Abstract
Plant-based antigen manufacturing procedures have transformed vaccine research and industry by offering a cost-effective, scalable, and safe alternative to traditional protein production systems. This chapter discusses genome editing applications for plant-based protein production systems, antigen, and antibody manufacturing, as well as their future and current developments. The chapter briefly summarizes the several advantages of plant-based protein manufacturing platforms, including lower production costs, faster response to developing risks, and the absence of animal-derived components, which contributes to a lower risk of contamination and allergic responses. The chapter provides a basic overview of recent advances in plant-based antigen production, with a focus on vaccine antigens generated from CRISPR/Cas9 genome edited Nicotiana benthamiana to improve immunogenicity by altering plant glycosylation patterns to be more compatible with human glycosylation. Solving this could revolutionize existing vaccine production from plants to meet sustainable production objectives while also benefiting human health. These applications of genome editing demonstrate how versatile the approaches may be, from basic science to improving human health.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Production of Plant-Based Therapeutic Antigens and Antibodies
The growing demand for recombinant proteins, particularly for therapeutic uses, drives the development of alternative efficient and cost-effective production systems. Current widely used platforms for recombinant protein production include bacterial, yeast, insect, and mammalian cells. These traditional systems are limited by high production costs, human pathogen contamination, improper protein folding, and glycosylation patterns [1].
1.1 Background to Plant-Based Protein Production Systems
Plant-based expression systems exhibit increasing interest due to their innate ability to generate complex proteins with precise folding patterns and post-translational modifications, and their potential for scalability and reduced risk of pathogenic contamination [2]. In 2012, the United States Food and Drug Administration (FDA) approved taliglucerase alfa, a therapeutic protein produced in plants, a groundbreaking milestone for plant-based protein production [3]. Numerous other biopharmaceuticals have been generated since and been through rigorous clinical evaluations, exhibiting the practicality and viability of plant-based protein manufacturing platforms [4]. Plant-based production can be based on transient, chloroplast, or stable nuclear expression [5]. Transient expression systems involve the temporary activity from transferred genes of interest into plant cells’ cytoplasm, typically via Agrobacterium-mediated infiltration or viral vectors [6]. This approach enables rapid protein production and has been used to produce a wide range of proteins, including monoclonal antibodies, vaccines, enzymes, and therapeutic proteins, such as human serum albumin and human somatotropin, in crops like rice and tobacco [7]. Stable transformation systems involve the integration of the target gene into the plant’s nuclear or chloroplast genomes, generating transgenic plants that express the protein of interest through their life span and following generations if transferred to offspring by classical cross-breeding [8].
1.2 The Use of Nicotiana benthamiana in Transient Expression Systems
Nicotiana benthamiana, a close relative of tobacco (Nicotiana tabacum), is a diploid herbaceous plant native to Australia, which can grow through 6–8 weeks from seed to harvest of vegetative parts. The small genome size, approximately 3 Gb, facilitates genetic modification, while its high rate of inbreeding promotes genetic uniformity [9]. Nicotiana benthamiana is there for one preferred model plant for plant-based protein production due to its high transformation frequencies, rapid growth, high biomass yield, and “accepting” immune system, making it susceptible to many common biotechnology tools [10].
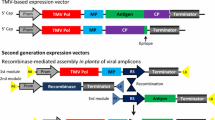
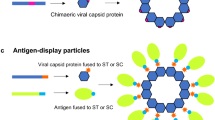
N. benthamiana has been widely employed for the expression of a diverse range of recombinant proteins, including antibodies, enzymes, and vaccine antigens. The transient expression system further allows for rapid production and evaluation of protein candidates, which is particularly valuable to respond to emerging infectious diseases, such as the production of ZMapp, a monoclonal antibody mix against Ebola virus [11], and generation of vaccine antigens in response to influenza viruses [12] (Fig. 8.1).
2 Antigen/Antibody Production for Sustainable Health Solutions
The N. benthamiana was decoded and compiled in 2012, offering an essential tool for exploring the functional genomics of N. benthamiana [13]. The primary assembly, Nb-1.0, spanned 3.1 Gb and comprised 46,220 anticipated protein-encoding genes. In 2023, a de novo whole-genome assembly was carried out in N. benthamiana using Hifi reads, resulting in 1668 contigs with a combined length of 3.1 Gb [14]. The 21 lengthiest scaffolds, considered pseudomolecules, held a 2.8-Gb sequence, covering 95.6% of the assembled genome. A sum of 57,583 gene sequences with high confidence was anticipated.
