Abstract
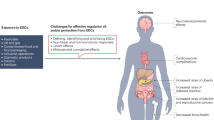
Endocrine-disrupting compounds (EDCs) as emerging pollutants and multi-target agents have accumulated in the environment at concentration levels inducing significant effects on planet and obviously on all living species so that public concern about the impact of EDCs is constantly growing.
Moreover, there are many contaminants in the environment which have never been examined. Even low-level exposure to these chemicals can have significant effects, and the same dose response can have different effects on individuals. Furthermore, the cumulative effects of these chemicals are yet to be studied, thus the effect on human beings is not fully understood. Anyway the health consequences of these chemicals have been particularly studied on reproductive system. Male reproductive health, especially, has represented ideal target for analysing the effects and mechanisms of damage to health of these chemical compounds. This field of health is, indeed, critical for the future of society, not only for interdisciplinary approach of several specialists and institutions involved but also for the educational mission of new generations especially in the vulnerable adolescent period; a mission, about lifestyle, diet, behaviour, personal and social awareness to reduce the exposure to EDCs and prevent non-communicable diseases (NCDs). In this chapter, we will discuss policy Implication and Community Interventions to reduce EDCs Exposure for minimisation health damages in the frame of more recent knowledge on these contaminants and proposing how hazard-based approach to guide and reach the regulations should be preferred to the risk-based one. This approach is particularly important to safeguard the male and female reproductive system, which is the most exposed one to environmental stress.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Endocrine-disrupting compounds (EDCs)
- Environmental exposure
- Reproductive health
- Male fertility
- Female fertility
- Human semen
- Sperm decline
- Sentinel organ system
- Policy management
- European Chemicals Agency (ECHA)
- Registration
- Evaluation
- Authorisation and Restriction of Chemicals (REACH)
- Plant Protection Products Regulation (PPPR)
- Biocidal Products Regulation (BPR)
- Policy management
- Hazard risk
10.1 Introduction
The group of molecules identified as endocrine disruptors (EDCs) is highly heterogeneous and includes synthetic chemicals used as industrial solvents/lubricants and their by-products, such as plastic compounds, plasticizers, pesticides, pharmaceutical agents, heavy metals, phthalates, bisphenol A, flame retardants, alkyl phenols, dioxins and polycyclic aromatic hydrocarbons have been identified as endocrine disruptors [1]. Currently, an endocrine disruptor (ED) assessment list is available at the European Chemicals Agency (ECHA) and includes chemicals undergoing an ED assessment under Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) or the Biocidal Products Regulation. These substances are listed on the ECHA website updated on 29 April 2022 for discussion to ECHA’s ED expert group [2].
Endocrine disruptor, term first introduced in 1991 [3], is defined as “an exogenous chemical, or mixture of chemicals, that can interfere with any aspect of endogenous hormone action” [4, 5]. These chemicals can bind to the body’s endocrine receptors to activate, block or alter natural hormone synthesis and degradation, which occur through a plethora of mechanisms resulting in “false” lack or abnormal hormonal signals that can increase or inhibit normal endocrine function [6]. These chemicals are also classified as emerging pollutants because they can be detected in the order of nanograms to micrograms (ng/L and μg/L) using gas chromatography with mass spectroscopy and high-performance liquid chromatography with mass spectroscopy; other methods, like enzyme-linked immune-sorbent assay, emergent new biological methods through biosensors, are also currently widely used [7]. EDCs group can be derivate from anthropogenic activities (synthetic endocrine disruptors) and from natural origins (natural endocrine disruptors [7]. An example of this classification of some EDCs is shown in Fig. 10.1.
In April 2016, the German Federal Institute for Risk Assessment (BfR) reached a consensus about the development of EDCs criteria in relevant EU legislation during a meeting [8]. Data from ecological studies, animal models, clinical observations in humans and epidemiological studies agree to consider endocrine-disrupting chemicals (EDCs) as a significant for wildlife and human health [9, 10].
EDCs are widespread in the environment, and the increase of non-communicable diseases (NCDs), such as cancer, diabetes, obesity, cognition deficit and neurodegenerative diseases, endometriosis, polycystic ovarian syndrome, early puberty, thyroid dysfunction, heart diseases and infertility, has all been linked to these substances exposure with costs in the hundreds of billions of Euros per year [11]. In particular, the exponential increase of cancer and metabolic disease, as well as obesity and diabetes worldwide, correlates with the widespread use of these substances and the costs in relation to morbidity and mortality are enormous [12,13,14,15,16,17,18,19,20,21,22,23].
The scientific research in the last three decades has solidified the knowledge of these chemicals and have been known the transgenerational effects through uterine exposure, for disease [24, 25].
However, the perturbative effects of EDCs on endogenous hormones, historically, have been focused on the reproductive system. In fact, already for some pesticides (thiocarbamates, chlororganics, imidazoles, triazoles and triazines), which determine an antiandrogenic action highlighted by the macroscopic sexual changes found in aquatic animals (particularly because of exposure to herbicides and fungicides) such as the demascolinization in rats and fish [26] and the production of estrogens and hermaphroditism in frogs [27] and other developmental disorder of the male gonad in alligators [28]. Certainly, the largest group of these substances accumulates in tissues and in the environment [29,30,31,32].
These substances cause an antiandrogenic effect in humans too, but they also mimic the estrogenic action, confirmed by both experiments in vivo and in vitro [33,34,35,36,37,38,39,40,41].
The great attention to the reproductive system underlines how it can represent a sentinel organ to environmental stresses, and the epidemiological and clinical data available today, in particular on male infertility, seem to confirm this sensitivity.
