Abstract
The seeds of oilseed rape (Brassica napus L.) are used in large-scale production of one of the most health-promoting plant oils in the food industry, as well as for animal feed and biofuel production. Thus, increasing the yield of this crop is of crucial economic and ecological importance. However, conventional breeding programs are slow, laborious and time-consuming. Hence, along with the discovery of the possibility to apply CRISPR/Cas technology to edit plant genomes and to accelerate the breeding process, much effort has been put into applying this technology to study specific genes and biosynthetic pathways, especially in species with many gene copies such as B. napus. Here, recent improvements in generating CRISPR/Cas-induced mutations in the B. napus genome, delivering CRISPR/Cas reagents into oilseed rape plant cells, fast-checking the efficiency of targeted mutagenesis of CRISPR/Cas reagents, and oilseed rape transformation and regeneration procedures are described. Finally, new applications of CRISPR/Cas tools in oilseed rape precision breeding are discussed, focusing mainly on applications verified in field.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Oilseed rape (canola; Brassica napus L.) belongs to the genus Brassica. It is a domesticated allotetraploid, which originated from spontaneous hybridization between two diploid species – turnip rape (B. rapa) and cabbage (B. oleraceae) – about 7500 years ago [1]. Today, oilseed rape is one of the most important oil crops worldwide, being cultivated in Europe and Asia predominantly as winter forms and in Australia, Canada and northern Europe as spring ones, providing food, feed and biofuel. This success is attributable to intensive breeding for seed quality traits in the last century. The first milestone in oilseed rape quality breeding was achieved by the introduction of the first low-erucic acid (EA – has a bitter taste and in high doses has been implicated in cardiac health problems) varieties (0-varieties), at the beginning of the 1970s, resulting in high-quality oil with high levels of desirable unsaturated fatty acids. The second milestone was the establishment (in the mid-1980s) of cultivars with low seed glucosinolates (GSL – in monogastric animals their digestion results in the release of toxic by-products that can cause liver and kidney damage along with lymph dysfunction) content (low EA and GSL together – 00-varieties), making possible the use of oilseed rape press residues as protein-rich fodder for animals [2].
However, these breeding programs have significantly reduced genetic diversity in modern breeding pools [3,4,5]. Both of the mentioned seed quality traits – low EA and low GSL content – originated from single genetic resources. Low EA derived from the German spring cultivar (cv.) “Liho”, which carried spontaneous mutations in two B. napus FATTY ACID ELONGASE 1 (FAE1) homologs, while low GSL content was first identified in the Polish spring-type cv. “Bronowski”, which was found to possess at least three recessive genes for low GSL content. Large-scale crossing programs facilitated the introgression of these traits into all the different ecogeographical forms of oilseed B. napus. The result was the release in 1974 of the first 00-quality spring variety, “Tower”, with zero EA and low GSL content (in 1978, the term “canola”, derived from “Canadian oil”, was adopted to identify these varieties). The first 00 winter oilseed rape cultivar, “Librador”, was released in Germany in 1981. Consequently, modern oilseed rape breeding material has a relatively narrow genetic diversity [2].
This narrow genetic diversity markedly restricts breeding progress, which relies on the availability of genetic variation to introduce new desirable traits into crops. Initially, breeders sought genetic variation from landraces and heirloom varieties. The emergence of mutation breeding in the 1940s allowed for the artificial induction of new genetic variation into plant genomes. This approach is extremely crude, as it introduces thousands of random mutations, both wanted and unwanted, with the latter requiring several rounds of backcrossing to remove. Moreover, in polyploid species, where most genes are multiple-copy genes with redundant functions, it is very inefficient to change traits through random mutations. However, all in all, this technology greatly increased the amount of genetic variation available to breeders, and, interestingly, falls within the definition of “conventional and traditional” breeding and thus is lightly regulated [6]. The first commercial varieties developed through mutation breeding were registered in the 1950s, and now over 3400 varieties are listed on the FAO/IAEA Mutant Variety Database, including 21 of B. napus obtained mainly by applying gamma rays [7].
New breeding technologies, such as genome editing, are providing plant breeders with access to a far broader range of genetic variation. Moreover, compared with the traditional random mutagenesis, genome editing not only reaches the same end point as conventional breeding but gets there with a greater: i) degree of precision – no/limited number of unexpected mutations (hence it being termed “precision breeding”), and ii) speed, which is very important as, with conventional plant breeding, it may take 10–15 years or more to get new crops to the market [6]. The current legislation in the EU and many other countries puts plants improved by means of genome editing “in the same box” as typical Genetically Modified Organisms (GMOs). However, very favorable legislature for the production and consumption of such plants in the USA, Japan, Australia, some countries from South and Central America, and others such as Canada [8] is stimulating changes in the legislation in the EU. It is important to understand that simple genome edited crops, i.e., where no foreign genetic sequence is introduced, are indistinguishable and equivalent to conventionally bred crops and therefore could be regulated in a similar way [6].
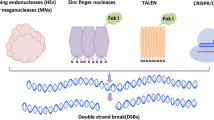
Genome editing technologies encompass sequence changes within an organism or incorporation of valuable sequences into a germplasm, both resulting in an alteration of genotype without extensive backcross. These technologies are based on sequence-specific nucleases (SSNs) such as zinc finger nucleases (ZFNs), TAL effector nucleases (TALENs), and CRISPR (Clustered, regularly interspaced, short, palindromic repeats)/Cas (CRISPR associated nuclease; with Streptococcus pyogenes Cas9 being the most commonly applied). The first two are relatively time-consuming to construct, as protein engineering of their DNA binding domains is required to achieve the requisite target specificity. The latter is the only RNA-guided endonuclease that targets DNA sites through nucleotide base pairing and, thus, has become the preferred SSN for genome editing in plants [9, 10]. SSNs create DNA double strand breaks (DSBs) at predefined genomic loci, which are then repaired through intrinsic erroneous, non-homologous end joining (NHEJ) or a donor DNA introducing homology-directed repair (HDR) pathways [11].
The CRISPR/Cas9 application uses a ~100-nt single guide RNA (gRNA or sgRNA), which is a combination of the crRNA and trans-activating crRNA (tracrRNA) originally encoded by the CRISPR loci [12], to direct Cas9 nuclease to a specific DNA site. The complex SpCas9-sgRNA targets with its 20 nt guide sequence, being part of the crRNA (the rest of the crRNA sequence and the tracrRNA is a scaffold), genomic sequence of 20 nt – protospacer – upstream to a 5’-NGG-3′ protospacer adjacent motif (PAM), and introduces a DSB near the PAM sequence and originates blunt ends [13] that, in plants, will be repaired preferentially by NHEJ, often introducing indel mutations.
