Abstract
The administration of intravenous (IV) fluids in critically ill patients remains a major therapeutic challenge. While there are many questions regarding the type, properties, speed, dose, and timing of fluid administration, there are only four major indications for their use: resuscitation, replacement, maintenance, and nutrition. In addition to these indications, IV fluids can also be used as carriers for medications. This chapter not only discusses the major indications for fluid therapy but also delves into different fluid management strategies, including early adequate goal-directed fluid management (EAFM), late conservative fluid management (LCFM), and late goal-directed fluid removal (LGFR). The concept of the six D’s of fluid therapy (diagnosis, drug, dosing, duration, de-escalation, and discharge) is also explored. This chapter aims to provide clinicians with a comprehensive understanding of IV fluid therapy beyond the traditional four indications.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Fluid therapy
- Critically ill
- Sepsis
- Indication
- Resuscitation
- Maintenance
- Replacement
- Nutrition
- Monitoring
- Fluid responsiveness
- Goal-directed
The administration of intravenous fluids is a crucial and one of the most frequent therapeutic options in critical care, but it is high time that clinicians begin to view fluids as drugs that require a systematic approach. To this end, a conceptual framework of the 7 D’s has been proposed to provide a comprehensive understanding of fluid therapy and monitoring.
The 7 D’s framework includes definitions of key terms, such as fluid status, preload, and fluid responsiveness. It also emphasizes the importance of accurate diagnosis, including hypo-, eu-, and hypervolemia, and monitoring organ and tissue perfusion. The framework also covers the drug aspect of fluid therapy, including the type of fluids, indications, contraindications, adverse effects, rate, objectives, and limits, followed by dose, duration, de-escalation, and discharge. Understanding these aspects is critical for selecting the appropriate fluids for patients and avoiding unnecessary complications.
The framework also addresses 4 critical questions, such as when to start and stop IV fluids, when to begin fluid evacuation, and when to stop fluid removal. The 4 indications for fluid administration are discussed: resuscitation, maintenance, replacement, and nutrition. It also outlines the four phases of fluid therapy, including resuscitation, optimization, stabilization, and evacuation (ROSE).
Furthermore, the framework explores the four hits and four compartments of fluid therapy. The four hits refer to the underlying conditions that may lead to fluid imbalances, including hypovolemia, redistribution, capillary leak, and interstitial edema. The four compartments refer to the various fluid compartments in the body and their unique characteristics, including intravascular, interstitial, intracellular, and transcellular fluids. Understanding these compartments is critical for selecting the appropriate fluids and monitoring their effectiveness.
Finally, the framework advocates for fluid stewardship, which refers to a coordinated series of interventions aimed at selecting the optimal fluid, dose, and duration of therapy to achieve the best clinical outcomes while minimizing adverse events and reducing costs. Fluid stewardship is critical in ensuring that patients receive the best possible care while minimizing the risks associated with fluid therapy.
In summary, the 7 D’s framework provides a comprehensive approach to fluid therapy and monitoring, emphasizing the importance of accurate diagnosis, drug selection, and appropriate monitoring. By adopting this approach, clinicians can optimize patient outcomes, minimize adverse events, and reduce healthcare costs.
FormalPara Learning ObjectivesIn this chapter we will discuss the different indications for intravenous (IV) fluid therapy in critically ill patients. After finishing this chapter, the reader will understand the differences between resuscitation, maintenance, replacement, and nutrition fluids. Each type of fluid has indications and contraindications and pros and cons, benefits, and adverse effects and the user must understand the different pitfalls that may affect the clinical outcome when one fluid is preferred over another. Resuscitation fluids should be given to save lives in patients with shock (trauma, sepsis, bleeding, burns, pancreatitis…). Maintenance fluids should be given to cover the daily needs of total body water, glucose, and electrolytes (mainly sodium and potassium). Replacement fluids should mimic the fluid that is lost (egammonium chloride or normal saline in case of gastrointestinal losses). And finally, nutrition fluids should cover the daily caloric needs with focus on proteins and/or nitrogen. In order to be able to administer the right dose of fluids, assessment of fluid status and hemodynamic function is mandatory, besides other monitoring techniques like indirect calorimetry or bioelectrical impedance analysis. Echocardiography should be seen as an additional tool or the modern stethoscope to assess cardiac function and to obtain a “volumetric” idea of preload in combination with cardiac function, afterload, and fluid responsiveness. Besides the four indications of fluid therapy, the reader will also understand other basic principles of fluid therapy like the four (or even six or seven) D’s, the four questions, the four hits, and the four phases.
FormalPara Case VignetteWoman, 75 years old, bedridden, weighs 50 kg
Previous History: minimental state evaluation (MMSE) 19/30, arterial hypertension, type 2 diabetes mellitus, diverticulitis
Current problem: fever, increasing confusion, blood pressure 99/55 mmHg, pulse rate 119/min
Lab results: CRP 99 mg/dl, creatinine 2.1 mg/dl, urea 102 mg/dl, Na 145 mmol/L, K 3.5 mmol/L
Diagnosis: urosepsis. The patient appears dehydrated and has acute renal failure RIFLE I (injury), presumably prerenal. Now follow some questions. We ask the reader not to think too much or too long, but to choose the answer that first pops up in his/her mind.
Questions
-
Q1. You decide to administer a fluid bolus, how much do you give? 100—250—500—1000 ml?
-
Q2. How fast do you administer the fluid bolus? 10 to 15 min—30 min—1 hour—2 hours?
-
Q3. What do you consider the most clinically relevant parameter to assess fluid responsiveness? Heart rate—mottled skin—capillary refill time—blood pressure—passive leg raise test—diuresis—respiratory rate?
-
Q4. What type of fluids did you administer during resuscitation phase? Normal saline (NaCl 0.9%)—Hypotonic saline (NaCl 0.45%)—Glucose 5%—Plasmalyte—Ringer’s lactate—Ringer’s acetate—Hartmann solution—Maintelyte—Volulyte—Gelatin—Albumin—Glucose 5% in NaCl 0.45%—Glucose 5% in NaCl 0.9%?
-
After fluid resuscitation (you gave twice 250 ml over 10 min), paracetamol (1 g IV in 100 ml bottle) and starting antibiotic therapy, the patient ameliorates, and on day 3, it is decided to transfer the patient to the normal ward. However, there are strong doubts about correct swallowing function, and the patient is kept nil per mouth until evaluation by ENT specialist.
-
Q5. What type of fluids did you administer as maintenance solution? Normal saline (NaCl 0.9%)—Hypotonic saline (NaCl 0.45%)—Glucose 5%—Plasmalyte—Ringer’s lactate—Ringer’s acetate—Hartmann solution—Maintelyte—Volulyte—Gelatin—Albumin—Glucose 5% in NaCl 0.45%—Glucose 5% in NaCl 0.9%?
-
Q6. How many ml of fluid do you administer to this patient on a daily basis? 500 ml—750 ml—1000 ml—1500 ml—2000 ml—2500 ml?
-
Q7. What is the daily need for sodium (mmol) in this patient? 15—25—50—75—100—150?
-
Q8. What is the daily need for potassium (mmol) in this patient? 15—25—50—75—100—150?
-
Q9. What is the daily need for glucose (g) in this patient? 15—25—50—75—100—150?
-
From the seventh day, the patient starts producing profound watery diarrhea (12 times a day) with nausea and vomiting (around 1500 ml of cumulative daily gastric aspirate volume). A lab result indicates a BE of 8.
-
Q10. What would be the best replacement fluid in addition to her daily maintenance solution? Normal saline (NaCl 0.9%)—Hypotonic saline (NaCl 0.45%)—Glucose 5%—Plasmalyte—Ringer’s lactate—Ringer’s acetate—Hartmann solution—Maintelyte—Volulyte—Gelatin—Albumin—Glucose 5% in NaCl 0.45%—Glucose 5% in NaCl 0.9%?
