Abstract
Orbital imaging with CT or MRI can be essential in the evaluation of many orbital conditions. Because of its superior bony characterization and fast acquisition, CT is imaging method of first choice in urgent situations like trauma, infection, and evaluation of lesions arising from the orbital wall. Through recent years, CT has also gained a prominent role in (pre)operative planning and navigation, especially through the development of postprocessing software. For the evaluation of more complex orbital disease, MRI is the preferred modality. With its superior soft-tissue differentiation, MRI is useful for determining the extent of orbital lesions, like inflammatory disease, vascular malformations, and orbital tumors. By adding functional MRI techniques, like diffusion and perfusion-weighted imaging, and by combining parameters of different imaging techniques in multiparametric imaging, it is possible to further improve characterization of orbital lesions. In this chapter, the optimal approach to orbital imaging is described, combining knowledge of orbital imaging techniques and imaging indications, together with a structured way of reviewing the orbital images, knowledge of radiological features of common, and more uncommon orbital pathology, and integrating this with the clinical features of the patient.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Learning Objectives-
To gain knowledge about state-of-the-art orbital imaging techniques and when to use them in clinical practice.
-
To review CT and MRI scans of the orbit in a systematic way in order to identify relevant findings.
-
To become familiar with radiological appearances of common and more uncommon orbital pathology.
Introduction
Many different conditions can affect the small anatomical space of the orbit, and imaging with computer tomography (CT) or magnetic resonance imaging (MRI) can be essential in the evaluation of orbital disease to make a correct diagnosis and guide and evaluate medical or surgical treatment. Common orbital diseases that may require imaging are orbital trauma, Graves’ orbitopathy (GO), orbital infection, and orbital soft tissue lesions like intraorbital vascular lesions or inflammation or lesions of the bony orbit. A structured way of reviewing the images and knowledge of orbital anatomy, imaging techniques, indications for orbital imaging, and the clinical and radiological presentation of orbital pathology are crucial for optimal evaluation.
Continuing technical developments created ample improvement in the imaging possibilities over the last decades. CT high-resolution images of the orbit, with the ability to reconstruct images in different planes, allow excellent depiction of the bony structures but also very reasonable delineation of soft tissues. However, MRI provides optimal soft-tissue contrast allowing for accurate depiction of the extension of pathology. Besides the standard anatomical imaging with MRI, it is also possible to look at biological phenomena of orbital pathology with functional MRI techniques like diffusion-weighted imaging (DWI) and perfusion-weighted imaging (PWI). Combining parameters from different imaging techniques in so-called multiparametric imaging creates additional value especially in the differential diagnosis of orbital soft-tissue masses. Moreover, ongoing developments in postprocessing tools strongly improve the usefulness of imaging, for example, in the presurgical planning and in the preoperative navigation during orbital reconstruction surgery. In the near future, also artificial intelligence (AI) will be applied to further improve orbital imaging and decision-making.
With the continuously improving possibilities in diagnostic imaging, evoking amplification of imaging indications, and the amount of orbital scans, the challenge is to keep considering principles like radiation protection and cost-effectiveness.
In this chapter, we give an overview of the current orbital imaging techniques, their indications and usefulness in clinical practice, some guidance to a structured approach of the images, and radiological features of some common and less common orbital conditions.
Imaging Techniques
CT and MRI are the main imaging modalities to evaluate orbital pathology. Imaging of the bony orbit through conventional radiographs has become obsolete and has been replaced by the much more sensitive CT scans. The only indication for a conventional radiograph of the orbit nowadays is to exclude metallic foreign bodies in patients who need to undergo an MRI scan and have an increased risk for orbital metallic foreign bodies. Ultrasound can be useful in preseptal and ocular lesions, but is of limited value for retrobulbar lesions. Ultrasound may provide information about tumor shape, internal reflectivity, and vascular flow, but is operator-dependent, less suited for follow-up, and has a low specificity identifying malignant tumors [1]. Nuclear medicine techniques like FDG-PET-CT have limited value in assessing orbital disease due to high FDG uptake in extraocular muscles and typical small volume of orbital disease. FDG-PET, however, might be useful in screening for systemic lymphoma or detecting the primary tumor in case of orbital metastasis. The use of 68Ga-DOTATATE PET-CT might be helpful to confirm the diagnosis of optic nerve sheet meningioma in selected cases when the diagnosis is not completely sure from the MRI [2].
CT
CT uses ionizing radiation (x-rays) to make cross-sectional images of the body. The X-rays are detected by a detector on the opposite side of the body. From the raw data, images are reconstructed from measurements of attenuation coefficients of the x-rays through the body. The reconstructed images reflect different densities of the body tissues, represented by Hounsfield units (HU) [3].
The advantages of CT are clear, it is a very fast and widely available imaging technique that gives excellent images of the bony orbit, but also information of the soft tissues. With the current multidetector CT (MDCT) machines, it is possible to scan the orbit with sub-millimetric and near isotropic (identical in all directions) resolution in only a few seconds, which makes it also suitable for restless patients in the emergency department [4].
Useful scan parameters for an orbital CT protocol on a state-of-the-art MDCT scanner are 120 kV, 90mAs, collimation 128–192 mm with 0.6 mm slice thickness, and pitch of 0.8. Parameters should be chosen with the ALARA principle (as low as reasonable achievable) in mind to keep the radiation dose as low as possible while maintaining diagnostic accuracy.
Because of the near isotropic resolution, the raw images from a single scan can be reconstructed in additional coronal or sagittal planes through multiplanar reconstructions (MPR). Moreover, different reconstruction filters can be applied to get optimal images from the orbital bone and from the soft tissues. Preferably, 1–2 mm reconstructions should be made for the bone setting and 1.5–3 mm for the soft-tissue images. Axial MPRs should be reconstructed when the original scan is not symmetric. Coronal MPRs should be made perpendicular to the orbital axis. Sagittal MPRs can be made on indication although for the assessment of orbital fractures these reconstructions are paramount. Metal artifact reduction (MAR) algorithms can be applied to improve image quality in patients with metal implants [5]. 3D reconstructions can be beneficial for surgical planning as they provide insight into the fracture malalignment prior to reconstructions (Fig. 4.1). In Part 5 of this book, treatment planning after trauma, including the use of CT-based reconstruction and planning software, will be discussed in more detail.
Axial (a), coronal (b), and 3D volumetric bone reconstructions (c) of the CT from a patient after extensive orbital trauma due to a shooting accident. Note the luxation of the left globe and fractures of all the orbital walls. 3D reconstructions can be beneficial for surgical planning of complex fractures as they provide insight into the fracture malalignment prior to reconstructions
Intravenous iodinated contrast can be used for optimizing delineation of normal and pathological structures. In orbital imaging, the main indication for CT with intravenous contrast is evaluation of orbital infection. For tumor imaging, MRI is imaging method of first choice. In patients with impaired renal function or a history of severe allergy to iodinated contrast agents, a CT without contrast or an MRI scan should be considered. When this is not possible, the patient should be prepared with medication in order to prevent a severe allergic reaction or with prehydration and posthydration to prevent postcontrast acute kidney injury (PC-AKI) [6, 7]. Every hospital should have a (local) protocol on prevention and management of adverse events in patients at risk receiving iodinated contrast.