Due to the possibility of introducing mutations at multiple sites concurrently, genome editing is an excellent option to significantly accelerate the breeding process of, especially, polyploid species such as B. napus, with many gene targets (at least one homolog from each of the A and C genomes) [1]. CRISPR/Cas technology offers great potential when working on complex traits such as crop yield and disease tolerance, which requires simultaneously targeting multiple loci, related or unrelated, within a single cell. The technology has already been successfully applied to generate mutations in multiple locations in oilseed rape genomes by co-expressing multiple guide RNAs [14,15,16,17].
Oilseed rape is a high-value commodity crop, which has already been modified and produced as traditional GMOs, despite the costs associated with regulatory compliance for such plants [6, 18]. Hence, it is not surprising that there is interest in investing in its precision breeding with the use of genome editing technologies. Here, recent works attempting to improve various steps on the way towards B. napus precision breeding are reviewed, as well as propositions/solutions that could potentially improve the system even further. Since reviews, some of them very comprehensive, on the application of CRISPR/Cas in oilseed rape for gene function research and genetic improvements have been published only recently [16, 17, 19,20,21], here, only some work that included field trials with Brassica CRISPR/Cas modified species will be described.
1 Optimization of CRISPR/Cas Reagents and Ways of Their Expression
Mutating homeologs from both subgenomes of B. napus is necessary due to their functional genetic redundancy. An optimization of CRISPR/Cas constructs/reagents is essential, as the gene editing efficiency depends to a large extent on the selection of a Cas protein and/or guide RNAs. Constructs encoding SpCas9 or Lachnospiraceae bacterium Cas12a (Cpf1; LbCas12a) endonucleases, with one or more guide RNA expression cassettes enabling production of either a single one-guide transcript, multiple one-guide transcripts or a single multi-guide transcript are the most widely used for CRISPR/Cas-mediated genome editing in most plant species. An example of the single, multi-guide transcript production is the CRISPR/Cas9 system based on endogenous tRNA processing that was used, for example, to introduce mutations in the genome of Brassica oleracea – a construct with tandemly arrayed tRNA-sgRNA architecture to express multiple sgRNAs [22]. Another example of the single, multi-guide transcript production is the CRISPR/Cas system, where the multi-guide transcript produced by a Pol III from only one promoter is processed by the same nuclease involved in the generation of DSBs – Cpf1 [23]. The variety of CRISPR/Cas based nucleases and their corresponding guide RNA backbones [24] can be explored applying expanded cloning toolkits that contain not only modules encoding some of the mentioned elements but a number of promoters that allow expression of CRISPR/Cas nucleases and their guide RNAs in monocots and dicots, e.g. [25].
In plants, like in other organisms, not all transformants containing Cas-guide RNA encoding sequences display high levels of mutations in the target gene, with efficiencies reported to vary from a few percent to close to 100% [26, 27]. The efficiency with which mutations are generated in target genes depends on multiple factors, including the choice of target sites in selected genes, as well as the nature of the coding and regulatory sequences of the Cas gene and guide RNA construct. In recent studies, several architectural parameters of Cas constructs designed for plants have been investigated, including the codon usage of the Cas gene, the number of nuclear localization signals (NLSs) in the Cas enzyme, the nature of the promoters and terminators of the Cas gene, the length and sequence of the conserved region of the guide RNA and the terminator sequence of the guide RNA, and the relative orientations of the various expression cassettes in the final transfer DNA (T-DNA) [24, 28].
Marked improvements in the efficiency of targeted mutagenesis (even up to 80% more mutated plants) of Brassica species – B. oleracea and B. napus – were observed when using a modified version of Cas9. The application of a plant-optimized Cas9 CDS with one intron coupled with a tRNA guide architecture [22, 29] and with two guides per gene targeting two unrelated B. oleracea genes helped to outperform the system targeting these genes with two constructs each carrying information on two guides, each under its own Pol III promoter, and the human-codon-optimized HsCas9. The potato intron IV was originally added to avoid expression in bacteria during cloning and, as a side effect, can also increase expression in planta [24]. The application of the improved construct resulted in 100% of plants mutated in two of the four loci [29]. The same improved system but with one guide to edit two copies of a gene was very successful in B. napus and outperformed the system targeting these copies with the same guide but not coupled with the tRNA guide architecture and instead using the HsCas9 [29]. It is worth noting that, in the improved system, the transcription of sgRNA and Cas9 was in head-to-head divergent orientation, which has been shown previously to often result in a highly active CRISPR/Cas system. It was proposed that a weak terminator after Cas9 enables Pol II read through that could interfere with Pol III transcription of sgRNAs in some T-DNA construct architectures, and that this limiting factor can be corrected by divergent transcription of Cas9 and sgRNAs [24]. Another version of Cas9 with 13 introns – ZCas9 + 13int – this time prepared by introducing 13 Arabidopsis thaliana introns into the Zea mays codon-optimized version [30] (again coupled with tRNA guide architecture, with a single guide targeting a B. oleracea gene GA4 or two copies of the gene in B. napus, with divergent transcription of Cas9 and sgRNAs) improved markedly the number of edited plants in comparison to the system not coupled with the tRNA guide architecture, head-to-tail orientation, and using the HsCas9 [29].
However, no matter what kind of modifications of the Cas9 are proposed, the CRISPR/Cas9 system still has some limitations. One of these is the strict PAM dependence, which constrains the availability of target sites, especially to coding regions, as noncoding regions are relatively poor in 5′-NGG-3′ sites. Other limitations of SpCas9 include its large size for viral delivery and the low efficiency in gene targeting caused by blunt DSBs. A solution comes with the CRISPR/Cas12a (Cpf1) system with i) a single crRNA (~42 nt, which is less than half that of Cas9, making it more suitable for multiplexed genome editing and packaging into viral vectors) and ii) Cas12a, an endonuclease smaller than SpCas9, which might facilitate viral delivery. This system requires a 5′-TTTN-3′ PAM sequence and introduces 5′ staggered ends, with 4–5 nt overhangs, at sites distal from 5′ T-rich PAM, at the end of the protospacer sequence, which has been proposed to favor gene insertions [31, 32].
Recently, Lachnospiraceae bacterium Cas12a (LbCas12a) was shown to generate edits in the genome of Brassica oleracea [29], which is a good indication that this endonuclease may function in B. napus as well. Working with this nuclease, four different constructs were analyzed for their efficiency in inducing mutations in a B. oleracea gene. In the case of all constructs, the nuclease encoding sequence was under the UBI10 promoter and was followed by the sequence of the Pisum sativum rbcS E9 terminator. These sequences were in a head-to-head orientation with the RNA guide expressing cassette under the AtU6–26 promoter.