The functional annotation of the N. benthamiana genome is a process that predicts gene functions by comparing them to known genes from other species and assessing experimental data. This method is vital for determining gene functionality and control, as well as aiding in pinpointing potential genome editing targets. Various tools have been established for the functional annotation of the N. benthamiana genome, such as the Sol Genomics Network (SGN) [15]. These repositories grant researchers access to genomic data, gene annotations, and functional projections, thus laying the groundwork for experimental inquiries guided by hypotheses and applications in biotechnology.
2.1 The Role of Glycosylation in Protein Function and Stability
Glycosylation is a post-translational modification of macromolecules, such as proteins and lipids, that involves the addition of carbohydrates to the final molecule. This modification affects the stability, activity, and immunogenicity of the molecule, and is particularly important in the production of recombinant proteins [16].
Despite the many benefits of plant-based protein manufacturing systems, the glycosylation patterns of plant and human proteins differ, which is a considerable challenge for plant-based production systems [17]. Protein folding, stability, and function depend on glycosylation, while such glycosylation patterns can affect plant-produced protein efficacy and immunogenicity in humans [18]. As such, the comprehension and modification of plant glycosylation patterns to emulate those of humans is essential and must be solved for efficient development and application of plant-derived biopharmaceuticals.
2.2 Key Differences Between Human and Plant Glycosylation Patterns
N-linked glycosylation, the covalent attachment of oligosaccharides to asparagine residues within the consensus sequence Asn-X-Ser/Thr, is a conserved modification among eukaryotes, including humans and plants [18]. However, despite conserving the core glycan structure, significant differences exist in the processing and maturation of N-glycans between humans and plants [19].
In humans, the intricate N-glycans exhibit prominent adornment with sialic acid residues and β1,4-galactose, which are nonexistent in plants. Additionally, the human N-glycans display core α1,6-fucosylation, while devoid of plant-specific β1,2-xylose and core α1,3-fucose residues (Fig. 8.2). These variances in N-glycan configurations can significantly impact the effectiveness and immunogenicity of recombinant proteins manufactured by plant-based expression systems, thus creating a significant obstacle that must be solved to develop plant-based biopharmaceuticals [20].
2.3 CRISPR/Cas9-Mediated Engineering of Glycosylation Patterns in N. benthamiana
In Nicotiana benthamiana, CRISPR/Cas9-mediated knockouts of the XylT and FucT genes have effectively eliminated plant-specific glycan structures, resulting in recombinant proteins with glycosylation profiles that more closely resemble human glycoproteins [21].
The study utilized CRISPR-Cas9 to create knockout lines of N. benthamiana plants to produce biopharmaceutical glycoproteins. The target genes XylT1, XylT2, FucT1, FucT2, FucT3, FucT4, and FucT5 were identified, and gRNAs were designed to target all genes in each group [21]. The gRNAs were tested by transient expression and then inserted into the binary pPAM vector carrying a plant-codon optimized cas9 cassette with intron and a gRNA expression cassette under the control of the A. thaliana U6 promoter. Three variants of the knockout construct were prepared, one targeting XylT1 and 2, one targeting FucT1-4, and one with all seven gRNAs combined. The CRISPR constructs were then transformed into N. benthamiana plants using agroinfiltration. High-resolution melt analysis and Western blotting were used to confirm successful knockout of the targeted genes [21].
The CRISPR-Cas9 system works by introducing a double-strand break at a specific location in the genome, which is then repaired by non-homologous end joining (NHEJ) or homology-directed repair (HDR). In the case of this study, the goal was to disrupt the function of specific genes involved in glycosylation, which can affect the efficacy of biopharmaceuticals. By creating knockout lines of N. benthamiana plants using CRISPR-Cas9, the researchers were able to produce glycoproteins with reduced or eliminated plant-specific glycans, which have comparable affinity to gold standard biopharmaceuticals produced in by using cells from Chinese hamster ovaries. (CHO).
A very recent paper utilizing this newly double knockout line of N. benthamiana investigated the impact of plant N-glycosylation on the immunogenic properties of a chimeric Hepatitis B Virus (HBV) S/L vaccine candidate produced in wild-type and XylT and FucT knockout lines of N. benthamiana [22]. The study found that prevention of b-1,2-xylose, and a-1,3-fucose attachment to the HBV antigen significantly increased the immune response in mice compared to the wild-type plant-produced counterpart. Notably, the antibodies triggered by the knockout-made antigen neutralized both wild-type HBV and a clinically relevant vaccine escape mutant more efficiently. The study validates the glycoengineered N. benthamiana as a substantially improved host for plant production of glycoprotein vaccines.