CT should be the preferential imaging technique when evaluation of the bony orbit is of main concern, like in trauma. Also in other urgent situations, such as evaluation of the extent of orbital cellulitis or postoperative complications, CT will be the imaging method of first choice in most hospitals. Moreover, CT is the preferred imaging method to evaluate orbital complications of chronic sinonasal disease, like silent sinus or sinonasal mucoceles involving the orbital wall [8].
Cone Beam CT
Cone beam CT (CBCT) is a relative recent CT imaging technique providing low-dose and high-spatial resolution visualization of high-contrast structures. In the head and neck, it is mainly used for dental and mastoid imaging. Although it gives excellent spatial resolution of the bony structures, the soft-tissues of the orbit cannot reliably be evaluated with CBCT because of its limited contrast resolution [9].
MRI
The advantage of orbital imaging with MRI, when compared to CT, is the excellent soft-tissue contrast, without the use of ionizing radiation. The latter makes it more suitable for children and young adults and for patient who need repeated orbital imaging. The downside, besides the higher costs, is the longer scanning time; different series and planes have to be scanned separately and take on average 2–4 min, making the average scanning time for an orbital MRI about 10–15 min for a standard protocol without contrast (i.e., for GO) up to 30 min for a more extensive tumor imaging protocol. Most children below the age of 6–7 will therefore need sedation or general anesthesia with adequate patient monitoring to undergo MRI scanning.
The superior visualization of soft-tissues makes MRI imaging the preferred method for imaging orbital soft-tissue masses and other more complex orbital disease. Orbital MRI scans can be obtained on both 1.5 T or 3 T MRI scanners and are usually performed with a head coil; only for superficial or globe lesions, a surface coil could have additional value. 3 T has the advantage of a higher signal-to-noise ratio, which can be translated into a better spatial resolution in the same acquisition time, but it also has more challenges regarding susceptibility artifacts.
A routine orbital MRI protocol includes a precontrast turbo spin-echo (TSE) T1- and T2-weighted imaging (WI) without fat suppression and a series with fat suppression (FS) that is relatively independent of field inhomogeneity like STIR (short-TI inversion recovery), T2-weighted TIRM (turbo inversion recovery), or Dixon technique. Images should be acquired with high in-plane spatial resolution and slice thickness not exceeding 3 mm. With the state-of-the-art MRI scanners, it is also possible to acquire volumetric scans in 3D, from which the other planes can be reconstructed.
A time of flight MR angiography (TOF MRA) without the need of IV contrast or a dynamic MRA after IV contrast could be added as an additional series when (high-flow) vascular lesions, such orbital arteriovenous malformation (AVM) or (dural) carotid cavernous fistula, are suspected.
The T1-WI and T2-WI without fat suppression give excellent anatomical detail and contrast between the orbital fat that is very bright and the other soft tissues, like the extraocular muscles (EOMs), the lacrimal gland and the optic nerve sheet complex. This facilitates assessing the extension of orbital disease. With fat-suppression techniques (i.e. STIR, TIRM, Dixon), edema is accentuated, facilitating the detection of active inflammation/disease. The fat suppression of the STIR technique is superior to most other fat-suppression techniques especially near metallic foreign bodies and near tissue interfaces with high susceptibility differences (like interface between the orbit, skull base, and paranasal sinus); moreover, it has the additional feature of additive T1 and T2 contrast [10]. The Dixon technique has the advantage to provide images with and without fat suppression from a single acquisition [11].
MRI protocol without intravenous contrast is generally adequate for evaluation of GO and follow-up of known benign orbital diseases like venous malformations. For more complex orbital pathology, like tumors, infectious or inflammatory disease, additional postcontrast series after injection of gadolinium-based contrast are indicated. The postcontrast T1-WI should be made with fat suppression; otherwise, it might be difficult to recognize enhancement within the bright orbital fat.
Functional/Advanced MRI Techniques
Besides the routine anatomical MRI series, more advanced techniques such as diffusion-weighted imagine (DWI) and perfusion-weighted imaging (PWI) allow studying of functional aspects of orbital lesions. This can help in further characterizing orbital pathology and a more accurate differentiation between malignant and benign disease.
Diffusion-Weighted Imaging (DWI)
The principle of DWI relies on the Brownian motion of water molecules in a voxel of tissue, i.e., within the intracellular and extracellular fluid of the voxel. In simplified terms, the higher the cellularity or cellular swelling, the more restricted the motion will be [12]. From the DWI, apparent diffusion coefficients (ADC) values are calculated and represented in the ADC maps. In active inflammation like GO, ADC values will be increased because of increased interstitial edema (increased diffusion), while in highly cellular tumors, like lymphoma or in purulent abscesses with highly packed inflammatory cells, ADC values will be low (restricted diffusion). DWI is therefore one of the techniques that can be used in further characterizing orbital lesions, especially in differentiating benign from malignant disease. The most frequently used DWI technique, especially in brain imaging, is the echo-planar imaging (EPI)-based sequence. The advantage is that it is a fast technique, but the imaging quality of this sequence in the orbit can be limited by the inhomogeneity artifacts caused by the interfaces between air, bone, and soft tissue in the orbit. When metallic reconstruction material is applied in the orbit, EPI-DWI sequences will be noninterpretable in most cases because of the susceptibility of artifacts resulting in severe distortions. Non-EPI-DWI like the turbo spin-echo (TSE) DWI sequence is an alternate technique with less susceptibility of artifacts and image distortion than on EPI-DWI scans. Although this sequence in general has a longer acquisition time, the non-EPI technique is preferable to the EPI-DWI technique when scanning the head and neck, including the orbit because of better image quality, both at 1.5 T and 3 T [13, 14]. Advisable is to use at least 3 b values, e.g., b = 0, b = 500 and b = 1000 s/mm2 to calculate reliable ADC values.
Perfusion-Weighted Imaging (PWI)
PWI is a technique that gives insight into the perfusion of tissues by blood. The main technique used in head and neck imaging is the dynamic contrast-enhanced (DCE) perfusion. It relies upon the T1 shortening induced by an intravenously injected gadolinium-based contrast bolus passing through tissue. Before (at baseline) and after injection of the contrast, fast repeated T1 images with a temporal resolution of about 3–4 s are obtained during 4–5 mins and the increase of the signal is measured. The increase of the signal (enhancement) is mainly caused by the accumulation of gadolinium in the extravascular extracellular space. This is dependent on the microvascular characteristics of the tissue, like blood volume, blood flow, permeability, and the availability of the extravascular space. In characterization of an orbital lesion, this can provide more information about angiogenesis and capillary permeability, features related to aggressive behavior, tumor grade, and prognosis [15]. With postprocessing software perfusion characteristics can be analyzed in a specific region of interest (ROI), for example, in an orbital mass. Cystic and hemorrhagic areas should be excluded. The scan can be analyzed in a qualitative way by looking at time-intensity curves (TICs), from which parameters like TTP (time-to-peak), iAUC (initial area under the curve), and washout rates can be calculated. In orbital imaging, three main TIC curves are usually recognized: persistent uptake pattern (mostly benign), plateau pattern (indeterminate), and washout pattern (mostly malignant); the latter can be subdivided into rapid uptake with rapid washout or with slow washout [15, 16]. This simple way of reading the DCE images is informative and easily applied in clinical practice.