In the case of the first construct, an Arabidopsis-optimized LbCas12a CDS carrying a “temperature tolerant’’ D156R mutation (ttAtCas12a) was encoded. The codon-optimized LbCas12a CDS carrying the mentioned mutation was shown to have increased activity in comparison to a non-mutated variant of the protein when Arabidopsis plants were grown for 2 weeks at 22°C or, especially, at 28°C. It is noteworthy that even the non-mutated variant, when put at 28°C, performed better at 4 of 5 loci tested [33]. Similarly, repeated heat stress treatments of Arabidopsis or Citrus plants had a major effect on the rate of mutagenesis by CRISPR/SpCas9 – in Arabidopsis it increased approximately five-fold in somatic tissues and up to 100-fold in the germline. It was also proposed that SpCas9 is more active in creating double-stranded DNA breaks at 37°C than at 22°C [34]. The first construct encoding the temperature-tolerant enzyme had a four guide (targeting one gene) expressing cassette, serving to produce transcript to be processed by the ttAtCas12a nuclease. The second construct encoded ttAtCas12a nuclease and a pre-gRNA transcript carrying information on one guide RNA (4 separate constructs each containing one of the four guides used in the first construct scenario). The pre-gRNA is a self-processing ribozyme-flanked guide expression cassette named Ribozyme-gRNA-Ribozyme (RGR). The RGR molecule contains ribozymes (possess nuclease activity) – Hammerhead (HH) type ribozyme at the 5′-end, and the terminal hepatitis delta virus (HDV) ribozyme at the 3′-end of the RGR, and thus undergoes self-catalyzed cleavage to generate the desired gRNA. However, the introduction of a self-cleavable ribozyme in the 5′ end of the transcript is unnecessary when a 5′-G is added to the Cas12a DR (direct repeat – region of the crRNA whose proper folding is important for nuclease activity), which is compatible with the Pol III promoter AtU6–26 transcription start site. The HDV ribozyme removes the poly-A tail from the transcript, leaving no additional nucleotides at the 3′ position of the crRNA with a classical construct carrying unprocessed U6 termination signal, which conserves a spurious tail of adenines at the 3′ position of the Cas12 crRNA [29, 32, 35,36,37]. The third construct contained the human-codon-optimized CDS, with modified CDS by inserting the D156R mutation to give ttHsCas12a, and the singular guide RGR expression cassette (one construct to target only one of the four target sites). The fourth construct contained ttAtCas12a, with an addition of 8 Arabidopsis introns generating ttAtCas12a + int, and the singular guide RGR expression cassette (one construct to target only one of the four target sites). The application of the singular guide architecture markedly increased the number of mutant plants, when applying constructs with ttAtCas12a, in two target regions from 0 to 10% and from 3 to 50% of the screened plants. The other two systems were applied only to compare the efficiency of the system in one target region, where the application of the second construct increased the number of mutant plants from 3 to 50%. By using the third constructs, where instead of ttAtCas12a, ttHsCas12a CDS was applied, researchers showed that both nucleases performed equally well. Next, the application of ttAtCas12a + int further increased the number of plants with edits in the target region from 50 to 68% [29].
Although it is clear that the temperature-tolerant version of the Cas nuclease works well in B. oleracea, it would be interesting to compare its activity with the Arabidopsis-codon-optimized version. Based on the results presented, it is hard to conclude whether the heat tolerant enzyme works better than the “wild type” version and it is not clear whether heat stress increases the efficiency of the CRISPR/Cas system in B. oleracea, and thus potentially in B. napus, as was shown for Arabidopsis [33, 34].
2 Delivery of CRISPR/Cas Reagents and Elements Carrying Information on CRISPR/Cas Reagents into Oilseed Rape Plant Cells
Oilseed rape plants bearing edited alleles have been predominantly obtained using Agrobacterium tumefaciens-mediated transformation. In one study, several essential factors that affect the transformation efficiency, such as Agrobacterium strains, selection marker genes and genotypes of oilseed rape were analyzed. Comparison of different Agrobacterium strains showed that the GV3101 had higher transformation efficiency than C58C1 and EHA105. The transformation efficiency was 3.7–4.8%, 2.2–22.5%, and 1.6–5.9% when the hypocotyl of Westar was infected by GV3101 and screened under hygromycin, kanamycin and basta, respectively. The transformation efficiency of Westar was highest and ZS11 was lowest when five different genotypes of oilseed rape (Westar, ZS9, ZS11, GY284 and WH3417) were infected by GV3101 [38].
The auxotrophic A. tumefaciens strain LBA4404 Thy-, which exhibits the inability to survive, proliferate or grow in the absence of thymidine, provides a method for the transformation and regeneration of plant cells that does not need an Agrobacterium counter-selective agent to cure plant tissue of Agrobacterium [39], and recently this strain has been used in a genotype-independent method of oilseed rape plant transformation and regeneration [40]. Another important element of this genotype-independent method is the application of the ternary pVir system with the T-DNA binary vector (with sequences encoding, e.g. CRISPR/Cas reagents) and an improved accessory plasmid [40] that is characterized by small size, enhanced vector stability, an improved bacterial selectable marker and amended vir genes. Application of the pVir system resulted in more efficient T-DNA delivery and stable plant transformation in difficult-to-transform maize elite inbreds [40, 41].
However, some drawbacks remain in these strategies, as they involve delivery of DNA-based CRISPR/Cas reagents that are first integrated into the genome, and expressed as a transgenic construct, and then segregated away by breeding as null segregants to leave only the desired edited allele/s [16]. T-DNA removal is crucial because regulatory constraints for gene-edited crops are likely to be less for those that do not contain foreign DNA [6]. Moreover, it can be important when a loss-of-function phenotype must be confirmed by complementation of the CRISPR/Cas-induced mutation. A CRISPR/Cas construct still present in the mutant can target the complementation transgene and interfere with the resulting phenotypes [24]. Furthermore, as long as the T-DNA encoding the reagents of the CRISPR/Cas system is present in a genome, a progressive gene-editing process can take place, both during selection on appropriate media with antibiotics (which may lead to chimeras) and during the growth of the transgenic plants, and even in next generations [16, 42]. Therefore, DNA-free gene editing has received extensive attention in recent years.
One of the commonly applied T-DNA free oilseed rape genome editing methods is protoplast transfection, which is a transient (no T-DNA integration) alternative for delivery of CRISPR/Cas vectors [43, 44]. CRISPR/Cas reagents can be delivered into plant cells as ribonucleoproteins (RNPs) composed of purified recombinant enzyme Cas and in vitro-transcribed or synthesized gRNA as well. Particle bombardment can be used to deliver RNPs into explants, whereas polyethylene glycol (PEG)-mediated transfection and lipofection can be used to deliver RNPs into protoplasts. Although CRISPR/Cas RNPs has become an attractive approach for genetic engineering, its editing efficiency remains modest [45]. As an example, although Murovec et al. [46] were able to obtain mutation frequencies of 0.09 to 2.25% and 1.15 to 24.51% in B. oleracea and B. rapa, respectively, no mutations were detected after PEG-mediated transfection of oilseed rape (cv. “Topaz”) protoplasts [46].