Introduction
In critically ill patients, the administration of intravenous (IV) fluids remains a major therapeutic challenge and one size does not fit all [1]. We are faced with many open questions regarding the type (crystalloids vs colloids), properties (balanced vs unbalanced), speed of administration, dose (intermittent bolus vs continuous), and timing (early vs late) of intravenous fluid administration [2, 3]. Figure 8.1 gives an overview of different IV fluids.
There are only four major indications for intravenous fluid administration: aside from resuscitation (in order to save lives), intravenous fluids have many other uses including maintenance (to cover the daily needs of total body water and electrolytes) and replacement (to replace the fluid that is lost) and for parenteral nutrition [4]. But there may even be more indications like fluids as carriers for medications (sedation, antibiotics, analgesics…). In this chapter, we discuss not only the 4 major indications for fluid therapy in the critically ill, but also the different fluid management strategies including early adequate goal-directed fluid management (EAFM), late conservative fluid management (LCFM), and late goal-directed fluid removal (LGFR) [4]. In addition, and in analogy to antibiotics, we expand on the concept of the six (seven) D’s of fluid therapy, namely (definitions), diagnosis, drug, dosing, duration, de-escalation, and documentation at discharge [5]. This chapter will elaborate further on the terms and definitions listed in Chap. 1.
The Four Indications
Resuscitation Fluids
Resuscitation fluids are given to correct an intravascular volume deficit in the case of absolute or relative hypovolemia [4]. In theory, the choice between colloids and crystalloids should take into account the revised Starling equation and the glycocalyx model of transvascular fluid exchange [4, 6]. When capillary pressure (or transendothelial pressure difference) is low, as in hypovolemia or sepsis and especially septic shock, or during hypotension (after induction and anesthesia), albumin or plasma substitutes have no advantage over crystalloid infusions, since they all remain intravascular. However, the glycocalyx layer is a fragile structure and is disrupted by surgical trauma-induced systemic inflammation or sepsis, but also by rapid infusion of fluids (especially saline). Under these circumstances, transcapillary flow (albumin leakage and risk of tissue edema) is increased; as is the risk to evolve to a state of global increased permeability syndrome (GIPS) [6]. Table 8.1 gives an overview of different resuscitation fluids.
Definitions and Key Messages
-
Isotonic resuscitation fluids are administered to save lives.
-
A fluid bolus (or better a fluid challenge) should be small (4 ml/kg) and given fast (in 5–15 min).
-
Do not administer fluids until the patient is no longer fluid-responsive
Maintenance Fluids
Maintenance fluids are given, specifically, to cover the patient’s daily basal requirements of water, glucose, and electrolytes. As such, they are intended to cover daily needs. The basic daily needs are water, in an amount of 1 ml/kg/h or 25–30 mL/kg/day of body weight, 1 mmol/kg/day potassium, 1–1.5 mmol/kg/day sodium per day, and glucose or dextrose 5 or 10% 1.4–1.6 g/kg/day (to avoid starvation ketosis) [7].
Some specific maintenance solutions are commercially available, but they are far from ideal. In Belgium, Glucion© 5% and Glucion© 10% are commercially available. During a previous ISICEM (International Symposium on Intensive Care and Emergency Medicine) meeting in Brussels, the Baxter company launched a new ready from the shelve maintenance solution called Maintelyte©. There is a lot of debate whether isotonic or hypotonic maintenance solutions should be used. Data in children showed that hypotonic solutions carry the risk for hyponatremia and neurologic complications [8, 9]. However, studies in adults are scarce and indicate that administration of isotonic solutions will result in a more positive fluid balance as compared to hypotonic solutions [10]. This was confirmed in a recent pilot study (MIHMoSA) in healthy volunteers showing that isotonic solutions (glucose 5% in NaCl 0.9% + 40 mmolKCl/L) caused lower urine output (and thus more positive cumulative fluid balance at 48 h), characterized by decreased aldosterone concentrations indicating (unintentional) volume expansion, than hypotonic solutions (Glucion© 5%) [11]. The cumulative fluid balance at 48 h is shown in Fig. 8.2.
Cumulative fluid balance over the course of each study period. Left panel shows the isotonic (glucose % + NaCl 0.9% + 40 mmol KCl/L) maintenance fluids (purple), while the right panel shows the effect of the hypotonic (Glucion© 5%, NaCl 0.32%) maintenance fluids (dark blue). Black lines are individual observations per subject. Colored lines are the marginal means estimated using the mixed effects model; the shaded areas represent 95% confidence intervals. Dashed lines are predicted values at 48 h (t48). §Outlier with exaggerated natriuresis. The positive fluid balance at t0 is attributable to oral fluid intake. Reproduced and adapted from Van Regenmortel et al. with permission according to the Open Access CC BY Licence 4.0 [11]
Despite their lower sodium and potassium content, hypotonic fluids were not associated with clinically significant hyponatremia or hypokalemia, as illustrated in Fig. 8.3 [11]. These results have been recently confirmed in 69 critically ill patients after major thoracic surgery (TOPMAST) using the same isotonic and hypotonic fluids as the MIHMoSA trial [12].
Serum concentration of electrolytes, sodium (Na, left panel) and chloride (Cl, right panel), over the course of both study periods. In-graph P-values are indicated for the difference between the two fluids. Hypotonic fluids are indicated in blue and isotonic fluids in purple box plots. #Significantly different from t0 on a fluid-specific level (P < 0.05). Black dashed lines represent the normal range of the electrolytes. Colored lines indicate the median value at t0 for each fluid. Reproduced and adapted from Van Regenmortel et al. with permission according to the Open Access CC BY Licence 4.0 [11]
Figure 8.4 shows the TOPMAST study results with respect to cumulative fluid balance. The tonicity of the maintenance fluids was responsible for the clinical impact on perioperative fluid retention (4.5 L in isotonic vs 3.1 L in hypotonic group), independent of the administered volume (around 2.65 L in both groups). Isotonic maintenance fluids resulted in an estimated difference at 72 h of 1369 mL (95%CI 601–2137). An isotonic maintenance fluid strategy was reported to cause hyperchloraemia (>109 mmol/L) in most patients (68.6% vs 11.8% p < 0.001), while hypotonic maintenance fluids decreased sodium levels and caused hyponatraemia (<135 mmol/L) in 11.8% vs 0% (p = 0.04), but no clinical effects were reported in adult surgical patients.
TOPMAST study results. Cumulative fluid balance over the course of each study period. Black lines are individual observations of cumulative fluid balance over time per subject. Colored lines are the marginal means estimated using the mixed effects model; the shaded areas represent 95% confidence intervals. Fluid balance was estimated at 72 h (dashed line), as this is a typical duration for maintenance fluid therapy in the perioperative setting and the maximum duration of study treatment in the current study. The positive fluid balance at baseline is fluids that were administered immediately before surgery. Adpated from Van Regenmortel et al., with permission according to the Open Access CC BY Licence 4.0 [13]
A recent study showed that maintenance and replacement fluids account for the largest amount (24.7%) of the average daily total fluid volume, exceeding by far resuscitation fluids (6.5%). Maintenance fluids are also the most important source of sodium and chloride [14]. Fluid creep caused by maintenance and replacement fluids represents on average one third of the daily fluid volume. In septic patients, non-resuscitation fluids have an even larger absolute impact on cumulative fluid balance compared to resuscitation fluids. Therefore, inadvertent daily loading of volume, sodium, and chloride should be considered when prescribing (isotonic) maintenance fluids [14]. Table 8.2 lists some common maintenance solutions.
Definitions and Key Messages
-
Maintenance fluids should always cover the daily needs.
-
The daily needs are 1 ml/kg/h or 20–25 ml/kg/day for water, 1–1.5 mmol/kg/day for sodium, 0.5–1 mmol/kg/day for potassium, 1–1.5 g/kg/day for glucose
-
Maintenance fluids are responsible for fluid creep, especially if they are isotonic.