Multiparametric Imaging and Future Directions
In multiparametric imaging, the outcomes of different imaging techniques, i.e., CT, anatomic and functional MRI techniques, are combined. The combining of functional characteristics of DWI and PWI additional to pure morphologic features can improve diagnostic accuracy, especially in the evaluation of orbital soft-tissue masses like vascular pathology, inflammatory disease, and neoplasms [16,17,18].
In recent years, an increasing amount of studies were performed on more quantitative analyses of MRI images. Examples of quantitative MRI include T2 mapping of the spin–spin relaxation time and quantifying the amount of edema and fatty infiltration using fat–water imaging with Dixon techniques that can be used to quantify clinical activity in GO [19]. However, the development in quantification of MRI is mainly focused on DWI and PWI parameters.
The frequently used ADC value derived from DWI is a quantitative parameter. However, the mean ADC value obtained from a manually drawn ROI cannot represent the heterogeneity of the whole lesion. Whole-tumor histogram analysis of the ADC maps can generate several diffusion parameters and could be valuable in differentiating orbital tumors [20, 21]. Moreover, ADC values calculated by a conventional monoexponential model cannot separate the pure water molecular diffusion from the water molecular diffusion in capillaries, and this can affect the measurements. Intravoxel incoherent motion (IVIM), based on a biexponential model by using multiple b values, enables quantification of both diffusion and perfusion in a single acquisition without the use of a contrast agent. Recent studies already showed that the true diffusion coefficient (D) and the perfusion fraction (f) might help to improve the characterization of orbital lesions [22, 23].
Quantitative perfusion parameters of PWI can be obtained through pharmacokinetic modeling with the modified Tofts model after assessing the arterial input function (AIF). With this model, perfusion parameters including tumor blood flow (TBF), ktrans (volume transfer constant between the plasma and the extracellular extravascular space), ve (extravascular extracellular volume fraction), and tumor flow residence time τ can be calculated from the DCE series [24,25,26]. However, DCE has the disadvantage that intravenous contrast agent is indispensable to obtain the parameters. Arterial spin labeling (ASL) is an emerging perfusion-weighted MRI technique that allows for absolute tissue perfusion quantification without the use of extrinsic contrast agents [27].
Besides quantification of imaging parameters, the use of postprocessing and planning software will take a leap forward in clinical practice. This will be more extensively discussed in the other chapters. Another promising future direction in diagnostic imaging is the development of AI that probably will play an important role in further improving orbital imaging and decision-making in next decades. An initial study already showed that an AI framework could accurately differentiate orbital cavernous venous malformation from schwannomas [28].
Structural Review of Orbital Imaging
Many different diseases can affect the orbit, and a systematic way to analyze an orbital CT or MRI scan will improve the quality of the review and narrow the differential diagnosis. Orbital lesions should be evaluated in multiple planes, at least in the axial and coronal planes. For some lesions, sagittal planes should be obtained for an optimal evaluation.
A structured review based on the compartment model can be a useful strategy to analyze orbital scans [29]. The first step is to localize the origin of the orbital pathology in one of the orbital compartments, i.e., paranasal sinus, bone, extraconal space, muscle cone, intraconal space, optic nerve, globe or lacrimal fossa. This allows significant reduction in the number of differential diagnoses as these compartments contain different tissues. However, there are also diseases that can present in multiple compartments.
Very important in further narrowing the differential diagnosis is to combine the imaging parameter with the demographic and clinical features of a patients. The quality of the radiological report highly depends on the quality of the clinical information and the questioning of the referring physician [30].
Structured reporting can be useful to improve the communication between radiologist and clinician and to improve the clinical significance of the radiological report [31]. The structured reporting should be tailored to the clinical question, a checklist can be helpful, and radiologist and clinicians could discuss together what is important to be mentioned in the radiological report.
Table 4.1 gives an overview of the preferred imaging techniques, the desired series, planes and a review checklist for most common orbital imaging indications.
Orbital Trauma
Imaging plays a key role in assessing the orbit after facial trauma, evaluating occult orbital injuries, the bony orbit and deep orbital structures, and identifying foreign bodies. High-resolution CT is imaging method of first choice in trauma, and the scan should be reconstructed in different planes and in both bone and soft-tissue reconstructions. Usually, in high-impact trauma, a CT scan of the whole skull is made from which separate brain and facial/orbital reconstructions can be made. On indication, CTA can be performed to asses for dissection, traumatic pseudoaneurysm, or carotid cavernous fistula. Plain radiography has very limited sensitivity in detecting orbital fractures and cannot asses the intraorbital soft tissues. MRI has limited value in the setting of acute trauma, but could be of additional value in complex cases or later in follow-up, especially when traumatic injury of the optic nerve or other soft tissues is suspected.
The first step in reviewing orbital trauma CT is to exclude foreign bodies. Metallic and glass foreign bodies have an increased attenuation and are best delineated on CT. Wooden foreign bodies can have the same attenuation as air, mimicking orbital gas, but within days this attenuation may increase.
When the orbital walls are fractured, the review of the bony structures should address the displaced fragments, the involvement of the orbital apex, and the infraorbital and the optic canal. The soft tissues of the orbit should be evaluated for intraorbital hematoma, fat stranding, and whether there is entrapment of the inferior and/or medial rectus muscles and fat. Also, the form and position of the globe should be evaluated, as well as the position of the lens. Signs of globe rupture include loss of volume and contour and intraocular gas. Retinal detachment is shown as a V-shaped hyperdense configuration, because the retina is relatively fixed at the level of the optic disc on the posterior side and at the ora serrata anteriorly. In choroidal detachment, lentiform hyperdense lesions are seen, not limited anteriorly by the ora serrata.
It is also important to analyze if fractures are limited to the orbit or part of more extensive maxillofacial fractures. Several classification systems and recognizable patterns exist for the imaging evaluation of maxillofacial fractures [32]. Knowledge of these classification systems can be important in effectively describing the fractures and aiding in clinical decision-making. Besides identifying all of the fractures and using the appropriate classification system, the radiologist also needs to recognize injuries that may be associated.
Isolated fractures to the orbital floor and orbital wall are often referred to as blowout fractures (BOF). This mainly involves the medial and/or inferior walls of the orbit and results in displacement of the fracture fragments into the ethmoid sinus, nasal cavity, or maxillary sinus. Indication for surgery is discussed in Chap. 10, but parameters that are taken into account for decision-making are size of the fracture fragments, displacement of orbital contents, like hypoglobus or enophthalmos, and entrapment of the extraocular muscles. On CT, kinking of the medial or inferior rectus muscle is a sign of entrapment. A specific type of BOF is the trapdoor fracture, where the bony fragment recoils back to its original position and entraps the muscle outside the orbital wall (Fig. 4.2). It is mainly seen in children and young adults due to the elasticity of the orbital floor and is considered a surgical emergency as the muscle can become ischemic. “Blow-up” fractures are a rare type of BOF that involve the orbital roof, and they are associated with traumatic intracranial injury. In “blow-in” fractures, the frontal bone is displaced inferiorly, resulting in decreased orbital volume.