Although researchers have used protoplast systems to show the potential of CRISPR/Cas reagents to edit a locus or loci of interest, lack of protoplast regeneration protocols to obtain edited plants has been a major bottleneck in this system [16, 43]. Although B. napus protoplast regeneration was shown to be possible before the era of genome editing [47], only recently was a protocol for regeneration of protoplasts of oilseed rape in combination with genome editing proposed. Targeted genes – BnGTR – controlling glucosinolate transport from the vegetative tissues to seeds were mutated with high frequency [48]. According to this protocol, relatively high concentrations of auxins are essential for protoplasts to form cell walls and maintain cell division, and thereafter auxin should be reduced for callus formation and shoot induction. For shoot regeneration, relatively high concentrations of cytokinin are required, with the best combinations resulting in up to 45% shoot regeneration [48].
3 Fast-Checking the Efficiency of Targeted Mutagenesis of CRISPR/Cas Reagents
The process of plant genome editing is usually time-consuming as, in most instances, there is a need for regeneration of plants from tissue culture. Therefore, to have confidence that specific genome editing components will work, it is important to test the CRISPR/Cas system of choice and, especially, the gRNAs, before plant genome editing.
To assess the efficiency of CRISPR/Cas mutagenesis, the plant protoplast system is commonly applied. This system was used to analyze the efficiency of CRISPR/Cas vectors designed and built to target oilseed rape loci encoding proteins involved in the metabolism of guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) – RelA/SpoT Homeologs (RSH]), which are likely to be involved in seed development, maturation and longevity [44, 49, 50]. Importantly, before checking the efficiency of editing, the transfection efficiency of the protoplast system can be determined with a GFP expressing vector, with the help of a microscope. Similarly, one can actually check whether CRISPR/Cas constructs were delivered into protoplasts and with what frequency. It has been shown that the efficiency of PEG-mediated transfection depends on cultivar and concentration of PEG used for the transfection. Using this approach, followed with multiplex amplicon sequencing, it was determined that the rate of particular CRISPR/Cas-mediated edits at one of the RSH loci was in the range of 1–3% [44]. Genome editing events can be detected in many different ways, many of which were mentioned in Shillito et al. [51].
Very recently, another interesting system was proposed to avoid the traditional lengthy explant transformation and regeneration process to study the gene-editing efficiency of various CRISPR/Cas constructs in oilseed rape – hairy-root cultures [52]. In this system, plant infection with Agrobacterium rhizogenes strains harboring a hairy-root-inducing (Ri) plasmid causes an abnormal rooting on hosts’ tissues. After an agrobacterial infection at wounded sites, a T-DNA from the Ri plasmid is transferred to the host cells, where it is stably integrated into the plant genome. Subsequently, the expression of T-DNA genes leads to the induction of hairy roots. Agrobacterial strains carrying both Ri plasmid and artificial binary vector have been widely used for delivering foreign DNA into plant cells. Recently, using this system, researchers examined the mutation efficiency of nine different CRISPR/Cas9 constructs to edit the auxin biosynthetic gene TRYPTOPHAN AMINOTRANSFERASE (BnTAA1; two paralogs). They showed that the plant-codon-optimized SpCas9 with the potato IV2 intron (pcoCas9) is more efficient in mutating the targeted loci than Staphylococcus aureus Cas9 (SaCas9; PAM – 5′-NNGRRT-3′) encoded by a gene that is about 1 kb shorter than pcoCas9 – 83.05% of mutated loci versus 47.98%. Moreover, they observed a slight increase in efficiency when using a longer version of 35S promoter (1.3 kb) compared to the shorter one (0.4 kb) – 67.29% mutated loci vs 58.7%, respectively. The efficiency of mutagenesis was also increased in the presence of the SV40 nuclear localization signal (NLS) – by 25% (75.82% with NLS-Cas9 versus 50.53% with Cas9). With the most efficient construct – NLSpcoCas9 – 96.95% loci were mutated, with less influence on the promoter choice. Next, among pcoCas9 constructs, NLSpcoCas9 induced homozygous mutations with the highest efficiency (33% of the mutated loci) [52].
The hairy-root system is a fast and straightforward system – it makes it possible to evaluate the most effective gene-editing construct within approximately 2 months after transformation. According to the protocol of Jedličková et al. [52], hypocotyls of 18-day-old seedlings are used for Agrobacterium injection, and the first calli and hairy roots are detected after 2 weeks. Another advantage of this system is the relatively high transformation efficiency (number of seedlings with emerging hairy roots over the number of injected seedlings). Three cultivars were tested (DH12075, Westar and Topas), and it was observed that the transformation efficiency was 97%, 84% and 42%. It is worth noting that Topas is a cultivar recalcitrant to petiole-based transformation. Hence, this system seems to work in a genotype-independent manner. Furthermore, hairy roots with homozygous/biallelic mutations can be used to some degree for functional gene studies, e.g. when the analyzed genes encode proteins involved in the production of metabolites, as the hairy roots have been used for the production of secondary metabolites or to investigate phytoremediation processes. Next, as it has been shown in the case of B. napus, using the edited hairy roots, one can regenerate plants applying appropriate protocols [52].
4 Oilseed Rape Plant Regeneration
One of the milestones of oilseed rape molecular breeding has been an efficient in vitro regeneration, though regeneration rates are genotype-dependent. Several elite B. napus varieties have not been easy to transform. At present, the reported varieties for A. tumefaciens-mediated genetic transformation include spring oilseed rape varieties (e.g., cv. “Westar”, “862” and “Haydn”) and semi-winter varieties (e.g., cv. “J9707”, “J9712” and “ZS6”) [17]. For many years, researchers aiming to improve B. napus plant regeneration protocols have focused on applying explant/chemical approaches, which are based on testing explants and different ratios of auxin and cytokinin to induce cells to differentiate into whole plants [53]. So far, the most widely used method for the development of genetically modified B. napus plants has been the Agrobacterium-mediated hypocotyl transformation [e.g., 16, 17, 38, 54,55,56,57]. However, this method, even after modifications and other methods applied so far are genotype-dependent [40, 58].
Recently, an alternative, genotype-independent Agrobacterium-mediated B. napus transformation method was developed that is rapid and amenable for high-throughput transformation and CRISPR/Cas-mediated genome editing; the method is based on epicotyl and higher stem (internodal) segments (3–4 mm), and it has been successfully implemented in multiple oilseed rape genotypes, though with varying transformation efficiencies. Epicotyl segments produced significantly higher rates of shoot formation compared to hypocotyl segments across all genotypes tested [40].