-
It is not (only) the volume, that causes fluid overload, it’s also the salt!
Replacement Fluids
Replacement fluids are administered to correct fluid deficits that cannot be compensated by oral intake. Such fluid deficits have a number of potential origins, like drains or stomata, vomiting, burns, diarrhea, vacuum dressings (like Abthera abdominal VAC, Acelity, USA), high output fistulas, fever or hyperthermia, open wounds, polyuria (salt wasting nephropathy, cerebral salt wasting, osmotic diuresis, or diabetes insipidus), during abdominal surgery, physical activity, and others [15].
Similar to maintenance fluids, data on replacement fluids are also scarce. Several recent guidelines advise matching the amount and composition of fluid and electrolytes as closely as possible to the fluid that is being or has been lost [16, 17]. An overview of the composition of the different body fluids can be found in the NICE guidelines [16]. Replacement fluids are usually isotonic balanced solutions. In patients with fluid deficit due to a loss of chloride-rich gastric fluid, high chloride solutions, like ammonium chloride (NH4Cl) or (ab)normal saline (0.9% NaCl), can be used as replacement fluid.
Definitions and Key Messages
-
Replacement fluids should mimic the fluid that is lost
-
Gastrointestinal losses may be the only indication left for (ab)normal saline
Nutrition Fluids
Often overlooked, it is about time to consider parenteral nutrition as another source of intravenous fluids that may contribute to fluid overload or accumulation. On the other hand, enteral nutrition can also contribute to fluid overload in critical illness as the adaptive mechanisms to avoid water and sodium retention may not always function properly. Likewise, nutritional therapy in the critically ill should be seen as “medication” helping the healing process. As such, we might consider also the four D’s of nutritional therapy in analogy to how we deal with antibiotics and fluids [3]: drug (type of feeding), dose (caloric and protein load), duration (when and how long), and de-escalation (stop enteral nutrition and/or parenteral nutrition when oral intake improves) [18]. It is noteworthy that in case of an open abdomen the patient is at risk for potentially significant fluid, electrolyte, and protein losses from the exposed viscera. In case of extensive fluid losses (open abdomen, abdominal drains, ascites paracentesis, or VAC dressings), there will be a substantial loss of nitrogen (on average 2g N/L fluid) [19].
Definitions and Key Messages
-
Nutrition fluids should cover daily caloric needs
-
On average, 2g N are lost per L drainage fluid
The Four Questions
When to Start IV Fluids?
Early adequate fluid management (EAFM) is the initial hemodynamic resuscitation of patients with (septic) shock by administering fluids during the first 6 h after the initiation of therapy. Most studies looking at treatment of septic shock define achieving the early goal as giving 25–50 mL/kg (on average 30 mL/kg) of fluids given within the first 3 h [1]. However, the recent surviving sepsis campaign guidelines define EAFM as 30 mL/kg given in the first hour [20, 21]. However, it has been hypothesized that fluid resuscitation using such large volumes of fluid may lead to “iatrogenic salt water drowning” and that more conservative strategies for fluid resuscitation might be warranted [22]. As stated above, the best fluid is the one that has not been given unnecessarily to the patient. Most patients with a good cardiac function are on the steep slope of their Frank-Starling curve. It is a misconception that in critical illness fluid responsiveness always means that actual fluids need to be administered. There is no high-quality evidence to support the use of IV fluids to optimize the circulation, and especially there is no evidence for the use of higher volumes as recommended by international guidelines [20, 23]. In contrast, data from cohort studies, small trials, and systematic reviews in sepsis and large trials in other settings and patient groups suggest potential benefits from restriction of IV fluids in patients with septic shock [24,25,26]. Therefore, fluids should only be given when needed, i.e., when the patient is in shock (DO2/VO2 imbalance with lactate production) and fluid-responsive [27, 28]. In all other cases, it may be wise to withhold IV fluids [29, 30].
Definitions and Key Messages
-
Early adequate fluid management (EAFM) is the initial hemodynamic resuscitation of patients with (septic) shock by administering adequate fluids during the first 6 h after the initiation of therapy.
-
Fluids should only be given when needed, i.e., when the patient is in shock (DO2/VO2 imbalance with lactate production) and fluid-responsive.
When to Stop IV Fluids?
Late Conservative Fluid Management (LCFM) describes a moderate fluid management strategy following the initial EAFM in order to avoid (or reverse) fluid overload. Recent studies showed that 2 consecutive days of negative fluid balance within the first week of the ICU stay is a strong and independent predictor of survival [31]. LCFM must be adapted according to the variable clinical course of septic shock during the first days of ICU treatment, e.g., patients with persistent systemic inflammation maintain transcapillary albumin leakage and do not reach the flow phase (see further) mounting up positive fluid balances. Once one has decided to administer IV fluids, one must immediately think of a fluid strategy stopping them. Fluids can be stopped when initial signs and symptoms of shock and hypovolemia have resolved. Usually this is based on normalization of macro-hemodynamic parameters like MAP, HR, CVP, GEDVI in combination with other clinical, biochemical, and/or imaging signs.
Definitions and Key Messages
-
Late Conservative Fluid Management (LCFM) is defined as 2 consecutive days of negative fluid balance within the first week of the ICU stay.
-
After the initial Ebb phase, most patients enter the Flow phase spontaneously
When to Start Fluid Removal?
Because of the very nature of (septic) shock, fluids may accumulate in the body. Late Goal-Directed Fluid Removal (LGFR) describes that in some patients more aggressive and active fluid removal by means of diuretics or renal replacement therapy with net ultrafiltration is needed either or not in combination with hypertonic solutions to mobilize the excess interstitial edema [32]. This is referred to as de-resuscitation, a term that was coined for the first time in 2014 [28].
Definitions and Key Messages
-
Late Goal-Directed Fluid Removal (LGFR) is defined as active fluid removal with diuretics or renal replacement therapy with net ultrafiltration and started within the first week of ICU stay also referred to as de-resuscitation
When to Stop Fluid Removal?
During de-resuscitation, it is important to assure adequate intravascular filling and to avoid hypovolemia and hypoperfusion as this may cause further harm. Hypertonic solutions can be used to “drive” fluids from the interstitium into the intravascular space. According to the revised Starling principle, this is usually obtained via lymphatic drainage.
Definitions and Key Messages
-
The benefits of fluid removal should always outweigh the potential risks
The Four (or Six) D’s
Diagnosis
Correct diagnosis of a state of shock, hypovolemia vs hypervolemia, and fluid responsiveness are all equally important. The American consensus definition states that shock is defined by a systolic blood pressure below 90 mmHg refractory to fluid administration. This definition is not useful at the bedside. The European definition states that shock is a situation of imbalance between oxygen delivery (DO2) and oxygen consumption (VO2) resulting in anaerobic metabolism and lactate production:
Standardizing and driving adoption of hypovolemia screening and assessment tools, including hemodynamic monitoring, is the cornerstone in initial management of any shocked patient. Figure 8.5 lists some clinical signs, and the laboratory, imaging, hemodynamic, and organ function signs and effects related to hypovolemia are summarized in Fig. 8.6.