Trapdoor fracture of the orbital floor on the right side. Due to repositioning of the fracture fragment in its original position, the inferior rectus muscle is entrapped outside the orbit in the maxillary sinus. This special type of blow-out fracture is mainly seen in children and young adults due to the elasticity of the orbital floor and is considered a surgical emergency as the muscle can become ischemic. Entrapment of the muscle is best seen in the soft-tissue reconstruction
The orbital fractures can also be part of naso-orbital-ethmoid fractures (NOE), zygomaticomaxillary complex fractures (ZMC), transfacial fractures (Le Fort type 2 or 3), or complex midfacial fracture (CMF). NOE fractures involve the nasal bones, the ethmoidal bones, and the medial orbital wall. They can be classified according to Markowitz into type I, with a single fracture fragment; type II, comminuted; and type III, comminuted with the medial canthal tendon disrupted from the bone [33].
In ZMC fractures, there is an isolated fracture of any part of the zygomatic bone, which includes the lateral and inferior orbital rims, the internal lateral orbital wall, the zygomaticomaxillary buttress, and the zygomatic arch [34]. In ZMC fractures, it is important to check what parts of the zygoma are involved, if fracture parts are displaced and if there are associated intraorbital injuries.
The characterizing features of Le Fort fractures are involvement of the maxilla and fracture of the pterygoid plates [35]. In type II and III, the orbit is involved. The type II Le Fort, there is a fracture of the pterygoid plates together with fractures of the inferior orbital rim, the medial orbital rim, and the nasal bone or nasofrontal suture. In this type, the maxilla and nasal regions are mobile from the rest of the face. A type III Le Fort fracture is a fracture of the pterygoid plates plus a fracture of the zygomatic arch, lateral and medial orbital rims, and nasal bones or nasofrontal sutures. In a type III Le Fort fracture, the entire midface is mobile.
Graves Orbitopathy (GO)
Besides the clinical parameters combined in the clinical activity score (CAS), imaging has important additional value in diagnosing and monitoring GO (also referred to as thyroid-associated orbitopathy or thyroid-associated ophthalmopathy) [36, 37]. Imaging also plays a role in the differential diagnosis, when the clinical presentation is atypical. The typical radiological presentation of GO is a bilateral proptosis due to bilateral enlargement of the extraocular muscles, with sparing of the tendons, as well as an increase in the orbital fat volume. Involvement of the extraocular muscles in decreasing order of frequency are the levator palpebrae superioris, the inferior rectus, the medial rectus, the superior rectus, the lateral rectus, and oblique muscles. The order can be memorized by the mnemonic I’M SLOW (except for the levator muscle). The most important differential diagnosis is inflammatory myositis, which often involves the tendinous insertion.
CT has been the preferred imaging method for GO for years. It can measure proptosis, detect muscle enlargement, increased orbital fat volume and lacrimal gland volumes, optic nerve stretching, apical crowding, and remodeling of the bony orbit [38,39,40]. A positive correlation can be seen between active inflammation and the volume and density of the intraorbital fat, EOMs, and lacrimal gland [41, 42]. The measurement of the orbital volume apex crowding index (ratio between soft tissue and orbital fat volume of the apex) and thickening of the medial rectus muscle could help to predict dysthyroid optic neuropathy (DON), a serious complication of GO [43, 44]. It is also shown that it is possible to reliable evaluate these CT characteristics of GO with low-dose CT with an iterative model reconstruction (IMR) algorithm to reduce radiation dose to the ocular lens [45].
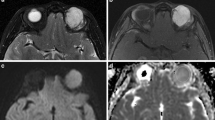
However, CT only provides limited information about disease activity and has limited correlation with the CAS. With MRI, it is possible to gain additional information about disease activity through its ability to reflect the edematous changes in the orbital soft tissues (Fig. 4.3). The STIR technique or T2-WI with fat suppression (i.e., TIRM, Dixon) is most reliable to show soft-tissue edema. The increased signal intensity (ratio) in the EOMs, and to a lesser extent in the orbital fat and lacrimal glands, reflecting edema and inflammation, positively correlate with the CAS and can be used to evaluate medicamentous treatment or to predict worsening of GO [19, 46,47,48,49].
Coronal CT with soft-tissue reconstruction (a) coronal STIR MRI (b) and axial T2-wighted MRI (c) in a patient with Graves’ orbitopathy. On CT, the bilateral thickening of the EOMs is appreciated, and the hyperintense signal on STIR and T2 in the EOMs and orbital fat reflects edema as a sign of active inflammation, which can be relevant additional information. Note the marked proptosis, and the sparing of the tendons, which is typical for GO
Besides signal intensity on the fat-suppressed series, DWI can be useful. An increased ADC value, reflecting increased diffusion is seen in the EOMs and lacrimal glands of active GO compared to inactive disease and healthy subjects [50, 51], and this can be detected even in an early stage of disease [52, 53]. Also, this technique can be helpful in monitoring treatment response [54].
A structural review of an orbital CT or MRI in GO patients should include measurement of the degree of proptosis, of the thickness of the EOMs, the orbital fat and lacrimal gland volumes and protrusion (through volumetric measurement or visual inspection), the degree of optic nerve stretching, and apical crowding. For MRI, additional evaluation of the edema of the EOMs and lacrimal gland should be performed, visually or more quantitative by measuring signal intensity ratios (SIRs) compared to the temporal muscle or with measurement of ADC values.
Orbital Infections
Preseptal cellulitis is mainly a clinical diagnosis that does not need additional imaging. In postseptal orbital cellulitis, however, imaging is often indicated to determine the extent of the disease and to make the decision between conservative and operative management. Imaging can be performed both with postcontrast CT of with MRI. The CT has the advantage that it is very fast and widely available, also besides office hours, thereby making it the preferential imaging method in most cases. CT can also reveal possible underlying sinusitis as the most common cause of orbital cellulitis and guide orbital or sinonasal surgery.
In children and young adults, MRI is the preferred imaging technique when available in orbital cellulitis because there is no ionizing radiation involved. In young kids, this advantage should be weighed against the use of general anesthesia often needed in children below 7 years old. Also, when limited availability of MRI could lead to significant delay, CT should be considered. An alternative strategy could be the use of a recently proposed rapid MRI in children without the use of intravenous contrast or general anesthesia [55]. Because of the superior soft-tissue contrast, it is easier to differentiate suppurative collections from orbital fat infiltration with MRI compared to CT. Especially, the differentiation between true intraorbital abscess from subperiosteal empyema (Fig. 4.4) or intraorbital fat infiltration is important because the first needs surgical intervention, while the other conditions can often be managed with conservative treatment [56]. Adding DWI to the MRI protocol further improves the diagnostic confidence in cases of subperiosteal empyema or orbital abscess, even without the use of intravenous contrast, because the purulent material will show diffusion restriction [57].