An efficient and likely genotype-independent system to obtain edited plants is plant regeneration from hairy roots. Recently, an optimized regeneration protocol for B. napus cultivar DH12075 was proposed [52]. Using this system, in combination with embryo rescue (21–28 days after pollination) from seeds containing torpedo-stage embryos or older, it is possible to obtain transgene-free T1 plants with desired mutations roughly 1 year after agrobacterial transformation. Moreover, the protocol was used for regenerating plants from hairy-root cultures of Topas, a variety referred to as being in many instances recalcitrant to transformation and plant regeneration. The system nonetheless has some disadvantages. The rol genes, which are crucial for hairy root formation, encoded on the T-DNA of the Ri plasmid, are integrated into the plant genome, and their presence in regenerated plants is responsible for altered growth characteristics called “Ri phenotype”. Thus, to obtain transgene free and Ri phenotype free plants, one has to carry out segregation analyses to obtain plants with neither the Ri T-DNA nor the CRISPR/Cas T-DNA [52].
Recently, to improve plant regeneration, molecular genetic-based methods have been studied [53]. These methods require transfer of sequences encoding morphogenic factors/developmental regulators, including WUSCHEL (WUS2) or BABY BOOM (BBM), that significantly improve the efficiency of plant regeneration and allow the regeneration of thus-far recalcitrant genotypes [53, 59]. However, in order to avoid genotype-specific pleiotropic effects, including abnormal plant growth and infertility, the expression of these genes must be regulated. To solve this problem, one can use the system based on overexpressing of a fusion protein combining transcription factor GROWTH REGULATING FACTOR 4 (GRF4) and its co-factor GRF-INTERACTING FACTOR 1 (GIF1) or of Arabidopsis GRF5 and/or its homologs, which helps to enhance plant regeneration and transformation without affecting plant growth and fertility [60, 61]. In oilseed rape (cv. “BNS3”), overexpression of AtGRF5, AtGRF6, AtGRF9 or BnGRF5-LIKE was shown to significantly increase transgenic callus production of hypocotyl explants; however, it had no significant impact of shoot formation [61].
Tissue culture procedures are often technically demanding, time-consuming and laborious. Hence, no-tissue-culture-required delivery methods, which are genotype-independent, such as nanoparticles [62] or virus delivery [63, 64], could be very helpful to further extend the application of CRISPR/Cas in oilseed rape genome editing. The floral-dip method, commonly used for Agrobacterium-mediated transformation of A. thaliana plants [65], is another such method. This approach was already applied to transform B. napus plant but not in combination with genome editing [66, 67]. The drawback of this method is that, to transform oilseed rape plants in this way, one must wait a relatively long time until the plants reach inflorescence, which is much longer for B. napus than for A. thaliana. To overcome this problem, it is possible to generate and use rapid flowering lines. One such example is fast-flowering mini maize, amenable to transformation and editing, with a seed-to-T1-seed time of 5.5 months compared to over 9 months for other genotypes [68].
5 New Applications of CRISPR/Cas Technology in Oilseed Rape Precision Breeding
CRISPR/Cas9 system was first applied in oilseed rape to target two ALCATRAZ (ALC) homologous genes for site-directed mutagenesis to avoid seed loss during mechanical harvest by increasing shatter resistance [69]. Since that time, this system has been applied for oilseed rape gene function research and genetic improvement relating to weed control, flowering, self-incompatibility, plant hormone biology, abiotic and biotic stress resistance, grain composition and pod shatter reduction. Since most of these achievements were well described in recent review reports [16, 17, 19,20,21], here, only the most recent applications of the CRISPR/Cas technology in B. napus (and closely related species) precision breeding that have been accompanied by field experiments will be summarized.
CRISPR/Cas9 technology was used to create targeted mutations on two homologous copies of the FAE1 gene (on the A08 and C03 chromosomes), which plays a decisive role in the synthesis of erucic acid, in three B. napus germplasms with high EA (>30%) and high oil (>50%). The EA content was significantly reduced by more than 10 percentage points in the mutant of BnC03.FAE1, while the double mutation of BnA08.FAE1 and BnC03.FAE1 resulted in nearly zero EA in three BnFAE1-edited germplasms, and the oleic acid content was increased in different degrees. The confirmed homozygous T2 mutant lines without Cas9 were grown in an experimental farm in China, and the field management was performed in line with standard breeding practice. The agronomic yield-related traits, including plant height, branch height, branch number, silique length, number of siliques per plant, 1000-seed weight and yield per plant, were measured. It was concluded that growth and yield of the mutant plants were not significantly different in comparison to wild type plants. These results provide a way for future low-EA breeding, broadening the resources of B. napus with low EA [57].
CRISPR/Cas9 MYB28-edited Brassica oleracea plants were the subject of the first CRISPR/Cas field trial in the United Kingdom approved and regulated by the UK Department for Environment, Food & Rural Affairs after the reclassification of gene-edited crops as genetically modified organisms by the European Court of Justice on July 25, 2018. The MYB28 gene encodes a transcription factor characterized as a key regulator of aliphatic glucosinolate (A-GSL) biosynthesis in Brassica genus. A-GSL derivatives may contribute to the putative health-promoting effects of cruciferous plant vegetables. Knocking out of MYB28 resulted in downregulation of A-GSL biosynthesis genes and reduction in accumulation of the methionine-derived glucosinolate – glucoraphanin, the precursor for isothiocyanate sulforaphane, which is believed to have health-promoting effects – in leaves and florets of field-grown broccoli plants [70]. These results demonstrate the potential for the gene-edited plants to express the improved traits when grown in field conditions.
6 Conclusions
CRISPR/Cas-mediated genome-editing offers great potential for both genetic improvement and biological research. Hence, this technology is being constantly developed, especially to make it more widely applicable and efficient for economically important crop species, such as oilseed rape. One of the improvements in the CRISPR/Cas technology is the application of new or improved CRISPR/Cas reagents (e.g., Cas12a, with its differing PAM requirement comparing to Cas9) and/or ways of their expression, which have increased the scope and efficiency of targeted mutagenesis. An important step was developing and improving methods of delivering CRISPR/Cas reagents, or of sequences encoding them, which has a tremendous impact on the final time that it takes to obtain transgene-free CRISPR/Cas edited plants and/or the efficiency of transformation and regeneration. Next, important protocols have been developed for analyses of different CRISPR/Cas variants/gRNAs to assess their efficiencies before applying to obtain edited plants. Lastly, setting up genotype-independent regeneration protocols to obtain mutations in a desired elite germplasm has been a tremendous achievement.