There is evidence that semen quality has declined in the last decades, and the incidence of male infertility has increased steadily in many countries [42,43,44]. An important decreasing trend has already been described for sperm concentration from 113 × 106/mL in 1940 to 66 × 106 in 1990 [45] and the same for testosterone levels [46, 47]. According to Levine, total sperm count had fallen by 59.3%/escalate between 1971 and 2011 in Europe, North America, Australia and New Zealand and sperm concentration/mL fell by 52.4% [48]. Other sperm decline is has reported also in China from 2001 to 2015 [49] in Africa, India, Brasil and Iran [50]. Changes in sperm production, initially thought to be due to maternal exposure to environmental oestrogens, corresponds precisely to the introduction of chemicals especially after 1940 [51], but with the most knowledge on experimental data, these effects seem to be due to different types of endocrine-disrupting chemicals (EDCs) [52, 53].
EDCs are now ubiquitous in the environment and their effects do not end with the exposed individual but are transmitted to future generation trough epigenetic changes to the germline, as reported in several studies [54,55,56,57,58,59,60,61,62,63].
However, if changes in behavioural factors and lifestyles, including the introduction and rapid growth of cell phones’ use, the large increase in the consumption of opiates and marijuana, the increase in the consumption of cigarettes and increasing physical inactivity, may have potentially induced alterations in seminal parameters and thus reduce male fertility [64], environmental and chemical contaminants in the workplace are recognized as major risk factors especially for male infertility in both epidemiological and experimental studies [65,66,67,68,69,70].
The incidence of genitourinary tract malformations and reduced sperm quality is indeed higher in people living in areas with a high rate of pollution or in individuals exposed to EDCs for professional reasons. More strikingly, especially in industrialized countries, the reduction of semen quality and/or semen count present differences in areas in the same country or even in the same region, supporting the idea that environmental factors, present in some areas but not in others, may be responsible for the decline in semen quality and sperm count [71,72,73,74,75,76,77,78,79,80,81,82,83,84]. Furthermore, different studies have reported that in high environmental pressure areas there is an increase of infertility, urogenital malformation and chronic disease (cancer, diabetes, etc.) [11, 77,78,79,80,81,82,83,84,85,86,87,88].
These epidemiological data are important to understand the shared biological mechanisms mediated by contaminants. There are therefore several evidences that show how ubiquitous presence of chemicals in the environment and in food is actually the root cause of increased health reproductive problems, especially for the reduction of semen quality, and the increased incidence in recent years, even of testicular dyskinesias, induce to believe that harmful environmental factors can have a much more important role than people think.
However, for ethical reasons, it is difficult to establish a causal relationship on human beings. Clinically, the most common manifestation of contaminants is a reduced sperm concentration, while its most severe form can include an increased risk of testicular cancer [89].
Associating both environmental data and chemical factors of exposure to the data found in the body, as well as verifying the consistency and the determinism or order of passage from the environment to the organism, is a crucial step for a better understanding of the environment-health relationship. In light of this, the male reproductive system is sensitive to a broad variety of environmental pollutants; therefore, it represents an optimal model for the study of environmental health. Spermatogenesis from puberty onward is continuously exposed to insults at the stages of continuous replication; as a consequence the male germline accumulates mutations [90, 91]. Sperm cells are more susceptible than eggs to the effects of oxidative damage, because they lack significant antioxidant protection because of reductive cytoplasmic space for an appropriate armoury of defensive enzymes and significant amounts of polyunsaturated fatty acids [92]. Simultaneously, in semen it is possible to measure environmental contaminants and in vivo effects on sperm cells, which are readily available, with features sensitive to environmental pollutants such as motility, morphology and the integrity of the DNA strand. If human semen seems an earlier and sensitive source of biomarkers than blood in monitoring high environmental pressure on human health, therefore it can be considered a reliable environmental sentinel [77, 78, 93]. More evidence in literature indicates the human semen as an important health marker. In fact the spermatogenesis cycle is extremely complex and vulnerable to endogenous and exogenous stress, so it is not surprising that it can be an important indicator of the state of well-being of the organism. Recent studies have demonstrated the association between semen quality and state of health [94,95,96], correlating the former with chronic degenerative diseases.
As a matter of fact, male infertility is becoming a public health priority, and it’s also related to an increased risk of later onset adult diseases, especially cancer [11, 97,98,99,100,101] not only testicular cancer [102,103,104], medical comorbidity [105], shorter life expectancy [106] and trans-generational effects [107, 108].
In this prospective, fertility assessment, sperm may be an indicator of overall health and the attention on maximum fertility age (18–35 years) can be important for chronic diseases prevention. In addition to the potential preventive and predictive role of reproductive biomarkers for chronic adult degenerative diseases, the growing interest on the transgenerational effects induced by pollution and lifestyles through epigenetic modifications on gametes shifts the interest of prevention as far as preconception; therefore, the interest for the reproductive system and biomarkers assumes a greater significance for safeguarding the health of future communities [107, 109].
However, given that a healthy environment and the mother’s lifestyle are crucial for the offspring’s health, and the utero window represents a field of study of Developmental Origins of Health and Disease (DOHaD) [110, 111], the Paternal Origins of Health and Disease paradigm (POHaD) should be taken into serious consideration [112,113,114,115,116,117,118]. Nevertheless, in spite of having few epidemiological studies on humans, the perspective opening the systematic study of reproductive biomarkers in environmental impact assessment and early and predictive health risk assessment is enormous [107].
Considering both the great impact of EDCs on the environment and health system and the need to protect and reduce their impact, policy implication and community interventions are mandatory. In this sense, the following chapters will point out the most important plan directions of principal public institutions in the frame of more recent knowledge on these contaminants.