Clinical signs and symptoms related to hypovolemia and hypoperfusion. CVP central venous pressure, EEO end-expiratory occlusion, FR fluid responsiveness, GRV gastric residual volume, HR heart rate, MAP mean arterial blood pressure, Na sodium, PAOP pulmonary artery occlusion pressure, PLR passive leg raising, PPV pulse pressure variation, Qs/Qt shunt fraction, RSB rapid shallow breathing, SMA superior mesenteric artery, SPV systolic pressure variation, SVV stroke volume variation, Vt/Vd dead space ventilation
Laboratory, imaging, hemodynamic and organ function signs and symptoms related to hypovolemia and hypoperfusion. Total body water accounts for 70% of body weight. Overt signs and symptoms of hypovolemia occur when circulating blood volume is reduced with more than 50%. AKI acute kidney injury, APP abdominal perfusion pressure, BIA bioelectrical impedance analysis, BNP brain natriuretic peptide, BVI blood volume index, CARS cardio-abdominal-renal syndrome, CBV circulating blood volume, CI cardiac index, CLI capillary leak index, COP colloid oncotic pressure, CRP C-reactive protein, CVP central venous pressure, ECW extracellular water, EIT electrical impedance tomography, EEO end-expiratory occlusion, EVWLI extravascular lung water index, FR fluid responsiveness, GEDVI global end-diastolic volume index, GEF global ejection fraction, GIPS global increased permeability syndrome, HR heart rate, IAP intra-abdominal pressure, ICW intracellular water, IVC inferior vena cava, IVCCI inferior vena cava collapsibility index, LVOT left ventricular outflow tract, MAP mean arterial blood pressure, Na sodium, P/F ratio pO2 over FiO2 ratio, PAOP pulmonary artery occlusion pressure, PLR passive leg raising, PPV pulse pressure variation, PVPI pulmonary vascular permeability index, RAAS renin angiotensin aldosterone system, RPP renal perfusion pressure, RVEDVI right ventricular end-diastolic volume index, RVEF right ventricular ejection fraction, ScvO2 mixed central venous oxygen saturation, SPV systolic pressure variation, SVV stroke volume variation, TBW total body water, TTE transthoracic echocardiography, US ultrasound, V/Q ventilation/perfusion, VTI velocity time integral
Drug
We should consider the different compounds: crystalloids vs. colloids, synthetic vs. blood derived, balanced vs. unbalanced, intravenous vs. oral; the osmolality, tonicity, pH, electrolyte composition (chloride, sodium, potassium, etc.) and levels of other metabolically active compounds (lactate, acetate, malate…). Clinical factors (underlying conditions, kidney or liver failure, presence of capillary leak, acid-base equilibrium, albumin levels, fluid balance…) must all be taken into account when choosing the type and amount of fluid for a given patient at a given time. Moreover, the type of fluid is different depending on the reason why they are administered.
Dose
As Paracelsus nicely stated: “All things are poison, and nothing is without poison; only the dose permits something not to be poisonous”. Like other drugs, it is the dose of fluids that make them poisonous. As stated before, the risk of excessive fluid overload is well established.
Similar to other drugs, choosing the right dose implies that we take into account the pharmacokinetics and pharmacodynamics of intravenous fluids (Table 8.3).
Pharmacokinetics describes how the body affects a drug resulting in a particular plasma and effect site concentration [33]. Pharmacokinetics of intravenous fluids depends on distribution volume, osmolality, tonicity, oncoticity, and kidney function. Eventually, the half time depends on the type of fluid, but also on the patient’s condition and the clinical context (Table 8.4). When administering one liter of fluid only, 10% of glucose solution, vs. 25% of an isotonic crystalloid solution, vs. 100% of a colloid solution will remain intravascularly after one hour, but as stated above the half-life is dependent on other conditions (like infection, inflammation, sedation, surgery, anesthesia, blood pressure,…) (Fig. 8.7) [34, 35].
Pharmacokinetics and pharmacodynamics fluids. Original artwork based on the work of Hahn R [34, 35]. (a) Volume kinetic simulation. Expansion of plasma volume (in ml) after intravenous infusion of 2 l of Ringer’s acetate over 60 min in an adult patient (average weight 80 kg), depending on normal condition as conscious volunteer (solid line —), during anesthesia and surgery (dashed line - -), immediately after induction of anesthesia due to vasoplegia and hypotension with decrease in arterial pressure to 85% of baseline, (mixed line — ▪) and after bleeding during hemorrhagic shock with mean arterial pressure below 50 mmHg (dotted line ∙∙∙∙), see text for explanation. (b) Volume kinetic simulation. Expansion of plasma volume (in ml) is 100, 300, and 1000 ml respectively after 60 min following intravenous infusion of 1 l of glucose 5% over 20 min in an adult patient (solid line —), vs. 1 l of crystalloid (dashed line - -), vs. 1 l of colloid (dotted line ∙∙∙∙), see text for explanation. (c) Volume kinetic simulation. Expansion of plasma volume (in ml) after intravenous infusion of 500 ml of hydroxyethyl starch 130/0.4 (Volulyte, solid line —) vs. 1 l of Ringer’s acetate (dashed line - -) when administered in an adult patient (average weight 80 kg), over 30 min (RED) vs 60 min (BLACK), vs 180 min (BLUE). When administered rapidly and as long as infusion is ongoing, the volume expansion kinetics are similar between crystalloids and colloids, especially in case of shock, after induction and anesthesia and during surgery (see text for explanation)
Volume kinetics is an adaptation of pharmacokinetic theory that makes it possible to analyze and simulate the distribution and elimination of infusion fluids [35]. Applying this concept, it is possible, by simulation, to determine the infusion rate that is required to reach a predetermined plasma volume expansion. Volume kinetics may also allow the quantification of changes in the distribution and elimination of fluids (and calculation of the half-life) that result from stress, hypovolemia, anesthesia, and surgery [34].
Pharmacodynamics relates the drug concentrations to its specific effect. For fluids, the Frank-Starling relationship between cardiac output and cardiac preload is the equivalent of the dose effect curve for standard medications. Because of the shape of the Frank-Starling relationship, the response of cardiac output to the fluid-induced increase in cardiac preload is not constant [36]. The effective dose 50 (ED50), in pharmacology, is the dose or amount of drug that produces a therapeutic response or desired effect in 50% of the subjects receiving it, whereas lethal dose 50 (LD50) will result in death of 50% of recipients. Translated to IV fluids, this would be the dose of fluid that induces, respectively, a therapeutic response or death in 50% of the patients. The problem is that the therapeutic response varies from one patient to another (Fig. 8.8).
Schematic representation of median IV Fluid doses. ED50: Median effective IV fluid dose: the dose of IV fluids required to achieve 50% of the desired response in 50% of the population. TD50: Median toxic IV fluid dose: the dose of IV fluids required to get 50% of the population reporting this specific toxic effect. LD50: Median lethal IV fluid dose: the dose of IV fluids required to achieve 50% mortality from toxicity
Fluid administration can be toxic (or even lethal) at a high enough dose, as demonstrated in 2007 when a California woman died of water intoxication (and hyponatremia) in a contest organized by a radio station (http://articles.latimes.com/2007/jan/14/local/me-water14). The difference between toxicity and efficacy is dependent upon the particular patient and the specific condition of that patient, although the amount of fluids administered by a physician should fall into the predetermined therapeutic window.
Unanswered questions remain: what is an effective dose of IV fluids? What is the exact desired therapeutic effect? What is the therapeutic window? In some patients, volume expansion increases the mean systemic filling pressure (the backward pressure of venous return), but it increases the right atrial pressure (the forward pressure of venous return) to the same extent, such that venous return and, hence, cardiac output does not increase [37]. Hence, venous congestion and backward failure may even play a more important and currently underestimated role [38]. The probability of the heart to “respond” to fluid by a significant increase in cardiac preload varies along the shock time course, and thus pharmacodynamics of fluids must be regularly evaluated. At the very early phase, fluid responsiveness is constant. After the very initial fluid administration, only one half of patients with circulatory failure respond to an increase in cardiac output [39].
Duration
The longer the delay in fluid administration, the more microcirculatory hypoperfusion and subsequent organ damage related to ischemia-reperfusion injury. In patients with sepsis [31], Murphy and colleagues compared outcomes related to early adequate vs. early conservative and late conservative vs. late liberal fluid administration and found that the combination of early adequate and late conservative fluid management carried the best prognosis [31] (Fig. 8.9).