However, although the presented tools and methods serve to make a major contribution to more efficient and rapid gene discovery and functional characterization, some CRISPR/Cas variants and methods applied in the frame of genome editing in plants await implementation in B. napus precision breeding. These include the application of editing technologies such as homology-directed gene editing or prime-editing to enable the most precise and defined edits in this crop. Furthermore, increasing the overall efficiency of regeneration protocols remains an active area of research.
The CRISPR/Cas system has even further scalability; one can use it to either regulate gene expression or introduce a single base change or introduce/remove epigenetic marks, by using a system where, respectively, a transcriptional activator/repressor or a base editor or a protein introducing/removing epigenetic marks is linked to a Cas endonuclease that functions as a nickase or a “dead” Cas [71, 72]. While some of these applications have already been applied in B. napus precision breeding [16, 20], some still await implementation.
The next reasonable step is to introduce the gene-edited traits through introgression breeding into elite varieties or, when genotype dependency does not play a role, apply the CRISPR/Cas technique to directly modify these elite varieties.
References
Chalhoub, B., Denoeud, F., Liu, S., et al.: Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science. 345, 950–953 (2014)
Friedt, W., Snowdon, R.: Oilseed rape. In: Vollmann, J., Rajcan, I. (eds.) Handbook of plant breeding, oil crops, pp. 91–126. Springer, New York (2009)
Hasan, M., Seyis, F., Badani, A.G., Pons-Kühnemann, J., Friedt, W., Lühs, W., Snowdon, R.J.: Analysis of genetic diversity in the Brassica napus L. gene pool using SSR markers. Genet. Resour. Crop Ev. 53, 793–802 (2006)
Bus, A., Körber, N., Snowdon, R.J., Stich, B.: Patterns of molecular variation in a species-wide germplasm set of Brassica napus. Theor. Appl. Genet. 123(8), 1413–1423 (2011)
Qian, L., Qian, W., Snowdon, R.J.: Sub-genomic selection patterns as a signature of breeding in the allopolyploid Brassica napus genome. BMC Genomics. 15, 1170 (2014)
Hundleby, P., Harwood, W.: Regulatory constraints and differences of genome-edited crops around the globe. In: Wani, S.H., Hensel, G. (eds.) Genome editing, pp. 319–341. Springer, Cham (2022)
FAO/IAEA: Mutant variety database, Vienna. https://mvd.iaea.org. Accessed 05 May 2023 (2022)
Halford, N.G.: Legislation governing genetically modified and genome-edited crops in Europe: the need for change. J. Sci. Food Agr. 99(1), 8–12 (2019)
Feng, Z., Zhang, B., Ding, W., Liu, X., Yang, D.L., Wei, P., Cao, F., Zhu, S., Zhang, F., Mao, Y., Zhu, J.K.: Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 23(10), 1229–1232 (2013)
Nekrasov, V., Staskawicz, B.J., Weigel, D., Jones, J.D.G., Kamoun, S.: Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 691–693 (2013)
Zhang, H., Zhang, J., Lang, Z., Botella, J.R., Zhu, J.-K.: Genome editing-principles and applications for functional genomics research and crop improvement. Crit. Rev. Plant Sci. 36, 291–309 (2017)
Jinek, M., Chylinski, K., Fonfara, I., Hauer, M., Doudna, J.A., Charpentier, E.: A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337(6096), 816–821 (2012)
Ran, F.A., Hsu, P.D., Wright, J., Agarwala, V., Scott, D.A., Zhang, F.: Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8(11), 2281–2308 (2013)
Zaman, Q.U., Chu, W., Hao, M., Shi, Y., Sun, M., Sang, S.F., Mei, D., Cheng, H., Liu, J., Li, C., Hu, Q.: CRISPR/Cas9-mediated multiplex genome editing of JAGGED gene in Brassica napus L. Biomolecules. 9(11), 725 (2019)
Zhang, K., Nie, L., Cheng, Q., Yin, Y., Chen, K., Qi, F., Zou, D., Liu, H., Zhao, W., Wang, B., Li, M.: Effective editing for lysophosphatidic acid acyltransferase 2/5 in allotetraploid rapeseed (Brassica napus L.) using CRISPR-Cas9 system. Biotechnol. Biofuels. 12(225), 1–18 (2019)
Gocal, G.F.W.: Gene editing in Brassica napus for basic research and trait development. In Vitro Cell Dev.-Pl. 57, 731–748 (2021)
Tian, Q., Li, B., Feng, Y., Zhao, W., Huang, J., Chao, H.: Application of CRISPR/Cas9 in rapeseed for gene function research and genetic improvement. Agronomy. 12(4), 824 (2022)
Jorasch, P.: The global need for plant breeding innovation. Transgenic Res. 28, 81–86 (2019)
Chang, T., Guan, M., Zhou, B., Peng, Z., Xing, M., Wang, X., Guan, C.: Progress of CRISPR/Cas9 technology in breeding of Brassica napus. Oil Crop Sci. 6(2), 53–57 (2021)
Li, J., Yu, X., Zhang, C., Li, N., Zhao, J.: The application of CRISPR/Cas technologies to Brassica crops: current progress and future perspectives. aBIOTECH. 3, 146–161 (2022)
Ali, E., Zhang, K.: CRISPR-mediated technology for seed oil improvement in rapeseed: challenges and future perspectives. Front. Plant Sci. 14, 1086847 (2023)
Ma, C., Zhu, C., Zheng, M., Liu, M., Zhang, D., Liu, B., Li, Q., Si, J., Ren, X., Song, H.: CRISPR/Cas9-mediated multiple gene editing in Brassica oleracea var. capitata using the endogenous tRNA-processing system. Hortic. Res. 6, 20 (2019)
Zetsche, B., Heidenreich, M., Mohanraju, P., Fedorova, I., Kneppers, J., DeGennaro, E.M., Winblad, N., Choudhury, S.R., Abudayyeh, O.O., Gootenberg, J.S., Wu, W.Y., Scott, D.A., Severinov, K., van der Oost, J., Zhang, F.: Multiplex gene editing by CRISPR-Cpf1 using a single crRNA array. Nat. Biotechnol. 35, 31–34 (2017)
Castel, B., Tomlinson, L., Locci, F., Yang, Y., Jones, J.D.G.: Optimization of T-DNA architecture for Cas9-mediated mutagenesis in Arabidopsis. PLoS One. 14(1), e0204778 (2019)