This work provides evidence that glyco-engineering of plants can significantly enhance the immunogenicity of plant-produced vaccines. Further research is ongoing to explore the potential of the CRISPR/Cas9 system in enhancing the immunogenicity of plant-produced antigens for human use. Using CRISPR/Cas9 in plant-based vaccine production provides an innovative avenue for developing novel cost-effective vaccines.
3 Socioeconomics of Plant-Based Protein Production Including Regulatory Issues
CRISPR/Cas9-mediated engineering of plant glycosylation patterns can produce human-like therapeutic proteins at lower cost, higher scalability, and with less contamination risk than traditional protein production platforms [23]. However, to ensure the safe and responsible development and use of plant-produced recombinant proteins, this technology’s introduction into the biopharmaceutical industry raises several ethical, regulatory, and commercial concerns.
CRISPR/Cas9 enables quick and easy alteration of glycosylation patterns in plants. Consequently, apprehensions regarding health and environmental hazards associated with genetically modified organisms (GMOs) emerge [24]. While not exclusive to plant-based protein production, considering long-term repercussions of introducing genetically altered flora into ecosystems and devising strategies to mitigate potential risks is vital. CRISPR/Cas9-mediated plant engineering also falls under GMO regulations, which exhibit considerable variation across nations. Such regulations impact the development, manufacture, and distribution of plant-made recombinant proteins. Businesses must adapt their operations to ensure compliance within this intricate framework. Streamlining international regulations and enhancing the transparency of the approval process could facilitate wider utilization of this technology.
Regulatory approval and patient safety are contingent upon the safety and quality of plant-produced recombinant proteins. Regulatory bodies, including the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), mandate comprehensive safety and efficacy data for the authorization of therapeutic proteins—even those generated in plants. Due to the lower costs associated with plant-based protein production, life-saving medications could become accessible in low-income regions where cutting-edge medical treatments often remain elusive. From an ethical perspective, it is crucial to ensure that technological advancements’ benefits are distributed equitably, preventing exacerbation of existing global health disparities.
The recent research into the intricacies of plant-specific glycosyltransferases, such as β1,2-xylosyltransferase (XylT) and core α1,3-fucosyltransferase (FucT), has laid the foundation for gene-editing methods to further engineer plant glycosylation patterns. By employing CRISPR/Cas9 technology, the modification of plant glycosylation patterns becomes more streamlined, enabling alterations to specific genes with exceptional accuracy and effectiveness, ultimately obtaining engineered plants that produce glycoproteins resembling their human equivalents. The ongoing fine-tuning of CRISPR/Cas9, which include the creation of new Cas9 variations, guide RNA designs, and the adoption of high-throughput screening methods, has the capacity to further advance gene-editing procedures within plants, making them even more precise and efficient.
The modification of glycosylation patterns in plants holds the promise to transform the biopharmaceutical industry. Plant-based expression systems can contribute to the discovery of innovative therapeutic proteins that boost enhanced pharmacological properties by offering an affordable, scalable, and secure alternatives to mammalian cell culture. Continued efforts in this area could potentially give rise to new treatments for a broad spectrum of illnesses.