Subperiosteal empyema due to infected mucoceles in the left ethmoid in a patient presenting with proptosis. Note the remodeling of the lamina papyracea due to the mucocele (arrow), best recognized on the bone reconstructions (a); the subperiosteal empyema is best visualized on the soft-tissue reconstructions after IV contrast (dashed arrow) (b)
MRI is also more sensitive in detecting possible complications, like superior orbital vein or intracranial (cavernous) sinus thrombosis, epidural empyema, or intracranial abscess [58, 59]. Therefore, in case of clinical presentation with a progressive orbital apex syndrome or neurological impairment, imaging with MRI is preferred. This also applies for rapidly progressive orbital infections in immunocompromised or diabetic patients when invasive fungal infections like angioinvasive aspergillus of rhino-orbital cerebral mucormycosis should be conceded. In these cases, urgent MRI is preferred to CT to precisely determine the extent of infection in surrounding tissues, because of the bad prognosis without aggressive treatment [60, 61].
Orbital Soft-Tissue Lesions
The most important role of imaging in orbital soft-tissue lesions or masses is to appoint the extent of disease and to make a correct (differential) diagnosis. The most common orbital soft-tissue masses include orbital inflammation, vascular or lymphatic malformations (of which the cavernous venous malformation is most common), developmental cysts, benign tumors like schwannoma, optic nerve sheet meningioma, solitary fibrous tumors (mostly benign, but up to 20% can be malignant) or pleomorphic adenoma of the lacrimal gland and malignant tumors like lymphoma, metastasis, and malignant tumors of the lacrimal gland. In children, most common orbital masses are infantile hemangioma, developmental cysts, optic nerve glioma, retinoblastoma, and rhabdomyosarcoma. For the differential diagnosis, radiological findings should be combined with clinical information, laboratory data, and information about other organs that might be involved.
Making the right diagnosis on orbital masses can be challenging, and it was even reported that ophthalmologists and radiologists could only give a correct diagnosis in less than 50% of the cases when compared to histology [62]. Orbital inflammation, for example, has many appearances mimicking other diseases like infection, Graves’ orbitopathy, optic nerve sheet meningioma, lymphoma, or other malignant tumors [63, 64]. Moreover, orbital inflammatory disease can be idiopathic (IOI) or non-specific orbital inflammation (NSOI) or can be related to a systemic inflammatory or granulomatous disease, like rheumatoid arthritis, seronegative spondyloarthropathies, sarcoidosis, IgG4-related ophthalmic disease (IgG4-ROD), or granulomatosis with polyangiitis (GPA). Although there are some distinctive imaging characteristics, it is often not possible to distinguish these different types of inflammation solely based on imaging. With MRI, the extent of the lesion can be determined and this helps to classify orbital inflammation according to the location including diffuse, focal mass-like, anterior, posterior and apical, or more specific if certain orbital structures are involved, like myositis, dacryoadenitis, periscleritis or perineuritis [65].
When the lesion is easily approached, a biopsy can be taken for histology, but it can be essential to have a radiological diagnosis before planning the biopsy or surgery. For apical lesions, it is hard to obtain a biopsy safely, so ophthalmologists could decide to start treatment based on the clinical and radiological diagnosis alone. Moreover, for lesions like pleomorphic adenomas or dermoid cysts, it is essential to know the diagnosis before surgical intervention. Biopsy can be avoided in most of these cases and the lesions should be removed in toto with their capsules to prevent recurrence or chemical inflammation.
CT, anatomic, and functional MRI techniques are often complementary in evaluating an intraorbital mass lesion. Combining different imaging techniques, in so-called multiparametric imaging (Fig. 4.5), showed to improve the diagnostic accuracy of orbital soft-tissue masses [16,17,18].
Multiparametric imaging of a lesion medial in the right orbit, combining parameters from CT (a), T1WI (b), T2WI (c), contrast-enhanced T1 with fat suppression (d), DWI (e) with the ADC map (f), and DCE (g) with the time-intensity curve (h). This lesion shows bone destruction, sclerosis with low signal on T2, homogeneous enhancement, no diffusion restriction (ADC = 1.1 × 10−3 mm2/s), and a persistent uptake on the TIC. This is suggestive of an benign mass, most likely a sclerosing form of orbital inflammation, which was proven by biopsy (histology: sclerosing inflammation, not otherwise specified)
Involvement of the bony orbit is best-evaluated with CT. When the orbital bone is involved, it is important to discriminate bone erosion or destruction from bone remodeling. Destruction is mainly seen in aggressive lesions like malignancy or osteomyelitis, while remodeling is mainly seen in slow-growing lesions like mucoceles, cavernous venous malformations, pleomorphic adenomas, or schwannomas. Osseous destruction is also frequently seen in GPA and is caused by necrosis. With CT, it also possible to appreciate calcifications within a lesion, for example, phleboliths, which are characteristic for low-flow venous malformations. Often, an CT scan is needed for per operative navigation.
The T1-WI, T2-WI, and T1-FS after contrast are used to evaluate the extent of the lesion in six directions and the involvement of the different orbital compartments and structures. Some lesions are characterized by a specific location like optic nerve gliomas originating from the optic nerve, optic nerve sheets meningiomas appearing with the typical ‘tram-track sign” surrounding the nonenhancing optic nerve, pleomorphic adenomas originating from the lacrimal gland, or myositis involving the EOMs. Other lesions, like most inflammatory and vascular of malignant lesions are not restricted to a specific location. Besides the origin and extent of the lesion, also potential extraorbital manifestation, like perineural spread or intracranial extension, should be evaluated with MRI. Further, lesion boundaries are appreciated, discerning well-circumscribed lesions from more infiltrative lesions, as well as potential perilesional edema.
Most of the orbital lesions are T1 iso-intense or hypointense, like in orbital inflammation and orbital tumors. Only lesions that contain fat, such as dermoid cysts (Fig. 4.6), melanin in case of melanotic melanomas, or that contains hemorrhagic components can show T1 hyperintense components.
Dermoid cyst lateral in the left orbit of a child. Note the hyperintense signal on both T2WI (a) and T1WI (b) due to fatty content (arrows) and the extension into the widened frontozygomatic suture (dashed arrow). Due to the typical appearance on MRI, presurgical biopsy can be avoided in most of these cases and the lesions should be removed in toto with their capsules to prevent recurrence or chemical inflammation
On T2-WI, the signal intensity depends on components like edema and fibrosis; edema in case of active inflammation being hyperintense, while fibrosis in case of sclerosing orbital inflammation appearing very hypointense. Inflammatory lesions that show very low signal on T2-WI, implicating extensive fibrosis, will be less responsive to steroid treatment than lesions that show hyperintense T2 signal due to active inflammation. Very low T2 signal can also be seen in IgG4-related orbital inflammation. Fluid-containing lesions like cysts and venous or lymphatic malformations are often very bright on T2, and this is exaggerated when applying fat saturation. Cavernous venous malformations can be recognized by the T2 hypointense pseudo-capsule.
A next step in the approach is to evaluate the enhancement pattern. In general, enhancement will be more homogeneous in case of inflammation and lymphoma than in case of venous malformations, infection, or other malignancies. In well-circumscribed ovoid orbital masses, the enhancement spread pattern over time can be used to differentiate orbital schwannomas, which show start of contrast-enhancement from a wide area, from cavernous venous malformations that demonstrate a heterogeneous moderate enhancement starting from one point or portion within the lesion [66].