Impact on outcome of appropriate timing of fluid administration. Bar graph showing outcome (mortality %) in different fluid management categories. Comparison of the data obtained from different studies: hospital mortality in 212 patients with septic shock and acute lung injury, adapted from Murphy et al. (black bars) [31], hospital mortality in 180 patients with sepsis, capillary leak and fluid overload, adapted and combined from 2 papers by Cordemans et al. (light grey bars) [32, 40], 90-day mortality in 151 adult patients with septic shock randomized to restrictive vs standard fluid therapy (CLASSIC trial), adapted from Hjortrup et al. (dark grey bars) [24]. See text for explanation. EA early adequate fluid management, defined as fluid intake >50 ml/kg/first 12–24 h of ICU stay, EC early conservative fluid management, defined as fluid intake <25 ml/kg/first 12–24 h of ICU stay, LC: late conservative fluid management, defined as 2 negative consecutive daily fluid balances within first week of ICU stay, LL late liberal fluid management, defined as the absence of 2 consecutive negative daily fluid balances within first week of ICU stay
De-escalation
As we will discuss below, the final step in fluid therapy is to consider withholding or withdrawing resuscitation fluids when they are no longer required [28, 41, 42].
Like for antibiotics (Table 8.3), the duration of fluid therapy must be as short as possible, and the volume must be tapered when shock is resolved. However, many clinicians use certain triggers to start, but are unaware of triggers to stop, fluid resuscitation, increasing the potential for fluid overload. As with duration of antibiotics, although there is no strong evidence, there is a trend towards shorter duration of intravenous fluids [24].
Discharge
Fluid therapy and prescription post-discharge from ICU or hospital must not be overlooked in order to assure adequate daily (oral) intake.
The Four Hits
First Hit: Initial Insult
After the initial insult (related to sepsis, burns, pancreatitis, trauma, haemorrhage…), the patient will enter the Ebb phase. This refers to the initial phase of septic shock when the patient shows hyperdynamic circulatory shock with decreased systemic vascular resistance due to vasodilation, increased capillary permeability, and severe absolute or relative intravascular hypovolemia. Fluids are mandatory and lifesaving in this phase. The patient in this stage needs EAFM [43].
Second Hit: Ischemia-Reperfusion
The second hit occurs within hours and refers to ischemia and reperfusion. Fluid accumulation reflects the severity of illness (and might be considered a “biomarker” for it). The greater the fluid requirement, the sicker the patient and the more likely organ failure (e.g., acute kidney injury) may occur.
Third Hit: Global Increased Permeability Syndrome
After the second hit, the patient can either further recover entering the “flow” phase with spontaneous evacuation of the excess fluids that have been administrated previously. Some patients will not transgress to the “flow” phase spontaneously and will remain in a “no flow” or persistent state of global increased permeability syndrome and ongoing fluid accumulation [44]. The global increased permeability syndrome can hence be defined as fluid overload in combination with new onset organ failure (Fig. 8.10). This is referred to as ‘the third hit of shock’ [32].
Potential adverse consequences of fluid overload on end-organ function. Adapted from Malbrain et al. with permission [4]. APP abdominal perfusion pressure, IAP intra-abdominal pressure, IAH intra-abdominal hypertension, ACS abdominal compartment syndrome, CARS cardio-abdominal-renal syndrome, CO cardiac output, CPP cerebral perfusion pressure, CS compartment syndrome, CVP central venous pressure, GEDVI global enddiastolic volume index, GEF global ejection fraction, GFR glomerular filtration rate, ICG-PDR indocyaninegreen plasma disappearance rate, ICH intracranial hypertension, ICP intracranial pressure, ICS intracranial compartment syndrome, IOP intraocular pressure, MAP mean arterial pressure, OCS ocular compartment syndrome, PAOP pulmonary artery occlusion pressure, pHi gastric tonometry, RVR renal vascular resistance, SV stroke volume
Fourth Hit: Hypoperfusion
Hypoperfusion is usually the result of hypovolemia. Hypovolemia is the term used to describe a patient with insufficient intravascular volume. It does not refer to total body fluid, but rather refers solely to the intravascular compartment. Total body fluid comprises approximately 60% of the body weight of men and 50% for women [45]. Blood volume can be estimated according to Gilcher’s rule of fives at 70 mL/kg for men and 65 mL/kg for women [46]. Blood loss is frequently followed by recruitment of interstitial fluid from compartments distant to the central compartment. Vasoconstriction of the splanchnic mesenteric vasculature is one of the first physiologic responses [47]. Sodium and water retention results from activation of the renin-angiotensin-aldosterone system (RAAS) which replenishes the interstitial reserves and maintains transcapillary perfusion [48]. As a result, the body may lose up to 30% of blood volume before hypovolemia becomes clinically apparent [44]. Therefore, undiagnosed hypovolemia may be present long before clinical signs and symptoms occur. Hypovolemia can also occur in edematous patients, where total body water in increased, but intravascular volume is reduced (e.g., eclamptic patients). Finally, some patients are fluid-responsive, but not necessarily hypovolemic. Even the most basic of paradigms, such as the description of early sepsis and distributive shock being a hypovolemic state needing aggressive fluid resuscitation, have recently been called into question, [49] with data suggesting improved outcomes with less or even no administered intravenous fluid [24, 49]. Greater focus on the health and function of the microcirculation and the endothelial glycocalyx, potential new treatment paradigms calling for less fluids, and earlier vasopressor use have become the focus [3, 4, 49, 50]. These elements make accurate assessment of fluid status in the critically ill a challenging task.
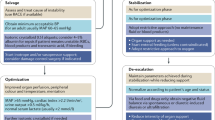
The Four Phases (ROSE Concept)
Recently, a three (or even four) hit model of septic shock was suggested in which we can recognize four (or even five) distinct dynamic phases of fluid therapy [40]: Resuscitation, Optimization, Stabilization, and Evacuation (de-resuscitation) at the end (the acronym R.O.S.E.) (Table 8.3). On the other hand, too aggressive de-resuscitation may result in hypoperfusion again increasing end-organ damage. Logically, this acronym or mnemonic describes the different clinical phases of fluid therapy, occurring over the time course during which patients experience a different impact on end-organ function (Fig. 8.11). Similar principles were also suggested by others confirming the need for a multicenter prospective clinical trial with a biphasic fluid therapy approach, starting with initial early adequate goal-directed treatment followed by late conservative fluid management in those patients not transgressing spontaneously from the Ebb to the Flow phase [41, 42, 51,52,53,54,55,56,57]. The RADAR (Role of Active De-resuscitation After Resuscitation) trial may help to find such answers (http://www.hra.nhs.uk/news/research-summaries/radar-icu/). Clinicians should take into account the revised Starling equation and the glycocalyx model of transvascular fluid exchange [6]. When capillary pressure (or transendothelial pressure difference) is low, as in hypovolemia or sepsis, albumin or plasma substitutes have no advantage over crystalloid infusions, since they all remain intravascular. However, the glycocalyx layer is a fragile structure and is disrupted by surgical trauma-induced systemic inflammation or sepsis, but also by rapid infusion of fluids (especially saline). Under these circumstances, transcapillary flow (and risk of tissue edema) is increased; as is the risk to evolve to a state of global increased permeability syndrome (GIPS) [6].