References
Tripathi, N.K., Shrivastava, A.: Recent developments in bioprocessing of recombinant proteins: expression hosts and process development. Front. Bioeng. Biotechnol. 7, 420 (2019)
Kulshreshtha, A., Sharma, S., Padilla, C.S., Mandadi, K.K.: Plant-based expression platforms to produce high-value metabolites and proteins. Front. Plant Sci. 13, 1043478 (2022)
Zimran, A., Wajnrajch, M., Hernandez, B., Pastores, G.M.: Taliglucerase alfa: safety and efficacy across 6 clinical studies in adults and children with Gaucher disease. Orphanet J. Rare Dis. 13, 36 (2018)
Shanmugaraj, B., Phoolcharoen, W.: Plant molecular farming: a viable platform for recombinant biopharmaceutical production. Plants (Basel). 9, 842 (2020)
Santoni, M., Gecchele, E., Zampieri, R., Avesani, L.: Plant-based systems for vaccine production. Methods Mol. Biol. 2412, 95–115 (2022)
Canto, T.: Transient expression systems in plants: potentialities and constraints. Adv. Exp. Med. Biol. 896, 287–301 (2016)
Fahad, S., et al.: Recent developments in therapeutic protein expression technologies in plants. Biotechnol. Lett. 37, 265–279 (2015)
Lorence, A., Verpoorte, R.: Gene transfer and expression in plants. Methods Mol. Biol. 267, 329–350 (2004)
Derevnina, L., Kamoun, S., Wu, C.-H.: Dude, where is my mutant? Nicotiana benthamiana meets forward genetics. New Phytol. 221, 607–610 (2019)
Eidenberger, L., Kogelmann, B., Steinkellner, H.: Plant-based biopharmaceutical engineering. Nat. Rev. Bioeng. 1, 426 (2023)
Budzianowski, J.: Tobacco against Ebola virus disease. Przegl. Lek. 72, 567–571 (2015)
Shanmugaraj, B., Rattanapisit, K., Manopwisedjaroen, S., Thitithanyanont, A., Phoolcharoen, W.: Monoclonal antibodies B38 and H4 produced in Nicotiana benthamiana neutralize SARS-CoV-2 in vitro. Front. Plant Sci. 11, 589995 (2020)
Bombarely, A., et al.: A draft genome sequence of Nicotiana benthamiana to enhance molecular plant-microbe biology research. Mol. Plant-Microbe Interact. 25, 1523–1530 (2012)
Kurotani, K.I., et al.: Genome sequence and analysis of Nicotiana benthamiana, the model Plant for Interactions between organisms. Plant Cell Physiol. 64, 248–257 (2023)
Fernandez-Pozo, N., et al.: The Sol Genomics Network (SGN)–from genotype to phenotype to breeding. Nucleic Acids Res. 43, D1036–D1041 (2015)
Berger, M., Kaup, M., Blanchard, V.: Protein glycosylation and its impact on biotechnology. Adv. Biochem. Eng. Biotechnol. 127, 165–185 (2012)
Schillberg, S., Spiegel, H.: Recombinant protein production in plants: a brief overview of strengths and challenges. Methods Mol. Biol. 2480, 1–13 (2022)
Liu, L.: Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins. J. Pharm. Sci. 104, 1866–1884 (2015)
Fogarty, C.A., Harbison, A.M., Dugdale, A.R., Fadda, E.: How and why plants and human N-glycans are different: insight from molecular dynamics into the “glycoblocks” architecture of complex carbohydrates. Beilstein J. Org. Chem. 16, 2046–2056 (2020)
Viinikangas, T., Khosrowabadi, E., Kellokumpu, S.: N-glycan biosynthesis: basic principles and factors affecting its outcome. Exp. Suppl. 112, 237–257 (2021)
Jansing, J., Sack, M., Augustine, S.M., Fischer, R., Bortesi, L.: CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking beta-1,2-xylose and core alpha-1,3-fucose. Plant Biotechnol. J. 17, 350–361 (2019)
Pantazica, A.M., et al.: The “humanized” N-glycosylation pathway in CRISPR/Cas9-edited Nicotiana benthamiana significantly enhances the immunogenicity of a S/preS1 Hepatitis B Virus antigen and the virus-neutralizing antibody response in vaccinated mice. Plant Biotechnol. J. 21, 1176 (2023)
Ahmad, A., Pereira, E.O., Conley, A.J., Richman, A.S., Menassa, R.: Green biofactories: recombinant protein production in plants. Recent Pat. Biotechnol. 4, 242–259 (2010)
Tsatsakis, A.M., et al.: Environmental impacts of genetically modified plants: a review. Environ. Res. 156, 818–833 (2017)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Evju, E., Opsahl-Sorteberg, HG. (2024). A Short Review of Advances in Plant-Based Antigen Production Strategies and the Production of Viral Vaccine Antigens Derived from CRISPR/Cas9 Genome Edited N. benthamiana Plants for Enhanced Vaccine Efficacy. In: Ricroch, A., Eriksson, D., Miladinović, D., Sweet, J., Van Laere, K., Woźniak-Gientka, E. (eds) A Roadmap for Plant Genome Editing . Springer, Cham. https://doi.org/10.1007/978-3-031-46150-7_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-46150-7_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-46149-1
Online ISBN: 978-3-031-46150-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)