DWI helps to distinguish benign from malignant orbital lesions, especially in differentiating orbital inflammatory disease from lymphoma. In general, inflammatory lesions have increased diffusion with high ADC values because of freely diffusible water molecules in the edema, while malignant lesions show more restricted diffusion with lower ADC values because of higher cellular content. Because lymphomas are very cellular tumors, they have low ADC values. A threshold value for ADC of 1.15 × 10−3 mm2/s was proposed to differentiate benign from malignant orbital masses with an accuracy of more than 90% [67]. Another study showed that lesions with an ADC of less than 0.93 × 10−3 mm2/s are likely to be malignant with a 90% probability, while lesions with an ADC value more than 1.35 × 10−3 mm2/s are likely to be benign with more than 90% probability [68].
The addition of PWI to the MRI protocol might further improve the lesion characterization, although there is less evidence for the impact of DCE on the diagnostic accuracy when compared to DWI [17]. While persistent uptake on the time-intensity curve is typical for benign lesions and washout is highly suspicious for malignant lesions, the curve quite often demonstrates a plateau, which is undetermined and not contributing to the differential diagnosis [69]. At least, PWI has proved to be useful in selected cases, i.e., to differentiate the rare orbital solitary fibrous tumors from other well-defined orbital lesions like schwannomas or cavernous vascular malformations due to their typical washout TIC pattern [70, 71].
Lesions of the Bony Orbit
The most common lesions of the orbital walls are related to trauma (discussed in previous paragraph) and related surgery. Other more common lesions are often related to sinonasal disease like mucoceles (Fig. 4.4) and long-standing polyposis with remodeling of the medial orbital wall leading to proptosis or the silent sinus syndrome with depression of the orbital floor leading to enophthalmos due to negative pressure in the maxillary sinus (Fig. 4.7). Also, sinonasal squamous cell carcinoma or esthesioneuroblastoma can involve the orbital walls. Primary lesions of the orbital bone can be classified into congenital lesions (i.e., dermoid, epidermoid), fibro-osseous lesions (i.e., fibrous dysplasia, osteoma, ossifying fibroma), benign tumors (i.e., meningioma, Langerhans cell histiocytosis, hemangioma of the bone, aneurysmal bone cyst, mucoceles), malignant primary (i.e., Ewing or osteosarcoma), and other malignant tumors (i.e., plasmacytoma, lymphoma, metastasis).
Silent sinus syndrome in a patient presenting with painless enophthalmos on the right side due to depression of the orbital floor (arrow). This is caused by a atelectasis of the maxillary sinus walls due to negative sinus pressure in a chronic opacified maxillary sinus. There is lateral displacement of the uncinate process in contact with the lamina papyracea(dashed arrow)
CT is the preferential imaging method in evaluating lesions of the bony orbit, but additional MRI can be desirable in complex cases to evaluate the bone marrow or when adjacent soft tissues are involved. A systematic approach is essential. The first step is to evaluate whether the lesion originates from the bone or whether the lesion arises from the soft tissues with secondary involvement of the bone. When the lesion originates from the bone, the next step is to analyze that the morphology is the lesion well-define or ill-defined, osteolytic, or sclerotic. Other clues are periosteal reaction, bone remodeling, cortical destruction, matrix calcification, and whether lesions are monostotic or polyostotic. Destruction is mainly seen in aggressive lesions like malignancy or osteomyelitis, while remodeling is mainly seen in slow-growing lesions like mucoceles, bone cysts, or fibrous dysplasia. Also, in bone lesions the clinical history and especially the age of the patient are very important for the differential diagnosis. A well-defined lytic lesion in a young child is suggestive of an (epi)dermoid cyst or Langerhans cell histiocytosis, while an ill-defined lytic lesion is suspicious of osteomyelitis, Ewing sarcoma, osteosarcoma, or leukemia. In elderly patients, a lytic lesion is most likely to be metastasis or plasmacytoma/ multiple myeloma.
A lesion where both CT and MRI are indicated is the spheno-orbital or sphenoid wing meningioma. They can be recognized by their typical appearance, centered within the greater sphenoid wing with reactive hyperostosis of the bone, best appreciated with CT, and a soft-tissue component with intraorbital and intracranial extension along the dura, best delineated with MRI (Fig. 4.8). These meningiomas often cause narrowing of the orbital apex due to the hyperostosis or due to the soft-tissue component with involvement of the superior orbital fissure and less frequently the optic nerve canal [72].
Typical example of a spheno-orbital or sphenoid wing meningioma in a middle-aged female, presenting with proptosis of the right eye. Note the additional value of both CT and MRI: the reactive hyperostosis of the sphenoid bone (arrow) is best appreciated with CT (a), while the soft-tissue component with intraorbital and intracranial extension is best reviewed on MRI (b). Narrowing of the orbital apex and optic nerve compression due to the combination of hyperostosis and soft tissue component
Conclusions
Imaging using CT or MRI will provide crucial additional information in the evaluation of many orbital conditions. Because of its superior bony characterization and fast acquisition, CT is the imaging method of first choice in urgent situations like trauma, infection, and when the lesions arise from the orbital wall. CT is also useful in preoperative planning and preoperative navigation. For evaluating complex orbital disease, MRI is the preferred modality. With its superior soft-tissue differentiation, MRI is ideal for determining extent of orbital lesions, including inflammatory disease, orbital vascular lesions, and tumors. By adding functional MRI techniques, like DWI and PWI, and by combining the parameters from different imaging techniques in so-called multiparametric imaging, it is possible to further improve biological characterization of these lesions. A structured way of reviewing the orbital images, knowledge of radiological appearance of most common orbital pathology and combining it with important clinical information is essential to create added value of orbital imaging for patient care.
References
Lanni V, Iuliano A, Fossataro F, Russo C, Uccello G, Tranfa F, et al. The role of ultrasonography in differential diagnosis of orbital lesions. J Ultrasound. 2021;24(1):35–40.
Al Feghali KA, Yeboa DN, Chasen B, Gule MK, Johnson JM, Chung C. The use of (68)Ga-DOTATATE PET/CT in the non-invasive diagnosis of optic nerve sheath meningioma: a case report. Front Oncol. 2018;8:454.
Ambrose J, Hounsfield G. Computerized transverse axial tomography. Br J Radiol. 1973;46(542):148–9.
Prokop M. New challenges in MDCT. Eur Radiol. 2005;15(Suppl 5):E35–45.
Katsura M, Sato J, Akahane M, Kunimatsu A, Abe O. Current and novel techniques for metal artifact reduction at CT: practical guide for radiologists. Radiographics. 2018;38(2):450–61.
Rosado Ingelmo A, Dona Diaz I, Cabanas Moreno R, Moya Quesada MC, Garcia-Aviles C, Garcia Nunez I, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26(3):144–55. quiz 2 p following 55
van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, et al. Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients : recommendations for updated ESUR contrast medium safety committee guidelines. Eur Radiol. 2018;28(7):2856–69.
Albadr FB. Silent sinus syndrome: interesting computed tomography a and magnetic resonance imaging findings. J Clin Imaging Sci. 2020;10:38.
Miracle AC, Mukherji SK. Conebeam CT of the head and neck, part 1: physical principles. AJNR Am J Neuroradiol. 2009;30(6):1088–95.
Krinsky G, Rofsky NM, Weinreb JC. Nonspecificity of short inversion time inversion recovery (STIR) as a technique of fat suppression: pitfalls in image interpretation. AJR Am J Roentgenol. 1996;166(3):523–6.