Hahn, F., Korolev, A., Sanjurjo Loures, L., Nekrasov, V.: A modular cloning toolkit for genome editing in plants. BMC Plant Biol. 20, 179 (2020)
Belhaj, K., Chaparro-Garcia, A., Kamoun, S., Nekrasov, V.: Plant genome editing made easy: targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods. 9, 39 (2013)
Ahmad, N., Rahman, M.U., Mukhtar, Z., Zafar, Y., Zhang, B.: A critical look on CRISPR-based genome editing in plants. J. Cell. Physiol. 235, 666–682 (2020)
Ordon, J., Bressan, M., Kretschmer, C., Dall’Osto, L., Marillonnet, S., Bassi, R., Stuttmann, J.: Optimized Cas9 expression systems for highly efficient Arabidopsis genome editing facilitate isolation of complex alleles in a single generation. Funct. Integr. Genomic. 20, 151–162 (2019)
Lawrenson, T., Chhetry, M., Clarke, M., Hundleby, P., Harwood, W.: Improved SpCas9 and LbCas12a genome editing systems in Brassica oleracea and Brassica napus. BioRxiv. (2022). https://doi.org/10.1101/2022.05.16.492057
Grützner, R., Martin, P., Horn, C., Mortensen, S., Cram, E.J., Lee-Parsons, C.W.T., Stuttmann, J., Marillonnet, S.: High-efficiency genome editing in plants mediated by a Cas9 gene containing multiple introns. Plant Commun. 2(2), 100135 (2020)
Zetsche, B., Gootenberg, J.S., Abudayyeh, O.O., Slaymaker, I.M., Makarova, K.S., Essletzbichler, P., Volz, S.E., Joung, J., van der Oost, J., Regev, A., Koonin, E.V., Zhang, F.: Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell. 163(3), 759–771 (2015)
Tang, X., Lowder, L.G., Zhang, T., Malzahn, A.A., Zheng, X., Voytas, D.F., Zhong, Z., Chen, Y., Ren, Q., Li, Q., Kirkland, E.R., Zhang, Y., Qi, Y.: A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants. 3, 17018 (2017)
Schindele, P., Puchta, H.: Engineering CRISPR/LbCas12a for highly efficient, temperature-tolerant plant gene editing. Plant Biotechnol. J. 18(5), 1118–1120 (2020)
Le Blanc, C., Zhang, F., Mendez, J., Lozano, Y., Chatpar, K., Irish, V.F., Yannick, J.: Increased efficiency of targeted mutagenesis by CRISPR/Cas9 in plants using heat stress. Plant J. 93(2), 377–386 (2017)
Gao, Y., Zhao, Y.: Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J. Integr. Plant Biol. 56(4), 343–349 (2014)
Bernabé-Orts, J.M., Casas-Rodrigo, I., Minguet, E.G., Landolfi, V., Garcia-Carpintero, V., Gianoglio, S., Vázquez-Vilar, M., Granell, A., Orzaez, D.: Assessment of Cas12a-mediated gene editing efficiency in plants. Plant Biotechnol. J. 17(10), 1971–1984 (2019)
Wolter, F., Puchta, H.: In planta gene targeting can be enhanced by the use of CRISPR/Cas12a. Plant J. 100(5), 1083–1094 (2019)
Dai, C., Li, Y., Li, L., Du, Z., Lin, S., Tian, X., Li, S., Yang, B., Yao, W., Wang, J., Guo, L., Lu, S.: An efficient Agrobacterium-mediated transformation method using hypocotyl as explants for Brassica napus. Mol. Breed. 40, 96 (2020)
Ranch, J.P., Liebergesell, M., Garnaat, C.W., Huffman, G.A.: Auxotrophic Agrobacterium for plant transformation and methods thereof. US Patent. 8334429 (2012)
Cao Chu, U., Kumar, S., Sigmund, A., Johnson, K., Li, Y., Vongdeuane, P., Jones, T.J.: Genotype-independent transformation and genome editing of Brassica napus using a novel explant material. Front. Plant Sci. 11, 579524 (2020)
Anand, A., Bass, S.H., Wu, E., Wang, N., McBride, K.E., Annaluru, N., Miller, M., Hua, M., Jones, T.J.: An improved ternary vector system for Agrobacterium-mediated rapid maize transformation. Plant Mol. Biol. 97, 187–200 (2018)
Yang, Y., Zhu, K., Li, H., Han, S., Meng, Q., Khan, S.U., Fan, C., Xie, K., Zhou, Y.: Precise editing of CLAVATA genes in Brassica napus L. regulates multilocular silique development. Plant Biotechnol. J. 16(7), 1322–1335 (2018)
Lin, C.S., Hsu, C.T., Yang, L.H., Lee, L.Y., Fu, J.Y., Cheng, Q.W., Wu, F.H., Hsiao, H.C., Zhang, Y., Zhang, R., Chang, W.J., Yu, C.T., Wang, W., Liao, L.J., Gelvin, S.B., Shih, M.C.: Application of protoplast technology to CRISPR/Cas9 mutagenesis: from single-cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 16(7), 1295–1310 (2018)
Boniecka, J., Turkan, S., Nekrasov, V., Ruttink, T., Eeckhaut, T., Van Laere, K.: Targeted mutagenesis in oilseed rape (Brassica napus L.) protoplasts using CRISPR/Cas. Presented at the 2nd PlantEd conference plant genome editing, the wide range of applications, Lecce, 20–22 September 2021
Zhang, Y., Iaffaldano, B., Qi, Y.: CRISPR ribonucleoprotein-mediated genetic engineering in plants. Plant Commun. 2(2), 100168 (2021)
Murovec, J., Guček, K., Bohanec, B., Avbelj, M., Jerala, R.: DNA-free genome editing of Brassica oleracea and B. rapa protoplasts using CRISPR-Cas9 ribonucleoprotein complexes. Front. Plant Sci. 9, 1594 (2018)
Hu, Q., Andersen, S.B., Hansen, L.N.: Plant regeneration capacity of mesophyll protoplasts from Brassica napus and related species. Plant Cell Tiss. Org. 59, 189–196 (1999)
Li, X., Sandgrind, S., Moss, O., Guan, R., Ivarson, E., Wang, E.S., Kanagarajan, S., Zhu, L.H.: Efficient protoplast regeneration protocol and CRISPR/Cas9-mediated editing of glucosinolate transporter (GTR) genes in rapeseed (Brassica napus L.). Front. Plant Sci. 12, 680859 (2021)
Boniecka, J., Prusińska, J., Dąbrowska, G.B., Goc, A.: Within and beyond the stringent response-RSH and (p)ppGpp in plants. Planta. 246(5), 817–842 (2017)
Boniecka, J., Kotowicz, K., Skrzypek, E., Dziurka, K., Rewers, M., Jędrzejczyk, I., Wilmowicz, E., Berdychowska, J., Dąbrowska, G.B.: Potential biochemical, genetic and molecular markers of deterioration advancement in seeds of oilseed rape (Brassica napus L.). Ind. Crop. Prod. 130, 478–490 (2019)
Shillito, R.D., Whitt, S., Ross, M., Ghavami, F., Vleesschauwer, D.D., D’Halluin, K., Hoecke, A.V., Meulewaeter, F.: Detection of genome edits in plants—from editing to seed. In Vitro Cell Dev.–Pl. 57, 595–608 (2021)