10.2 Europe Police Priorities on EDCs: REACH Regulation, European Plant Protection Products Regulation (PPPR), Biocidal Products Regulation (BPR)
In Europe, general future priorities for protecting humans and wildlife from adverse effects of EDCs were reported at the Weybridge meeting in 1996 [119] and later in 1999 [120] a community strategy for EDCs was adopted. This was a fundamental step for addressing a regulatory basis on health and environmental effects caused by EDCs. European policy embraces the precautionary principle aimed at limiting exposure to agents harmful to humans, animals and the environment even in the absence of scientific certainties. The precautionary principle in the rules of the UE gives particular attention in the comparison of the danger of exposure of the fetus in utero and in its subsequent development. The EDC strategy, indeed, was characterized by a series of actions for monitoring programs and estimating exposure and effects of EDCs, to define and check testing methods and other actions for research on EDCs and consequently to develop regulatory actions. All instruments adopted for long-term actions under ED research group of European Union (EU), for example the 7th Environment Action Programs (EAPs) [121] were meant to protect all living species in EU. In 2006, REACH, officially Regulation No. 1907/2006, a regulation of the European Union, dated 18 December 2006, concerning the registration, validation, authorization and restriction of chemical substances [122], defines EDCs as substances of very high concern (SVHCs) for both health and environment, in order to reduce their use and replace them with other safer substances. Before 2007, EDCs had been considered responsible for the development of reproductive problems and cancer lesions, and therefore regulated. Currently, the EDCs risk assessment is specifically applied in the context of chemical classes in use. REACH requires companies to register substances and give data for ensuring safe handling; if the chemical is identified as an SVHC, it is included in a list of restricted chemicals under consideration of REACH for possible authorization. The European Chemicals Agency (ECHA), indeed, evaluates in first instance the chemicals included in the Authorization List for allowing their entry into the market after evaluation on the basis of article 57 of REACH regulation, which refers to the toxicity, carcinogenic bio accumulative, environment persistence properties and possibility to be replaced with safer alternatives.
The European Plant Protection Products Regulation (PPPR) on EDCs [123], although approved after REACH regulation, it was the first (EU) to take into account health effects and non-target organisms, evaluating the substances at mutagenic, carcinogenetic or toxic level on the basis of the regulatory system of classification, labelling and packaging (CLP). Nevertheless, the PPPR does not contain indications to define a substance with endocrine-interfering properties, consequently it referred with amendments to the regulation based on the WHO IPCS regarding the definition of "endocrine disorder" and "adverse health" (Table 10.1) [124]. Two derogations to the PPPR on EDCs were applied, but no agreement has been reached on the handling of these derogations until now (Derogations: 1. the necessity for an active substance to control a serious danger to plant health (Article 4, paragraph 7). 2: negligible exposure towards an active substance, safener or synergist (Annex II, point 3.6.5) [125]
In 2009, Plant Protection Products Regulation banned EDCs in pesticides and in the 2018 the European Food Safety Authority and European Chemical Agency produce a guideline for identifying EDCs present in pesticides.
The Biocidal Products Regulation (Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products) [126], has been the second European Regulation on substances used in biocidal products and has been applicable since 4 June 2018 (European Commission, Commission Delegated Regulation (EU) 2017/2100 of 4 September 2017 setting out scientific criteria for the determination of endocrine-disrupting properties pursuant to Regulation (EU) No 528/2012 of the European Parliament and Council, 2018) [127]. The most important difference compared to PPPR is that, in case of a substance having endocrine-disrupting properties, the application is approved for 5 years only and a product containing them it cannot be authorized for public use.
For medical and in vitro diagnostics devices, an EU regulation consolidated data on 24 April 2020 (Consolidated text: Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA relevance) [128] and individualized only substances that in contact with the body pass to higher concentrations 0.1% weight by weight and have a justified endocrine interference property. These findings have been reported and discarded. A consolidated text of EU regulation for in vitro diagnostic devices on 28 February 2022 (Consolidated text: Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU (Text with EEA relevance) [129], labelling the presence of endocrine interfering properties must be mandatory. For cosmetics, in the 2009 regulation, there are no reported endocrine interfering properties, although the problem was later addressed in 2018 leading to a commitment of the ED commission to define these ED properties and possibly limit or in any case avoid their use. In the light of this, REACH regulation should also address the environmental matter.
In order to release these substances in contact with food, although Regulation (EC) No. 1935/2004 does not generally allow it the release, much is handed over postponed to national laws; however, greater attention is paid to the release of plastic material, which is absolutely not allowed to be used in children’s food; the same level of Bisphenol A release was reduced from 0.6 mg kg−1 food to 0.05 mg kg−1 food (Commission Regulation (EU) No 10/2011 of 14 January 2011, on plastic materials and articles intended to come into contact with food). Bundesinstitut fu¨r Risikobewertung (BfR), Database BfR Recommendations on Food Contact Materials) [130].
The Water Framework Directive (WFD) 2000/60/EC regulate known or suspected EDCs in water. In fact there are substances that are detected and that have already been banned from the market for some time. In 2020, 205 substances were included in the substances of very high concern (SVHC) list, 16 of these substances were included due to their endocrine-disrupting characteristics, currently these are included in a list of 45 chemical substances. Another list of substances including pharmaceuticals are monitored and under evaluation for health and environmental risk substances.
10.3 Other EDCs Regulations
EDCs have been also identified as an emerging policy issue by the UN Environment Programme (UNEP), which oversees global policy through strategic alliance for International Chemical Management. In 2015, the alliance welcomed the 2012 WHO and UNEP State of the Science report on EDCs [131]. In this report, tests exclusively focus on the estrogen, androgen and thyroid pathways [132] and do not take into account neither other receptors nor many other receptors mechanisms of action [133]. In 2017, UNEP identified 28 Policy actions, worldwide characterized by the variability to regulate the use of hazardous EDCs [134].
Currently, the various existing regulations share an agreement to limit a subset of persistent organic pollutants, including many EDCs. At the same time, the USA do not take into account the agreement and continue to market chemicals that the agreement has banned.
10.4 Economic Burden
The Food and Drug Administration has identified more than 1800 chemicals; today, medical societies and governmental agencies are experiencing an increase in health problems created by the action of EDCs and their effect seems to be transgenerational, occurring over at least two or three generations.
Considering nutrition is the main route of exposure to EDCs in human beings, we are currently witnessing a global and massive process of orientation and categorization of food consumption, through customs and lifestyles. This widespread exposure to EDCs and their consequent pathologies are diversified not only by gender characteristics but also by different age groups as well as economic income.