Simon J, Szumowski J, Totterman S, Kido D, Ekholm S, Wicks A, et al. Fat-suppression MR imaging of the orbit. AJNR Am J Neuroradiol. 1988;9(5):961–8.
Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(Suppl 1):S205–23.
Hirata K, Nakaura T, Okuaki T, Kidoh M, Oda S, Utsunomiya D, et al. Comparison of the image quality of turbo spin echo- and echo-planar diffusion-weighted images of the oral cavity. Medicine (Baltimore). 2018;97(19):e0447.
Feeney C, Lingam RK, Lee V, Rahman F, Nagendran S. Non-EPI-DWI for detection, disease monitoring, and clinical decision-making in thyroid eye disease. AJNR Am J Neuroradiol. 2020;41(8):1466–72.
Jittapiromsak N, Hou P, Liu HL, Sun J, Schiffman JS, Chi TL. Dynamic contrast-enhanced MRI of orbital and anterior visual pathway lesions. Magn R eson Imaging. 2018;51:44–50.
Xu XQ, Hu H, Liu H, Wu JF, Cao P, Shi HB, et al. Benign and malignant orbital lymphoproliferative disorders: differentiating using multiparametric MRI at 3.0T. J Magn Reson Imaging. 2017;45(1):167–76.
Russo C, Strianese D, Perrotta M, Iuliano A, Bernardo R, Romeo V, et al. Multi-parametric magnetic resonance imaging characterization of orbital lesions: a triple blind study. Semin Ophthalmol. 2020;35(2):95–102.
Ro SR, Asbach P, Siebert E, Bertelmann E, Hamm B, Erb-Eigner K. Characterization of orbital masses by multiparametric MRI. Eur J Radiol. 2016;85(2):324–36.
Das T, Roos JCP, Patterson AJ, Graves MJ, Murthy R. T2-relaxation mapping and fat fraction assessment to objectively quantify clinical activity in thyroid eye disease: an initial feasibility study. Eye (Lond). 2019;33(2):235–43.
Ren J, Yuan Y, Wu Y, Tao X. Differentiation of orbital lymphoma and idiopathic orbital inflammatory pseudotumor: combined diagnostic value of conventional MRI and histogram analysis of ADC maps. BMC Med Imaging. 2018;18(1):6.
Xu XQ, Hu H, Su GY, Liu H, Hong XN, Shi HB, et al. Utility of histogram analysis of ADC maps for differentiating orbital tumors. Diagn Interv Radiol. 2016;22(2):161–7.
Jiang H, Wang S, Li Z, Xie L, Wei W, Ma J, et al. Improving diagnostic performance of differentiating ocular adnexal lymphoma and idiopathic orbital inflammation using intravoxel incoherent motion diffusion-weighted MRI. Eur J Radiol. 2020;130:109191.
Lecler A, Duron L, Zmuda M, Zuber K, Berges O, Putterman M, et al. Intravoxel incoherent motion (IVIM) 3 T MRI for orbital lesion characterization. Eur Radiol. 2021;31(1):14–23.
Khalifa F, Soliman A, El-Baz A, Abou El-Ghar M, El-Diasty T, Gimel’farb G, et al. Models and methods for analyzing DCE-MRI: a review. Med Phys. 2014;41(12):124301.
Erb-Eigner K, Asbach P, Ro SR, Haas M, Bertelmann E, Pietsch H, et al. DCE-MR imaging of orbital lesions: diagnostic performance of the tumor flow residence time tau calculated by a multi-compartmental pharmacokinetic tumor model based on individual factors. Acta Radiol. 2019;60(5):643–52.
Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–32.
Eissa L, Abdel Razek AAK, Helmy E. Arterial spin labeling and diffusion-weighted MR imaging: utility in differentiating idiopathic orbital inflammatory pseudotumor from orbital lymphoma. Clin Imaging. 2020;71:63–8.
Bi S, Chen R, Zhang K, Xiang Y, Wang R, Lin H, et al. Differentiate cavernous hemangioma from schwannoma with artificial intelligence (AI). Ann Transl Med. 2020;8(11):710.
Goh PS, Gi MT, Charlton A, Tan C, Gangadhara Sundar JK, Amrith S. Review of orbital imaging. Eur J Radiol. 2008;66(3):387–95.
Castillo C, Steffens T, Sim L, Caffery L. The effect of clinical information on radiology reporting: a systematic review. J Med Radiat Sci. 2021;68(1):60–74.
Ganeshan D, Duong PT, Probyn L, Lenchik L, McArthur TA, Retrouvey M, et al. Structured reporting in radiology. Acad Radiol. 2018;25(1):66–73.
Patel R, Reid RR, Poon CS. Multidetector computed tomography of maxillofacial fractures: the key to high-impact radiological reporting. Semin Ultrasound CT MR. 2012;33(5):410–7.
Markowitz BL, Manson PN, Sargent L, Vander Kolk CA, Yaremchuk M, Glassman D, et al. Management of the medial canthal tendon in nasoethmoid orbital fractures: the importance of the central fragment in classification and treatment. Plast Reconstr Surg. 1991;87(5):843–53.
Kelley P, Hopper R, Gruss J. Evaluation and treatment of zygomatic fractures. Plast Reconstr Surg. 2007;120(7 Suppl 2):5S–15S.
Rhea JT, Novelline RA. How to simplify the CT diagnosis of Le fort fractures. AJR Am J Roentgenol. 2005;184(5):1700–5.
Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73(8):639–44.
Lo C, Ugradar S, Rootman D. Management of graves myopathy: orbital imaging in thyroid-related orbitopathy. J AAPOS. 2018;22(4):256 e1–9.
Nugent RA, Belkin RI, Neigel JM, Rootman J, Robertson WD, Spinelli J, et al. Graves orbitopathy: correlation of CT and clinical findings. Radiology. 1990;177(3):675–82.
Harris MA, Realini T, Hogg JP, Sivak-Callcott JA. CT dimensions of the lacrimal gland in Graves orbitopathy. Ophthalmic Plast Reconstr Surg. 2012;28(1):69–72.
Tan NYQ, Leong YY, Lang SS, Htoon ZM, Young SM, Sundar G. Radiologic parameters of orbital bone remodeling in thyroid eye disease. Invest Ophthalmol Vis Sci. 2017;58(5):2527–33.
Le Moli R, Pluchino A, Muscia V, Regalbuto C, Luciani B, Squatrito S, et al. Graves’ orbitopathy: extraocular muscle/total orbit area ratio is positively related to the clinical activity score. Eur J Ophthalmol. 2012;22(3):301–8.
Byun JS, Moon NJ, Lee JK. Quantitative analysis of orbital soft tissues on computed tomography to assess the activity of thyroid-associated orbitopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(2):413–20.
Goncalves AC, Silva LN, Gebrim EM, Matayoshi S, Monteiro ML. Predicting dysthyroid optic neuropathy using computed tomography volumetric analyses of orbital structures. Clinics (Sao Paulo). 2012;67(8):891–6.
Weis E, Heran MK, Jhamb A, Chan AK, Chiu JP, Hurley MC, et al. Quantitative computed tomographic predictors of compressive optic neuropathy in patients with thyroid orbitopathy: a volumetric analysis. Ophthalmology. 2012;119(10):2174–8.