Graph showing the four-hit model of shock with evolution of patients’ cumulative fluid volume status over time during the five distinct phases of resuscitation: Resuscitation (R), Optimization (O), Stabilization (S), and Evacuation (E) (ROSE), followed by a possible risk of Hypoperfusion in case of too aggressive deresuscitation. On admission, patients are hypovolemic, followed by normovolemia after fluid resuscitation (EAFM, early adequate fluid management), and possible fluid overload, again followed by a phase going to normovolemia with late conservative fluid management (LCFM) and late goal-directed fluid removal (LGFR) or deresuscitation. In case of hypovolemia O2 cannot get into the tissues because of convective problems, in case of hypervolemia O2 cannot get into the tissue because of diffuse problems related to interstitial and pulmonary edema, gut edema (ileus and abdominal hypertension). Adapted according to the Open Access CC BY Licence 4.0 with permission from Malbrain et al. [4]
Resuscitation
After the first hit which can be sepsis (but also burns, pancreatitis, or trauma), the patient will enter the “ebb” phase of shock. This phase of severe circulatory shock, that can be life-threatening, occurs within minutes and is characterized by a low mean arterial pressure and microcirculatory impairment and can be accompanied with high cardiac output (hyperdynamic circulatory shock) or low cardiac output (e.g., septic shock with severe hypovolemia or septic shock with cardiomyopathy). Early adequate fluid management is not only useful but also lifesaving in this phase, but the goal should be individualized for every patient; also considering the patient’s premorbid conditions [1, 58,59,60].
The lower autoregulatory threshold of the most vulnerable organs (kidney and brain) should be minimally reached [41]. In this phase, we try to find an answer to the first question: “When to start fluid therapy?”, addressing the benefits of fluid resuscitation (restoration of organ perfusion).
Optimization
The second hit occurs within hours and refers to ischemia and reperfusion. Fluid accumulation reflects the severity of illness (and might be considered a “biomarker” for it) [55]. The greater the fluid requirement, the sicker the patient. In this phase, we try to find an answer to the second question: “When to stop fluid therapy?”, avoiding potential risks of fluid administration (fluid overload).
Stabilization
After the Optimization phase follows the Stabilization phase (homeostasis) evolving over the next days. As previously described, the focus now is on organ support and this phase reflects the point at which a patient is in a stable steady state [28, 57]. Fluid therapy is now only used for ongoing maintenance and replacement fluids either in setting of normal fluid losses (i.e., renal, gastrointestinal, insensible), but this could also be fluid infusion (including rehydration) if the patient was experiencing ongoing losses because of unresolved pathologic conditions [28, 57]. However, this stage is distinguished from the prior two by the absence of shock (compensated or uncompensated) or the imminent threat of shock. Since persistence of a positive daily fluid balance over time is strongly associated with a higher mortality rate in septic patients [61], clinicians should also be aware of the hidden obligatory fluid intake, as it may contribute more than a liter each day [62].
Evacuation
After the second hit, the patient can either further recover entering the “flow” phase with spontaneous evacuation of the excess fluids that have been administrated previously, or, as is the case in many ICU patients, the patient remains in a “no flow” state followed by a third hit usually resulting from GIPS with ongoing fluid accumulation due to capillary leak [6, 63]. Further fluid administration at this stage becomes toxic. Peripheral and generalized edema is not only of cosmetic concern, as believed by some [64], but harmful to the patient as a whole as it results in organ dysfunction [28, 42]. In any case, the patient enters a phase of “de-resuscitation” (Table 8.5). This term was first suggested in 2012 [32] and finally coined in 2014 [28]. It specifically refers to Late Goal-Directed Fluid Removal and Late Conservative Fluid Management. Estimation of fluid overload measured by bioelectrical impedance (vector) analysis seems to predict mortality risk and is safe and easy to perform at the bedside [45, 65].
A vicious cycle may be established with further fluid loading. This will cause even more intestinal edema and visceral swelling leading to venous hypertension and deteriorating renal function (Fig. 8.12).
The vicious cycle of septic shock resuscitation. Adapted from Peeters et al. with permission [66]. IAH intra-abdominal hypertension
At this stage, testing preload responsiveness may still be useful, since it is safe to remove fluid in patients who have no preload dependence [67]. The use of albumin seems to have positive effects on vessel wall integrity, facilitates achieving a negative fluid balance in hypoalbuminemia, and is less likely to cause nephrotoxicity [68]. In this phase, we try to find an answer to the third and fourth question: “When to start fluid removal?” and “When to stop fluid removal?” in order to find the balance between the benefits (reduction of second and third space fluid accumulation and tissue edema) and risk (hypoperfusion) of fluid removal and subsequent organ failure (Fig. 8.13).
Graph illustrating the four-hit model of shock corresponding to the impact on end-organ function in relation to the fluid status. On admission patients are hypovolemic [1], followed by normovolemia [2] after fluid resuscitation, and fluid overload [3], again followed by a phase going to normovolemia with deresuscitation [4] and hypovolemia with risk of hypoperfusion [5]. In case of hypovolemia (phase 1 and 5), O2 cannot get into the tissues because of convective problems, in case of hypervolemia (phase 3) O2 cannot get into the tissue because of diffusion problems related to interstitial and pulmonary edema, gut edema (ileus and abdominal hypertension). See text for explanation
The Other Fours
The Four Compartments
This is tricky one and could be fat—water—protein—and minerals, while water also is also distributed into four compartments: intracellular water (ICW)—Interstitial—intravascular—and transcellular, with extracellular water (ECW) calculated as the sum of interstitial + intravascular + transcellular water content.
The Four Spaces
There are traditionally four fluid spaces: 1st space = intravascular—2nd space = interstitial—3rd space = pleural or peritoneal space—and 4th space = transcellular fluid. And not to forget the lymphatic system.
The Four Losses
Traditionally, four ways can be taken into account with regard to fluid losses: insensible loss—urine output—gastrointestinal losses—and third space. Additional losses can occur in trauma with overt bleeding.
Case Vignette
Below the correct answers are given for the case vignette.
-
Q1: You decide to administer a fluid bolus, how much do you give?
-
A1: 100–250 are correct. The lower dose would fit for a mini-fluid challenge as well.
-
Q2: How fast do you administer the fluid bolus?
-
A2: Bolus given in 10–15 min is the best answer.
-
Q3: What do you consider the most clinically relevant parameter to assess fluid responsiveness?
-
A3: The correct answer is passive leg raising test.
-
Q4: What type of fluids did you administer during resuscitation phase?
-
A4: The best fluid is a balanced isotonic solution like Plasmalyte–Ringer’s lactate–Ringer’s acetate–Hartmann solution.
-
Q5: What type of fluids did you administer as maintenance solution?
-
A5: The best fluid for maintenance (if at all needed) is a hypotonic balanced solution like Maintelyte–Glucion.
-
Q6: How many mls of fluid do you administer to this patient on a daily basis?
-
A6: In view of the body weight of 50 kg 20–25 mL/kg/day should be enough are thus around 1000 mL.
-
Q7: What is the daily need for sodium (mmol) in this patient?
-
A6: Sodium need is 1–1.5 mmol/kg/day or thus 50–75 mmol/day.
-
Q8: What is the daily need for potassium (mmol) in this patient?
-
A8: Potassium need is 1 mmol/kg/day or thus 50 mmol/day.
-
Q9: What is the daily need for glucose (grams) in this patient?
-
A9: Glucose need is 1–1.5 g/kg or thus 50–75 g/day.
-
Q10: What would be the best replacement fluid in addition to her daily maintenance solution?
-
A10: Normal saline (NaCl 0.9%) can be given in case of metabolic alkalosis as saline will induce hyperchloremic metabolic acidosis. This is the only indication left for “abnormal” saline, besides TBI.
Conclusions
There are only four major indications for fluid administration in the critically ill: resuscitation, maintenance, replacement, and nutrition (enteral or parenteral). In this chapter, a conceptual framework is presented looking at fluids as drugs by taking into account the four D’s (drug selection, dose, duration, and de-escalation) and the four phases of fluid therapy within the ROSE concept (resuscitation, optimization, stabilization, evacuation). The four hits model is presented herein. This will provide answers to the four basic questions surrounding fluid therapy: (1) when to start IV fluids? (2) when to stop fluid administration? (3) when to start fluid removal, and finally (4) when to stop fluid removal? In analogy to the way we deal with antibiotics in critically ill patients, it is time for fluid stewardship.