As previously stated, the most common way one comes into contact to EDCs is through food; however, people who work using these chemicals are exposed to them daily. EDCs can have long term and severe adverse effects on unborn babies’ hormone system too, as shown over the last generation. The next generation will experience the same, or worse, if no future regulatory strategy for prevention is adopted. Indeed, the presence of EDCs in the environment and people’s daily habits can expose the unborn child, from the embryonic stage onward, to these chemicals through feta–placental contact with the mother. The severity of the pathologies created range from pathologies of the male and female reproductive systems, to diabetes, cancer and diseases that affect the cognitive system by conditioning the level of IQ [135,136,137,138].
The Global Burden of Disease project uses an approach that calculates disability-adjusted life-year (DALY) [139], thanks to which the costs [140] of intellectual diseases and disabilities are also assessed. Nevertheless, due to its complexity, the DALY system remains insufficient to evaluate the intellectual damage to the human being [141].
Recently, several assessments have been carried out on the costs of diseases and damage caused by exposure to EDCs, such as neurobehavioural defects, disorder of the male and female reproductive systems, obesity and diabetes [135,136,137,138]. The economic burden results in 163 billion euro for EU, and 340 billion dollars for the USA annually, which means that not only this evaluation has been underestimated but also that only a few EDCs and their effects on health have been investigated [142,143,144].
It is therefore clear that a regulation aimed at identifying EDCs facilitates their replacement and prevents exposure by informing the population on how to avoid EDCs.
10.5 Limiting Exposure to EDCs
To give a clear indication for the hoped regulations is to promote the EDCs definition given by Endocrine Society as “any chemical or mixture of chemicals that interferes with any aspect of hormone action” [4]. This definition has the possibility to be applied anywhere globally: in all sectors of the economy and jurisdictions of the world. It also has the clear potential to deeply analyze what the damage is to the general population and to the specific exposed workers. It has the ability to calculate what the costs of treating the diseases are and the inconveniences caused to humankind, surrounding nature and the environment.
Differently, the definition given by WHO specifically requires an adverse effect to be documented [6, 145, 146]. Pointing out an adverse effect to define an EDC is problematic because regulatory agencies often disagree on which outcomes are adverse [147].
Perhaps, the time has come to consider EDCs among global burden diseases; this assessment becomes more evident if we consider the health costs paid by people exposed to EDCs and the costs that are paid by communities to treat the diseases [148].
We are realizing day by day that the population’s exposure to EDCs creates pathologies involving the female and male reproductive system, which reduce their abilities and also, in some minority groups of the population [149], create disabilities, including neurocognitive ones [138].
We should avoid searching with great difficulty the critical levels of tolerability as a risk-based approach assumes [150]. Furthermore, many EDCs can act at low concentrations and often present non-linear dose–responses; these properties represent a regulatory challenge.
Indeed, for several EDCs, it’s not possible to evaluate a safe threshold for the toxicity, especially for neuro developmental deficits. In this regard, in a Danish report, the aim of obtaining an additional and pragmatic choice for the evaluation of safety was discussed [151].
Differently, in a hazard-based approach, once identified the hazardous properties of a chemical, it becomes sufficient to prohibit market use regardless of the exposure and economic cost. It is difficult to imagine that the regulation of an EDC must wait for full-blown pathological effect instead of preventing it. If we consider pathologies such as cancer, diabetes, or all malfunctions of the male and female reproductive apparatus and neurocognitive problems, it becomes very complicated to accept a logic that exposes humans and the surrounding environment to suffer inert the snare of EDCs.
10.6 Policy Management and Recommendations
We need regulatory recommendation of EDCs, pointing on their identification, a policy geared to monitor and reduce exposure to better protect humans and the environmental health.
The first recommendation is on the identification of EDCs. This is the necessary basis for an effective action.
The Endocrine Disruptor Screening and Testing Advisory Committee [152] promotes evaluation of estrogen, androgen and thyroid receptor disruptions.
Despite these clear indications, in the USA, regulation requires testing for estrogen only for few materials in use.
To obtain better EDCs identification, we would probably need more sensitive tests to some EDCs and clarify more pathways for each estrogen, androgen and thyroid receptor.
For thyroid axis, identification disruptors are scarce, and, for many pathways, they are completely absent. Furthermore, we need to modify some test modalities for disruptor identification, which could give misleading results, for example, the changes in uterine weight test under high concentrations of estrogenic EDCs [153, 154]. At the same time, to this day, a whole series of capable tests for a broader number of nuclear receptors, and other receptor types, is available, which is also capable of assessing several mechanisms of action for EDCs [155]. This capable test should be inserted in regulatory requirement after validation. The Organisation for Economic Cooperation and Development guidance provides new assays to test more pathways than those required from EU and USA regulation.
As soon as possible, we need tests to identify EDCs involved in adipocyte development, steroidogenesis and all reproductive functions to begin from spermatogenesis and other female reproductive system pathologies or dysfunctions (e.g. endometriosis, polycystic ovary syndrome).
Human diseases should become the first-tier management to identify EDCs and their adverse impact [156]. The difficulty to recognize the effects induced by EDCs is found in the regulations that encourages just in vivo observation. This means that we need to use vertebrate animals or epidemiological evidence, but the regulatory authorities have proposed strong limitations to use mammals for regulatory testing and at the same time we do not have sufficient in vitro guidelines for this shortcoming. Likewise, the non-mammalian vertebrate and invertebrate models have not the possibility to overcome the shortcoming information.
We need more typical mammalian models (egg rodents) evidence to validate in vitro testing and have the possibility to examine complex effect on health.
All this limitation takes risk of EDCs identification. A determination of adverse effect should be sufficient for identification as an EDC and subsequent regulation.