Jedličková, V., Mácová, K., Štefková, M., Butula, J., Staveníková, J., Sedláček, M., Robert, H.S.: Hairy root transformation system as a tool for CRISPR/Cas9-directed genome editing in oilseed rape (Brassica napus). Front. Plant Sci. 13, 919290 (2022)
Gordon-Kamm, W., Barone, P., Svitashev, S., Sander, J.D., Kumar, S., Jones, T.: Strategies for CRISPR/Cas9-mediated genome editing: from delivery to production of modified lants. Burleigh Dodds Science Publishing Limited, Cambridge (2021)
De Block, M., De Brouwer, D., Tenning, P.: Transformation of Brassica napus and Brassica oleracea using Agrobacterium tumefaciens and the expression of the bar and neo genes in the transgenic plants. Plant Physiol. 91(2), 694–701 (1989)
Jonoubi, P., Mousavi, A., Majd, A., Salmanian, A.H., Jalali Javaran, M., Daneshian, J.: Efficient regeneration of Brassica napus L. hypocotyls and genetic transformation by Agrobacterium tumefaciens. Biol. Plantarum. 49(2), 175–180 (2005)
Boniecka, J., Rybicka, A., Schweighofer, A., Trejgell, A.: Rape (Brassica napus L.) transformation and shoot organogenesis: important steps towards successful genome editing. Presented at the 1st PlantEd Conference Plant Genome Editing, State of the Art, Novi Sad, 6–7 November 2019
Liu, Y., Du, Z., Lin, S., Li, H., Lu, S., Guo, L., Tang, S.: CRISPR/Cas9-targeted mutagenesis of BnaFAE1 genes confers low-erucic acid in Brassica napus. Front. Plant Sci. 13, 848723 (2022)
Rani, T., Yadav, R.C., Yadav, N.R., Rani, A., Singh, D.: Genetic transformation in oilseed brassicas – a review. Indian J. Agr. Sci. 83(4), 367–373 (2013)
Gordon-Kamm, B., Sardesai, N., Arling, M., Lowe, K., Hoerster, G., Betts, S., Jones, T.: Using morphogenic genes to improve recovery and regeneration of transgenic plants. Plants. 8(2), 38 (2019)
Debernardi, J.M., Tricoli, D.M., Ercoli, M.F., Hayta, S., Ronald, P., Palatnik, J.F., Dubcovsky, J.: A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 38(11), 1274–1279 (2020)
Kong, J., Martin-Ortigosa, S., Finer, J., Orchard, N., Gunadi, A., Batts, L.A., Thakare, D., Rush, B., Schmitz, O., Stuiver, M., Olhoft, P., Pacheco-Villalobos, D.: Overexpression of the transcription factor GROWTH-REGULATING FACTOR5 improves transformation of dicot and monocot species. Front. Plant Sci. 11, 572319 (2020)
Demirer, G.S., Silva, T.N., Jackson, C.T., Thomas, J.B., Ehrhardt, D.W., Rhee, S.Y., Mortimer, J.C., Landry, M.P.: Nanotechnology to advance CRISPR-Cas genetic engineering of plants. Nat. Nanotechnol. 16(3), 243–250 (2021)
Dinesh-Kumar, S.P., Voytas, D.F.: Editing through infection. Nat. Plants. 6(7), 738–739 (2020)
Ellison, E.E., Nagalakshmi, U., Gamo, M.E., Huang, P.J., Dinesh-Kumar, S., Voytas, D.F.: Multiplexed heritable gene editing using RNA viruses and mobile single guide RNAs. Nat. Plants. 6(6), 620–624 (2020)
Clough, S.J., Bent, A.F.: Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16(6), 735–743 (1998)
Verma, S.S., Chinnusamy, V., Bansa, K.C.: A simplified floral dip method for transformation of Brassica napus and B. carinata. J. Plant Biochem. Biot. 17(2), 197–200 (2008)
Li, J., Tan, X., Zhu, F., Guo, J.: A rapid and simple method for Brassica napus floral-dip transformation and selection of transgenic plantlets. Int. J. Biol. 10(1), 127–131 (2010)
McCaw, M.E., Lee, K., Kang, M., Zobrist, J.D., Azanu, M.K., Birchler, J.A., Wang, K.: Development of a transformable fast-flowering mini-maize as a tool for maize gene editing. Front. Genome Ed. 2, 622227 (2021)
Braatz, J., Harloff, H.J., Mascher, M., Stein, N., Himmelbach, A., Jung, C.: CRISPR-Cas9 targeted mutagenesis leads to simultaneous modification of different homoeologous gene copies in polyploid oilseed rape (Brassica napus). Plant Physiol. 174(2), 935–942 (2017)
Neequaye, M., Stavnstrup, S., Harwood, W., Lawrenson, T., Hundleby, P., Irwin, J., Troncoso-Rey, P., Saha, S., Traka, M.H., Mithen, R., Østergaard, L.: CRISPR-Cas9-mediated gene editing of MYB28 genes impair glucoraphanin accumulation of Brassica oleracea in the field. CRISPR J. 4(3), 416–426 (2021)
Razzaq, A., Saleem, F., Kanwal, M., Mustafa, G., Yousaf, S., Arshad, H.M.I., Hameed, M.K., Khan, M.S., Joyia, F.A.: Modern trends in plant genome editing: an inclusive review of the CRISPR/Cas9 toolbox. Int. J. Mol. Sci. 20(16), 4045 (2019)
Van Eck, J.: Applying gene editing to tailor precise genetic modifications in plants. J. Biol. Chem. 295(38), 13267–13276 (2020)
Acknowledgments
The research experiments conducted by JB, financed from the funds of the National Science Centre in Poland (2017/01/X/NZ1/01981) and the Ministry of Science and Higher Education in Poland (1189-B research grant from the Faculty of Biological and Veterinary Sciences at the Nicolaus Copernicus University in Toruń), were the inspiration to write this publication. The Author acknowledges the European COST Action CA18111 – Genome Editing in Plants for providing an excellent platform to discuss genome editing-related topics.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Boniecka, J. (2024). CRISPR/Cas-Based Precision Breeding of Oilseed Rape (Brassica napus L.) – Recent Improvements. In: Ricroch, A., Eriksson, D., Miladinović, D., Sweet, J., Van Laere, K., Woźniak-Gientka, E. (eds) A Roadmap for Plant Genome Editing . Springer, Cham. https://doi.org/10.1007/978-3-031-46150-7_18
Download citation
DOI: https://doi.org/10.1007/978-3-031-46150-7_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-46149-1
Online ISBN: 978-3-031-46150-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)