Take Home Messages
-
There are only 4 indications for fluid therapy: Resuscitation, maintenance, replacement, nutrition
-
Resuscitation fluids should save lives
-
Fluid bolus should be small (4 ml/kg) and given fast (in 5–15 min)
-
Do not administer fluids until the patient is no longer fluid responsive
-
-
Maintenance fluids should always cover the daily needs
-
The daily need for water is 1 ml/kg/h or 20–25 ml/kg/day
-
The daily need for sodium is 1–1.5 mmol/kg/day
-
The daily need for potassium is 0.5–1 mmol/kg/day
-
The daily need for glucose is 1–1.5 g/kg/day
-
Fluid creep is real: subtract unintended fluid administration (to dilute drugs in) from daily water need
-
-
Maintenance fluids are responsible for fluid creep, especially if they are isotonic. It is not (only) the volume, that causes fluid overload, it’s the salt!
-
Replacement fluids should mimic the fluid that is lost
-
Gastrointestinal losses may be the only indication left for (ab)normal saline
-
-
Nutrition fluids should cover the daily caloric needs
-
On average 2 g N are lost per L drainage fluid
-
-
The best fluid is the one that has not been given unnecessarily
-
The presence of fluid responsiveness does not mean that fluids need to be administered
-
One must always try to find answers to the four basic questions
-
When to start fluids, or the benefits of fluid therapy
-
Early adequate fluid management (EAFM) is the initial hemodynamic resuscitation of patients with (septic) shock by administering adequate fluids during the first 6 h after the initiation of therapy.
-
Fluids should only be given when needed, i.e., when the patient is in shock (DO2/VO2 imbalance with lactate production) and fluid-responsive.
-
-
When to stop fluids, or the dangers of fluid therapy (fluid overload)
-
Late Conservative Fluid Management (LCFM) is defined as 2 consecutive days of negative fluid balance within the first week of the ICU stay.
-
After the initial Ebb phase, most patients enter the Flow phase spontaneously
-
-
When to start fluid removal, or the benefits of deresuscitation
-
Late Goal-Directed Fluid Removal (LGFR) is defined as active fluid removal with diuretics or renal replacement therapy with net ultrafiltration, and started within the first week of ICU stay also referred to as de-resuscitation
-
-
When to stop fluid removal, or the dangers of deresuscitation (hypoperfusion)
-
The benefits of fluid removal should always outweigh the potential risks
-
-
-
Consider the 4-hit model during fluid therapy
-
1st hit: initial insult
-
2nd hit: following ischemia and reperfusion
-
3rd hit: global increased permeability syndrome (GIPS) and fluid accumulation
-
4th hit: hypoperfusion during deresuscitation
-
-
Fluids are drugs, consider the 6 D’s in analogy with antibiotic therapy
-
1st D—Diagnosis: depending on underlying conditions, different types of fluids need to be administered
-
2nd D—Drug: fluids are drug with indications and contraindications and possible adverse effects
-
3rd D—Dose: the dose depends on the condition and indication
-
4th D—Duration: stop IV fluids when they are no longer needed
-
5th D—De-escalation: taper fluids when oral intake has resumed and remove excess fluids in case of fluid overload with diuretics or ultrafiltration
-
6th D—Discharge: make sure the patient is able to cover his daily needs
-
-
Fluid therapy is not static but dynamic, consider the four phases during critical illness (ROSE)
-
Resuscitation: early adequate goal-directed fluid therapy to rescue the patient and to save lives
-
Optimization: monitoring and support organ function
-
Stabilization: late conservative fluid management (2 consecutive negative daily fluid balances within first week of ICU stay)
-
Evacuation: late goal-directed fluid removal by means of diuretic therapy or ultrafiltration
-
References
Vandervelden S, Malbrain ML. Initial resuscitation from severe sepsis: one size does not fit all. Anaesthesiol Intensive Ther. 2015;47:44–55.
Malbrain ML, Van Regenmortel N, Owczuk R. The debate on fluid management and haemodynamic monitoring continues: between Scylla and Charybdis, or faith and evidence. Anaesthesiol Intensive Ther. 2014;46(5):313–8.
Malbrain ML, Van Regenmortel N, Owczuk R. It is time to consider the four D’s of fluid management. Anaesthesiol Intensive Ther. 2015;47:1–5.
Malbrain MLNG, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal P-J, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. 2018;8(1):66.
Malbrain MLNG, Rice TW, Mythen M, Wuyts S. It is time for improved fluid stewardship. ICU Manage Pract. 2018;18(3):158–62.
Woodcock TE, Woodcock TM. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: an improved paradigm for prescribing intravenous fluid therapy. Br J Anaesth. 2012;108(3):384–94.
Herrod PJ, Awad S, Redfern A, Morgan L, Lobo DN. Hypo- and hypernatraemia in surgical patients: is there room for improvement? World J Surg. 2010;34(3):495–9.
McNab S, Duke T, South M, Babl FE, Lee KJ, Arnup SJ, et al. 140 mmol/L of sodium versus 77 mmol/L of sodium in maintenance intravenous fluid therapy for children in hospital (PIMS): a randomised controlled double-blind trial. Lancet. 2015;385(9974):1190–7.
Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373(14):1350–60.
Lobo DN, Stanga Z, Simpson JA, Anderson JA, Rowlands BJ, Allison SP. Dilution and redistribution effects of rapid 2-litre infusions of 0.9% (w/v) saline and 5% (w/v) dextrose on haematological parameters and serum biochemistry in normal subjects: a double-blind crossover study. Clin Sci. 2001;101(2):173–9.
Van Regenmortel N, De Weerdt T, Van Craenenbroeck AH, Roelant E, Verbrugghe W, Dams K, et al. Effect of isotonic versus hypotonic maintenance fluid therapy on urine output, fluid balance, and electrolyte homeostasis: a crossover study in fasting adult volunteers. Br J Anaesth. 2017;118(6):892–900.
Hendrickx S, Van Vlimmeren K, Baar I, Verbrugghe W, Dams K, Van Cromphaut S, et al. Introducing TOPMAST, the first double-blind randomized clinical trial specifically dedicated to perioperative maintenance fluid therapy in adults. Anaesthesiol Intensive Ther. 2017;49(5):366–72.
Van Regenmortel N, Hendrickx S, Roelant E, Baar I, Dams K, Van Vlimmeren K, et al. 154 compared to 54 mmol per liter of sodium in intravenous maintenance fluid therapy for adult patients undergoing major thoracic surgery (TOPMAST): a single-center randomized controlled double-blind trial. Intensive Care Med. 2019;45(10):1422–32.
Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018;44(4):409–17.
Van Regenmortel N, Jorens PG, Malbrain ML. Fluid management before, during and after elective surgery. Curr Opin Crit Care. 2014;20(4):390–5.
Padhi S, Bullock I, Li L, Stroud M, National Institute for H, Care Excellence Guideline Development G. Intravenous fluid therapy for adults in hospital: summary of NICE guidance. BMJ. 2013;347:f7073.
Soni N. British consensus guidelines on intravenous fluid therapy for adult surgical patients (GIFTASUP): cassandra’s view. Anaesthesia. 2009;64(3):235–8.
De Waele E, Honore PM, Malbrain M. Does the use of indirect calorimetry change outcome in the ICU? Yes it does. Curr Opin Clin Nutr Metab Care. 2018;21(2):126–9.
Cheatham ML, Safcsak K, Brzezinski SJ, Lube MW. Nitrogen balance, protein loss, and the open abdomen. Crit Care Med. 2007;35(1):127–31.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Dellinger RP, Schorr CA, Levy MM. A users’ guide to the 2016 surviving sepsis guidelines. Intensive Care Med. 2017;43(3):299–303.
Marik PE. Iatrogenic salt water drowning and the hazards of a high central venous pressure. Ann Intensive Care. 2014;4:21.