Obviously, the search for already tested chemical safer substitutes should be supported and developed by different governments. Before chemicals get into the market to be used by the population, they should be tested in vitro and in vivo in order to give a guarantee not to alter the state of human health and the surrounding environment. Unfortunately, some substitutions used up to now for polybrominated diphenyl or BPA remain questionable and dangerous in other pathways. Another recommendation concerns the exposure to EDCs. Once identified, an EDC should be excluded from the possibility of human exposure. A risk-based regulatory approach currently prevails in which the effects are evaluated on the basis of exposure degree. This means that we need a specific monitoring for each single EDC conjugated to each environmental reality, economic development system lifestyle and profession.
It is essential that decision makers know how chemicals are being used.
Information campaigns could be organized for the general population or population particularly exposed to EDCs. An action of this dimension could change exposure levels by informing and raising awareness of how to avoid the possible and different contact with individual EDCs and defend health from the sneaky snare of EDCs.
10.7 Conclusion
EDC policies are justified on economic grounds and to further environmental justice.
The endocrine disruptor is constantly expanding its range of influence on the globe population. We are currently witnessing what’s happening in the surrounding environment.
On the scientific level that works on the identification of chemical substances with altering characteristics of the functional systems of living organisms, it is necessary to expand the possibilities of directly investigating altered processes in vivo on animal models. The hazard-based approach, meant to guide and reach the regulations, should be preferred to the risk-based one.
The health risks are considerable as the social costs associated with poor health conditions are, in this sense, representative signs are emerging both in the number of women and men involved and in the developed dysfunctions.
In particular, since it is better measurable, great evidence of how much human activities are seriously impacting the quality of the planet, and, consequently, of human health, can be precisely deduced from the progressive semen quality decline, which probably represents the impact best. The reproductive system, in particular the male one, can be represented as a “Sentinel Organ System” due to its extreme sensitivity to environmental stress; recent evidence indicates human semen as an early and sensitive source of exposure, therefore a useful tool for measuring the presence and effects of chemical substances not only on the classic seminal parameters (number, motility, morphology and DNA sperm damage) but also on others that are now better studied in vivo with molecular biology techniques. Exposure assessment, in conjunction with information on the inherent toxicity of the chemical (i.e. the expected response at a given level of exposure), plays a key role in predicting the likelihood, nature and magnitude of adverse health effects. The use of reproductive biomarkers for early risk detection represents a possible new methodological approach where they could be exploited as early indicators of environmental toxicity and enhanced risk of chronic adverse effects not only for reproductive health. In particular, human semen as an early and reliable source of biomarkers, giving information on biologically active exposures, can be very useful for preventive health surveillance programs, especially in environmental risk areas. This approach appears very promising, above all, in young people (maximum fertile age:18–35 years), considering the possibility to reduce the chronic-degenerative diseases in future adults. In this context, many scientific findings regard the association between pollution and fertility problems. Therefore, the safeguard of germ cells is a new challenge to reduce the burden of epigenetically transmitted diseases.
References
Yilmaz B, Terecia H, Sandal S, Kelestimur F. Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev Endocr Metab Disord. 2020;21(1):127–47. https://doi.org/10.1007/s11154-019-09521-z.
ECHA. Endocrine disruptor assessment list of European Chemical Agency (ECHA). endocrine disruptor assessment list. 2023. https://echa.europa.eu/it/ed-assessment.
Colborn T, Vom Saal FS, Soto AM. Review developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101(5):378–84.
Gore AC, Chappell VA, Fenton SE, et al. EDC-2: the Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr Rev. 2015;36:e1–150.
Solecki R, Kortenkamp A, Bergman Å, Chahoud I, Degen GH, Dietrich D, Greim H, Håkansson H, Hass U, Husoy T, Jacobs M, Jobling S, Mantovani A, Marx-Stoelting P, Piersma A, Ritz V, Slama R, Stahlmann R, Martin van den Berg R, Zoeller T, Boobis AR. Scientific principles for the identification of endocrine-disrupting chemicals: a consensus statement. Arch Toxicol. 2017;91(2):1001–6. https://doi.org/10.1007/s00204-016-1866-9.
WHO. State of the science of endocrine disrupting chemicals. http://www.who.int/ceh/publications/endocrine/en/. Accessed 20 Nov 2020.
Pironti C, Ricciardi M, Proto A, Bianco PM, Montano L, Motta O. Endocrine-disrupting compounds: an overview on their occurrence in the aquatic environment and human exposure. Water. 2021;13(10):1347. https://doi.org/10.3390/w13101347.
Myers JP, Guillette LJ Jr, Palanza P, Parmigiani S, Swan SH, Vom Saal FS. The emerging science of endocrine disruption. In: Ragaini RC, editor. International Seminars on Planetary Emergencies, 30th Session. London: World Scientific Publishing; 2004. p. 105–12.
Heindel JJ, vom Saal FS, Blumberg B, Bovolin P, Calamandrei G, Ceresini G, Cohn BA, Fabbri E, Gioiosa L, Kassotis C, et al. Parma consensus statement on metabolic disruptors. Environ Health. 2015;14:5.
Trasande L, Zoeller RT, Hass U, Kortenkamp A, Grandjean P, Myers JP, DiGangi J, Bellanger M, Hauser R, Legler J, Skakkebaek NE, Heindel JJ. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European union. J Clin Endocrinol Metab. 2015;100(4):1245–55. https://doi.org/10.1210/jc.2014-4324.
Jagai JS, Messer LC, Rappazzo KM, Gray CL, Grabich SC, Lobdell DT. County-level cumulative environmental quality associated with cancer incidence. Cancer. 2017;123(15):2901–8. https://doi.org/10.1002/cncr.30709.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2014;63:11–30.
National Heart, Lung, and Blood Institute. Fact book: fiscal year 2008. Bethesda, MD: National Institutes of Health; 2009.
Tomasetti C, Vogelstein B, Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347:78–81.
Wallace TA, Martin DN, Ambs S. Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis. 2011;32:1107–21.
Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529:43–7.
