi.org/10.1002/adma.201804936
Kim Y, Kim GT, Jeong S, Dou X, Geng C, Kim Y, Passerini S (2019) Large-scale stationary energy storage: seawater batteries with high rate and reversible performance. Energy Storage Mater 16:56–64. https://doi.org/10.1016/j.ensm.2018.04.028
Kim Y, Künzel M, Steinle D, Dong X, Kim GT, Varzi A, Passerini S (2022) Anode-less seawater batteries with a Na-ion conducting solid-polymer electrolyte for power to metal and metal to power energy storage. Energy Environ Sci 15:2610–2618. https://doi.org/10.1039/D2EE00609J
Debe MK (2012) Electrocatalyst approaches and challenges for automotive fuel cells. Nature 486:43–51. https://doi.org/10.1038/nature11115
Acknowledgments
F.P. and F.S. acknowledge the following grants: HyFlow Project (European Union’s Horizon 2020 research and innovation program under grant agreement No. 963550), CO2CARBON – Upscaling carbon nanomaterial production from CO2 emissions (KAVA Call 8, 01.01.2022 – 31.12.2023); SMART – Sustainable MAterials for Redox-flow Technology (EIT Raw Materials Booster Call, Project Agreement N. 15099-SCLC-2021-5, 07/2021 – 12/2021); Contest M. Startup (2019); EIT Raw Materials Battery Challenge 2019; H2020-SMEInst-2018-2020-1 (Grant Agreement n. 837091, Nessox. 01/01/2019 – 30/06/2019); Climate-KIC; MOST – Sustainable Mobility Center and received funding from the European Union Next Generation EU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNNR) Missione 4 Componente 2, Investimento 1.4 D.D. 1033 17/06/2022, CN00000023). E.S. acknowledges European Union (BEST project, Grant agreement No. 101069676) and Basque Government (GV-ELKARTEK2022 KK-2022/00043) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Diéz, E.S., Poli, F., Soavi, F. (2024). Open Battery Systems. In: Passerini, S., Barelli, L., Baumann, M., Peters, J., Weil, M. (eds) Emerging Battery Technologies to Boost the Clean Energy Transition. The Materials Research Society Series. Springer, Cham. https://doi.org/10.1007/978-3-031-48359-2_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-48359-2_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-48358-5
Online ISBN: 978-3-031-48359-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)