Lee HJ, Kim J, Kim KW, Lee SK, Yoon JS. Feasibility of a low-dose orbital CT protocol with a knowledge-based iterative model reconstruction algorithm for evaluating Graves’ orbitopathy. Clin Imaging. 2018;51:327–31.
Higashiyama T, Nishida Y, Morino K, Ugi S, Nishio Y, Maegawa H, et al. Use of MRI signal intensity of extraocular muscles to evaluate methylprednisolone pulse therapy in thyroid-associated ophthalmopathy. Jpn J Ophthalmol. 2015;59(2):124–30.
Higashiyama T, Iwasa M, Ohji M. Quantitative analysis of inflammation in orbital fat of thyroid-associated ophthalmopathy using MRI signal intensity. Sci Rep. 2017;7(1):16874.
Chen W, Hu H, Chen HH, Su GY, Yang T, Xu XQ, et al. Utility of T2 mapping in the staging of thyroid-associated ophthalmopathy: efficiency of region of interest selection methods. Acta Radiol. 2020;61(11):1512–9.
Tachibana S, Murakami T, Noguchi H, Noguchi Y, Nakashima A, Ohyabu Y, et al. Orbital magnetic resonance imaging combined with clinical activity score can improve the sensitivity of detection of disease activity and prediction of response to immunosuppressive therapy for Graves’ ophthalmopathy. Endocr J. 2010;57(10):853–61.
Abdel Razek AA, El-Hadidy M, Moawad ME, El-Metwaly N, El-Said AA. Performance of apparent diffusion coefficient of medial and lateral rectus muscles in Graves’ orbitopathy. Neuroradiol J. 2017;30(3):230–4.
Razek AA, El-Hadidy EM, Moawad ME, El-Metwaly N, El-Said AAE. Assessment of lacrimal glands in thyroid eye disease with diffusion-weighted magnetic resonance imaging. Pol J Radiol. 2019;84:e142–e6.
Kilicarslan R, Alkan A, Ilhan MM, Yetis H, Aralasmak A, Tasan E. Graves’ ophthalmopathy: the role of diffusion-weighted imaging in detecting involvement of extraocular muscles in early period of disease. Br J Radiol. 2015;88(1047):20140677.
Politi LS, Godi C, Cammarata G, Ambrosi A, Iadanza A, Lanzi R, et al. Magnetic resonance imaging with diffusion-weighted imaging in the evaluation of thyroid-associated orbitopathy: getting below the tip of the iceberg. Eur Radiol. 2014;24(5):1118–26.
Lingam RK, Mundada P, Lee V. Novel use of non-echo-planar diffusion weighted MRI in monitoring disease activity and treatment response in active Grave’s orbitopathy: an initial observational cohort study. Orbit. 2018;37(5):325–30.
Jain SF, Ishihara R, Wheelock L, Love T, Wang J, Deegan T, et al. Feasibility of rapid magnetic resonance imaging (rMRI) for the emergency evaluation of suspected pediatric orbital cellulitis. J AAPOS. 2020;24(5):289e1–4.
Van der Veer EG, van der Poel NA, de Win MM, Kloos RJ, Saeed P, Mourits MP. True abscess formation is rare in bacterial orbital cellulitis; consequences for treatment. Am J Otolaryngol. 2017;38(2):130–4.
Sepahdari AR, Aakalu VK, Kapur R, Michals EA, Saran N, French A, et al. MRI of orbital cellulitis and orbital abscess: the role of diffusion-weighted imaging. AJR Am J Roentgenol. 2009;193(3):W244–50.
Branson SV, McClintic E, Yeatts RP. Septic cavernous sinus thrombosis associated with orbital cellulitis: a report of 6 cases and review of literature. Ophthalmic Plast Reconstr Surg. 2019;35(3):272–80.
Cumurcu T, Demirel S, Keser S, Bulut T, Cavdar M, Dogan M, et al. Superior ophthalmic vein thrombosis developed after orbital cellulitis. Semin Ophthalmol. 2013;28(2):58–60.
Raab P, Sedlacek L, Buchholz S, Stolle S, Lanfermann H. Imaging patterns of rhino-orbital-cerebral mucormycosis in immunocompromised patients : when to suspect complicated mucormycosis. Clin Neuroradiol. 2017;27(4):469–75.
Thurtell MJ, Chiu AL, Goold LA, Akdal G, Crompton JL, Ahmed R, et al. Neuro-ophthalmology of invasive fungal sinusitis: 14 consecutive patients and a review of the literature. Clin Exp Ophthalmol. 2013;41(6):567–76.
Koukkoulli A, Pilling JD, Patatas K, El-Hindy N, Chang B, Kalantzis G. How accurate is the clinical and radiological evaluation of orbital lesions in comparison to surgical orbital biopsy? Eye (Lond). 2018;32(8):1329–33.
Ferreira TA, Saraiva P, Genders SW, Buchem MV, Luyten GPM, Beenakker JW. CT and MR imaging of orbital inflammation. Neuroradiology. 2018;60(12):1253–66.
Gordon LK. Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye (Lond). 2006;20(10):1196–206.
Yesiltas YS, Gunduz AK. Idiopathic orbital inflammation: review of literature and new advances. Middle East Afr J Ophthalmol. 2018;25(2):71–80.
Tanaka A, Mihara F, Yoshiura T, Togao O, Kuwabara Y, Natori Y, et al. Differentiation of cavernous hemangioma from schwannoma of the orbit: a dynamic MRI study. AJR Am J Roentgenol. 2004;183(6):1799–804.
Razek AA, Elkhamary S, Mousa A. Differentiation between benign and malignant orbital tumors at 3-T diffusion MR-imaging. Neuroradiology. 2011;53(7):517–22.
Sepahdari AR, Politi LS, Aakalu VK, Kim HJ, Razek AA. Diffusion-weighted imaging of orbital masses: multi-institutional data support a 2-ADC threshold model to categorize lesions as benign, malignant, or indeterminate. AJNR Am J Neuroradiol. 2014;35(1):170–5.
Yuan Y, Kuai XP, Chen XS, Tao XF. Assessment of dynamic contrast-enhanced magnetic resonance imaging in the differentiation of malignant from benign orbital masses. Eur J Radiol. 2013;82(9):1506–11.
Yang BT, Wang YZ, Dong JY, Wang XY, Wang ZC. MRI study of solitary fibrous tumor in the orbit. AJR Am J Roentgenol. 2012;199(4):W506–11.
Zhang Z, Shi J, Guo J, Yan F, Fu L, Xian J. Value of MR imaging in differentiation between solitary fibrous tumor and schwannoma in the orbit. AJNR Am J Neuroradiol. 2013;34(5):1067–71.
Kirollos RW. Hyperostosing sphenoid wing meningiomas. Handb Clin Neurol. 2020;170:45–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
de Win, M.M.L. (2023). Imaging of the Orbit: “Current Concepts”. In: Gooris, P.J., Mourits, M.P., Bergsma, J. (eds) Surgery in and around the Orbit. Springer, Cham. https://doi.org/10.1007/978-3-031-40697-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-40697-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-40696-6
Online ISBN: 978-3-031-40697-3
eBook Packages: MedicineMedicine (R0)