Papalou O, Kandaraki EA, Papadakis G, Diamanti-Kandarakis E. Endocrine disrupting chemicals: an occult mediator of metabolic disease. Front Endocrinol. 2019;10:112. https://doi.org/10.3389/fendo.2019.00112. eCollection 2019.
Ogden CL, Carroll MD, Kit BK, Flegal KMJAMA. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–14.
International Diabetes Federation. IDF Diabetes Atlas. 8th ed; 2017. https://www.idf.org/e-library/epidemiology-research/diabetes-atlas.html
Lehnert T, Sonntag D, Konnopka A, Riedel-Heller S, Konig HH. Economic costs of overweight and obesity. Best Pract Res Clin Endocrinol Metab. 2013;27:105–15. https://doi.org/10.1016/j.beem.2013.01.002.
Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Int Med. 2014;174:251–8. https://doi.org/10.1001/jamainternmed.2013.129569.
Anneet B, Raphael R. Endocrine disruptor chemicals. In: Endotext. South Dartmouth (MA): MDText.com, Inc; 2000.
American Diabetes. Economic costs of diabetes in the US. in 2017. Diabetes Care. 2018;41:917–28. https://doi.org/10.2337/dci18-0007.
Predieri B, Alves CAD, Iughetti L. New insights on the effects of endocrine-disrupting chemicals on children. J Pediatr. 2022;98(Suppl 1):S73–85. https://doi.org/10.1016/j.jped.2021.11.003.
Johnson RA, Harris RE, Wilke RA. Are pesticides really endocrine disruptors? WMJ. 2000;99(8):34–8.
Guillette LJ Jr. Organochlorine pesticides as endocrine disruptors in wildlife. Cent Eur J Public Health. 2000;8:34–544.
Guillette LJ, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994;102(8):680–8.
Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci U S A. 2002;99(8):5476–80.
Hayes TB, Anderson LL, Beasley VR, de Solla SR, Iguchi T, Ingraham H, Kestemont P, Kniewald J, Kniewald Z, Langlois VS, Luque EH, McCoy KA, Muñoz-de-Toro M, Oka T, Oliveira CA, Orton F, Ruby S, Suzawa M, Tavera-Mendoza LE, Trudeau VL, Victor-Costa AB, Willingham E. Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes. J Steroid Biochem Mol Biol. 2011;127(1–2):64–73. https://doi.org/10.1016/j.jsbmb.2011.03.015. Epub 2011 Mar 23.
Grilo TF, Rosa R. Intersexuality in aquatic invertebrates: prevalence and causes. Sci Total Environ. 2017;592:714–28. https://doi.org/10.1016/j.scitotenv.2017.02.099. Epub 2017 Mar 18.
Baatrup E, Junge M. Antiandrogenic pesticides disrupt sexual characteristics in the adult male guppy Poecilia reticulate. Environ Health Perspect. 2001;109(10):1063–70.
Singleton DW, Khan SA. Xenoestrogen exposure and mechanisms of endocrine disruption. Front Biosci. 2003;1(8):S110–8.
Akingbemi BT, Hardy MP. Oestrogenic and antiandrogenic chemicals in the environment: effects on male reproductive health. Ann Med. 2001;33(6):391–403.
Sultan C, Balaguer P, Terouanne B, Georget V, Paris F, Jeandel C, Lumbroso S, Nicolas J. Environmental xenoestrogens, antiandrogens and disorders of male sexual differentiation. Mol Cell Endocrinol. 2001;178(1–2):99–105.
Hodges LC, Bergerson JS, Hunter DS, Walker CL. Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol Sci. 2000;54(2):355–64.
Chrustek A, Hołyńska-Iwan I, Dziembowska I, Bogusiewicz J, Wróblewski M, Cwynar A, Olszewska-Słonina D. Current research on the safety of Pyrethroids used as insecticides. Medicina (Kaunas). 2018;54(4):E61. https://doi.org/10.3390/medicina54040061.
Garey J, Wolff MS. Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochem Biophys Res Commun. 1998;251(3):855–9.
Bell EM, Hertz-Picciotto I, Beaumont JJ. A case-control study of pesticides and fetal death due to congenital anomalies. Epidemiology. 2001;12(2):148–56.
Quesada I, Fuentes E, Viso Leon MC, Soria B, Ripoll C, Nadal A. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17β-estradiol rapidly activate transcription factor CREB. FASEB J. 2002;16:1671–3.
Takeuchi T, Tsutsumi O. Serum bisphenol a concentrations showed gender differences, possibly linked to androgen levels. Biochem Biophys Res Commun. 2002;291:76–8.
Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Enciron Health Perspect. 2000;108(10):961–6.
Andersson AM, Jørgensen N, Main KM, Toppari J, Meyts ER, Leffers H, Juul A, Jensen TK, Skakkebæk NE. Adverse trends in male reproductive health: We may have reached a crucial ‘tipping point’. Int J Androl. 2008;31(2):74–80.
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. https://doi.org/10.1371/journal.pmed.1001356. Epub 2012 Dec 18.
Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13.
Travison TG, Araujo AB, O'Donnell AB, Kupelian V, John B, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92(1):196–202. https://doi.org/10.1210/jc.2006-1375. Epub 2006 Oct 24.
Atlantis E, Fahey P, Martin S, O'Loughlin P, Taylor AW, Adams RJ, Shi Z, Wittert G. Predictive value of serum testosterone for type 2 diabetes risk assessment in men. BMC Endocr Disord. 2016;16(1):26. https://doi.org/10.1186/s12902-016-0109-7.
Levine H, Jørgensen N, Andrade AM, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646–59. https://doi.org/10.1093/humupd/dmx022.
Huang C, Li B, Xu K, Liu D, Hu J, Yang Y, Nie H, Fan L, Zhu W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil Steril. 2017;107(1):83–88.e2. https://doi.org/10.1016/j.fertnstert.2016.09.035.
Montano L, Maugeri A, Volpe MG, Micali S, Mirone V, Mantovani A, Navarra M, Piscopo M. Mediterranean diet as a shield against male infertility and Cancer risk induced by environmental pollutants: a focus on flavonoids. Int J Mol Sci. 2022;23(3):1568. https://doi.org/10.3390/ijms23031568.