Machado FR, Levy MM, Rhodes A. Fixed minimum volume resuscitation: pro. Intensive Care Med. 2016;43:1678–80.
Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Pettila V, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42(11):1695–705.
Perner A, Singer M. Fixed minimum fluid volume for resuscitation: Con. Intensive Care Med. 2016;43:1681–2.
Myles PS, Bellomo R, Corcoran T, Forbes A, Peyton P, Story D, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med. 2018;378(24):2263–74.
Marik PE, Malbrain M. The SEP-1 quality mandate may be harmful: how to drown a patient with 30 mL per kg fluid! Anaesthesiol Intensive Ther. 2017;49(5):323–8.
Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–80.
Marik PE, Farkas JD, Spiegel R, Weingart S. POINT: should the surviving sepsis campaign guidelines be retired? Yes. Chest. 2019;155(1):12–4.
Spiegel R, Farkas JD, Rola P, Kenny JE, Olusanya S, Marik PE, et al. The 2018 surviving sepsis campaign’s treatment bundle: when guidelines outpace the evidence supporting their use. Ann Emerg Med. 2019;73(4):356–8.
Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–9.
Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Martin G, et al. Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: a pilot study looking at the effects of PAL-treatment. Ann Intensive Care. 2012;2(Suppl 1):S15.
Elbers PW, Girbes A, Malbrain ML, Bosman R. Right dose, right now: using big data to optimize antibiotic dosing in the critically ill. Anaesthesiol Intensive Ther. 2015;47(5):457–63.
Hahn RG, Lyons G. The half-life of infusion fluids: an educational review. Eur J Anaesthesiol. 2016;33(7):475–82.
Hahn RG. Volume kinetics for infusion fluids. Anesthesiology. 2010;113(2):470–81.
Monnet X, Marik P, Teboul JL. Prediction of fluid responsiveness: an update. Ann Intensive Care. 2017;6(1):111.
Guerin L, Teboul JL, Persichini R, Dres M, Richard C, Monnet X. Effects of passive leg raising and volume expansion on mean systemic pressure and venous return in shock in humans. Crit Care. 2015;19:411.
Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WH, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol. 2013;62(6):485–95.
Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298–309.
Cordemans C, De Laet I, Van Regenmortel N, Schoonheydt K, Dits H, Huber W, et al. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak and fluid balance. Ann Intensive Care. 2012;2(1):S1.
Benes J, Kirov M, Kuzkov V, Lainscak M, Molnar Z, Voga G, et al. Fluid therapy: double-edged sword during critical care? Biomed Res Int. 2015;2015:729075.
O'Connor ME, Prowle JR. Fluid overload. Crit Care Clin. 2015;31(4):803–21.
Cuthbertson DP. Observations on disturbance of metabolism produced by injury to the limbs. Quart J Med. 1932;25:233–46.
Duchesne JC, Kaplan LJ, Balogh ZJ, Malbrain ML. Role of permissive hypotension, hypertonic resuscitation and the global increased permeability syndrome in patients with severe hemorrhage: adjuncts to damage control resuscitation to prevent intra-abdominal hypertension. Anaesthesiol Intensive Ther. 2015;47(2):143–55.
Malbrain MLNG, Huygh J, Dabrowski W, De Waele J, Wauters J. The use of bio-electrical impedance analysis (BIA) to guide fluid management, resuscitation and deresuscitation in critically ill patients: a bench-to-bedside review. Anaesthesiol Intensive Ther. 2014;46(5):381–91.
Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–32.
Miller TE, Raghunathan K, Gan TJ. State-of-the-art fluid management in the operating room. Best Pract Res Clin Anaesthesiol. 2014;28(3):261–73.
Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM, Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103(2):128–33.
Marik P, Bellomo R. A rational approach to fluid therapy in sepsis. Br J Anaesth. 2016;116(3):339–49.
Myburgh JA, Mythen MG. Resuscitation fluids. N Engl J Med. 2013;369(25):2462–3.
McDermid RC, Raghunathan K, Romanovsky A, Shaw AD, Bagshaw SM. Controversies in fluid therapy: type, dose and toxicity. World J Crit Care Med. 2014;3(1):24–33.
Rivers EP. Fluid-management strategies in acute lung injury-liberal, conservative, or both? N Engl J Med. 2006;354(24):2598–600.
Bellamy MC. Wet, dry or something else? Br J Anaesth. 2006;97(6):755–7.
Bagshaw SM, Bellomo R. The influence of volume management on outcome. Curr Opin Crit Care. 2007;13(5):541–8.
Bagshaw SM, Brophy PD, Cruz D, Ronco C. Fluid balance as a biomarker: impact of fluid overload on outcome in critically ill patients with acute kidney injury. Crit Care. 2008;12(4):169.
Vincent JL, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726–34.
Hoste EA, Maitland K, Brudney CS, Mehta R, Vincent JL, Yates D, et al. Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014;113(5):740–7.
Perel A, Saugel B, Teboul JL, Malbrain ML, Belda FJ, Fernandez-Mondejar E, et al. The effects of advanced monitoring on hemodynamic management in critically ill patients: a pre and post questionnaire study. J Clin Monit Comput. 2015;30(5):511–8.
Saugel B, Trepte CJ, Heckel K, Wagner JY, Reuter DA. Hemodynamic management of septic shock: is it time for “individualized goal-directed hemodynamic therapy” and for specifically targeting the microcirculation? Shock. 2015;43(6):522–9.
Saugel B, Malbrain ML, Perel A. Hemodynamic monitoring in the era of evidence-based medicine. Crit Care. 2016;20(1):401.
Acheampong A, Vincent JL. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015;19:251.
Bashir MU, Tawil A, Mani VR, Farooq U. Hidden obligatory fluid intake in critical care patients. J Intensive Care Med. 2016;32(3):223–7.
Malbrain ML, De Laet I. AIDS is coming to your ICU: be prepared for acute bowel injury and acute intestinal distress syndrome. Intensive Care Med. 2008;34(9):1565–9.
Pinsky MR. Hemodynamic evaluation and monitoring in the ICU. Chest. 2007;132(6):2020–9.
Samoni S, Vigo V, Resendiz LI, Villa G, De Rosa S, Nalesso F, et al. Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit Care. 2016;20:95.
Peeters Y, Lebeer M, Wise R, Malbrain ML. An overview on fluid resuscitation and resuscitation endpoints in burns: past, present and future. Part 2 - avoiding complications by using the right endpoints with a new personalized protocolized approach. Anaesthesiol Intensive Ther. 2015;47:15–26.
Monnet X, Cipriani F, Camous L, Sentenac P, Dres M, Krastinova E, et al. The passive leg raising test to guide fluid removal in critically ill patients. Ann Intensive Care. 2016;6(1):46.
Vincent JL, De Backer D, Wiedermann CJ. Fluid management in sepsis: the potential beneficial effects of albumin. J Crit Care. 2016;35:161–7.
Acknowledgments
Parts of this chapter were published previously as open access under the creative commons Attribution 4.0 International Licence (CC BY 4.0) [4]. The European Commission announced it has adopted CC BY 4.0 and CC0 to share published documents, including photos, videos, reports, peer-reviewed studies, and data. The Commission joins other public institutions around the world that use standard, legally interoperable tools like Creative Commons licenses and public domain tools to share a wide range of content they produce. The decision to use CC aims to increase the legal interoperability and ease of reuse of authors’ own materials.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Malbrain, M.L.N.G. et al. (2024). The 4-indications of Fluid Therapy: Resuscitation, Replacement, Maintenance and Nutrition Fluids, and Beyond. In: Malbrain, M.L., Wong, A., Nasa, P., Ghosh, S. (eds) Rational Use of Intravenous Fluids in Critically Ill Patients. Springer, Cham. https://doi.org/10.1007/978-3-031-42205-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-42205-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-42204-1
Online ISBN: 978-3-031-42205-8
eBook Packages: MedicineMedicine (R0)