Sharpe RM. The “oestrogen hypothesis”- where do we stand now? Int J Androl. 2003;26:2–15.
Knez J. Endocrine-disrupting chemicals and male reproductive health. Reprod BioMed Online. 2013;26:440–8. https://doi.org/10.1016/j.rbmo.2013.02.005.
Del-Mazo J, Brieño-Enríquez MA, García-López J, López-Fernández LA, De-Felici M. Endocrine disruptors, gene deregulation and male germ cell tumors. Int J Dev Biol. 2013;57:225–39.
Sifakis S, Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharmacol. 2017;51:56–70. https://doi.org/10.1016/j.etap.2017.02.024.
Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS One. 2014;9:e102091.
Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One. 2012;7:e46249.
Skinner MK, Anway MD. Seminiferous cord formation and germ-cell programming: epigenetic transgenerational actions of endocrine disruptors. Ann N Y Acad Sci. 2005;1061:18–32.
Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9.
Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2)-ethylhexyl phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88:112.
Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol A exposure disrupts genomic imprinting in the mouse. PLoS Genet. 2013;9:e1003401.
Goldsby JA, Wolstenholme JT, Rissman EF. Multi- and transgenerational consequences of bisphenol A on sexually dimorphic cell populations in mouse brain. Endocrinology. 2016;158:21–30.
Hao C, Gely-Pernot A, Kervarrec C, Boudjema M, Becker E, Khil P, et al. Exposure to the widely used herbicide atrazine results in deregulation of global tissue-specific RNA transcription in the third generation and is associated with a global decrease of histone trimethylation in mice. Nucleic Acids Res. 2016;44:9784–802.
Horan TS, Marre A, Hassold T, Lawson C, Hunt PA. Germline and reproductive tract effects intensify in male mice with successive generations of estrogenic exposure. PLoS Genet. 2017;2(23):e1006885. https://doi.org/10.1371/journal.pgen.1006885.
Viluksela M, Pohjanvirta R. Multigenerational and transgenerational effects of dioxins. Int J Mol Sci. 2019;20(12):2947.
Barazani Y, Katz BF, Nagler HM, Stember DS. Lifestyle, environment, and male reproductive health. Urol Clin North Am. 2014;41:55–66.
Selevan SG, Borkovec L, Slott VL, Zudova Z, Rubes J, Evenson DP, Perreault SD. Semen quality and reproductive health of young Czech men exposed to seasonal air pollution. Environ Health Perspect. 2000;108:887–94.
Rubes J, Selevan SG, Evenson DP, Zudova D, Vozdova M, Zudova Z, Robbins WA, Perreault SD. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum Reprod. 2005;20:2776–83.
Guven A, Kayikci A, Cam K, Arbak P, Balbay O, Cam M. Alterations in semen parameters of toll collectors working at motorways: does diesel exposure induce detrimental effects on semen? Andrologia. 2008;40:346–51.
Deng Z, Chen F, Zhang M, Lan L, Qiao Z, Cui Y, An J, Wang N, Fan Z, Zhao X, Li X. Association between air pollution and sperm quality: a systematic review and meta-analysis. Environ Pollut. 2016;208:663–9.
Hammoud A, Carrell DT, Gibson M, Sanderson M, Parker-Jones K, Peterson CM. Decreased sperm motility is associated with air pollution in Salt Lake City. Fertil Steril. 2010;93(6):1875–9. https://doi.org/10.1016/j.fertnstert.2008.12.089.
Fathi Najafi T, Latifnejad Roudsari R, Namvar F, Ghavami Ghanbarabadi V, Hadizadeh Talasaz Z, Esmaeli M. Air pollution and quality of sperm: a meta-analysis. Iran Red Crescent Med J. 2015;17(4):e26930. https://doi.org/10.5812/ircmj.17(4)2015.26930.
Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertilemen in Paris during the past 20 years. N Engl J Med. 1995;332((9)):281–5.
Mendiola J, Jorgensen N, Andersson AM, Stahlhut RW, Liu F, Swan SH. Reproductive parameters in young men living in Rochester, New York. Fertil Steril. 2014;101:1064–71.
Le Moal J, Rolland M, Goria S, Wagner V, De Crouy-Chanel P, Rigou A, et al. Semen quality trends in French regions are consistent with a global change in environmental exposure. Reproduction. 2014;147:567.
Hauser R, Sokol R. Science linking environmental contaminant exposures with fertility and reproductive health impacts in the adult male. Fertil Steril. 2008;89:e59–e6.
Akre O, Cnattingius S, Bergstrom R, Kvist U, Trichopoulos D, Ekbom A. Human fertility does not decline: evidence from Sweden. Fertil Steril. 1999;71:1066–9.
Menchini-Fabris F, Rossi P, Palego P, Simi S, Turchi P. Declining sperm counts in Italy during the past 20 years. Andrologia. 1996;28:304–32.
Nordkap L, Joensen UN, Blomberg Jensen M, Jørgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355(2):221–30.
Bergamo P, Volpe MG, Lorenzetti S, Mantovani A, Notari T, Cocca E, et al. Human semen as an early, sensitive biomarker of highly polluted living environment in healthy men: a pilot biomonitoring study on trace elements in blood and semen and their relationship with sperm quality and RedOx status. Reprod Toxicol. 2016;66:1–9.
Lettieri G, D'Agostino G, Mele E, Cardito C, Esposito R, Cimmino A, Giarra A, Trifuoggi M, Raimondo S, Notari T, Febbraio F, Montano L, Piscopo M. Discovery of the involvement in DNA oxidative damage of human sperm nuclear basic proteins of healthy young men living in polluted areas. Int J Mol Sci. 2020;21(12):E4198. https://doi.org/10.3390/ijms21124198.
Zhou N, Cui Z, Yang S, Han X, Chen G, Zhou Z, et al. Air pollution and decreased semen quality: a comparative study of Chongqing urban and rural areas. Environ Pollut. 2014;187:145–52.
Vecoli C, Montano L, Borghini A, Notari T, Guglielmino A, Mercuri A, Turchi S, Andreassi MG. Effects of highly polluted environment on sperm telomere length: a pilot study. Int J Mol Sci. 2017;18:1703. https://doi.org/10.3390/ijms18081703.
Bosco L, Notari T, Ruvolo G, Roccheri MC, Martino C, Chiappetta R, Carone D, Lo Bosce G, Carrillo L, Raimondo S, Guglielmino A, Montano L. Sperm DNA fragmentation: an early and reliable marker of air pollution. Environ Toxicol Pharmacol. 2018;58:243–9. https://doi.org/10.1016/j.etap.2018.02.001.
Zani C, Zani D, Donato F, Marullo M, Viola GCV, Lorenzetti S, Montano L. Lifestyle, environmental exposures and male fertility in healthy young men in North Italy. Eur J Pub Health. 2019;29(Supplement_4):ckz186–459. https://doi.org/10.1093/eurpub/ckz186.459.
Montano L, Ceretti E, Donato F, Bergamo P, Zani C, Viola GCV, Notari T, Pappalardo S, Zani D, Ubaldi S, Bollati V, Consales C, Leter G, Trifuoggi M, Amoresano A, Lorenzetti S. Effects of a lifestyle change intervention on semen quality in healthy young men living in highly polluted areas in Italy: the FASt randomized controlled trial. Eur Urol Focus. 2021;8(1):351–9. https://doi.org/10.1016/j.euf.2021.01.017.
Pirastu R, Comba P, Conti S, Iavarone I, Fazzo L, Pasetto R, Zona A, Crocetti E, Ricci P. Sentieri-Epidemilogical study of residents in National Priority Contaminated Sites:mortality, cancer incidence and hospital discharges. Epidemiol Prev. 2014;38:25–33.
Tagliabue G, Borgini A, Tittarelli A, van Donkelaar A, Martin RV, Bertoldi M, Fabiano S, Maghini A, Codazzi T, Scaburri A, Favia I, Cau A, Barigelletti G, Tessandori R, Contiero P. Atmospheric fine particulate matter and breast cancer mortality: a population-based cohort study. BMJ Open. 2016;6(11):e012580. https://doi.org/10.1136/bmjopen-2016-012580. PMID: 2807627528–32.
Martuzzi M, Mitis F, Bianchi F, Minichilli F, Comba P, Fazzo L. Cancer mortality andcongenital anomalies in a region of Italy with intense environmental pressure due to waste. Occup Environ Med. 2009;66:725–32. https://doi.org/10.1136/oem.2008.044115.
Pasetto R, Zengarini N, Caranci N, De Santis M, Minichilli F, Santoro M, Pirastu R, Comba P. Environmental justice in the epidemiological surveillance system of residents in Italian National Priority Contaminated Sites (SENTIERI project). Epidemiol Prev. 2017;41(2):134–9.
Bay K, Asklund C, Skakkebaek N, Andersson AM. Testicular dysgenesis syndrome: possible role of endocrine disrupters. Best Pract Res Clin Endocrinol Metab. 2006;20(1):77–90.
Ségurel L, Wyman MJ, Przeworski M. Determinants of mutation rate variation in the human germline. Annu Rev Genomics Hum Genet. 2014;15:47–70.
Blumenstiel JP. Sperm competition can drive a male-biased mutation rate. J Theor Biol. 2007;249(3):624–32.
Aitken RJ, Gibb Z, Baker MA, Joel Drevet J, Parviz Gharagozloo P. Causes and consequences of oxidative stress in spermatozoa. Reprod Fertil Dev. 2016;28(2):1–10. https://doi.org/10.1071/RD15325.
Montano L, Bergamo P, Andreassi MG, Lorenzetti S. The role of human semen as an early and reliable tool of environmental impact assessment on human health. In: Spermatozoa—Facts and Perspectives. Rijeka: InTechOpen; 2018. https://doi.org/10.5772/intechopen.73231.
Choy JT, Eisenberg ML. Male infertility as a window to health. Fertil Steril. 2018;110(5):810–4. https://doi.org/10.1016/j.fertnstert.2018.08.015.
Barnhart KT. Introduction: fertility as a window to health. Fertil Steril. 2018;110(5):781–2. https://doi.org/10.1016/j.fertnstert.2018.08.031.
Pisarska MD. Fertility status and overall health. Semin Reprod Med. 2017;35(3):203–4. https://doi.org/10.1055/s-0037-1603728. Epub 2017 Jun 28.
Eisenberg ML, Li S, Brooks JD, Cullen MR, Baker LC. Increased risk of cancer in infertile men: analysis of U.S. claims data. J Urol. 2015;193(5):1596–601. https://doi.org/10.1016/j.juro.2014.11.080.
Hanson BM, Eisenberg ML, Hotaling JM. Male infertility: a biomarker of individual and familial cancer risk. Fertil Steril. 2018;109(1):6–19. https://doi.org/10.1016/j.fertnstert.2017.11.005.
Glazer CH, Bonde JP, Eisenberg ML, Giwercman A, Hærvig KK, Rimborg S, Vassard D, Pinborg A, Schmidt L, Bräuner EV. Male infertility and risk of nonmalignant chronic diseases: a systematic review of the epidemiological evidence. Semin Reprod Med. 2017;35(3):282–90. https://doi.org/10.1055/s-0037-1603568.
Brinton LA. Fertility status and cancer. Semin Reprod Med. 2017;35(3):291–7. https://doi.org/10.1055/s-0037-1603098.
Rogers MJ, Walsh TJ. Male infertility and risk of cancer. Semin Reprod Med. 2017;35:298–303.
Baker JA, Buck GM, Vena JE, Moysich KB. Fertility patterns prior to testicular cancer diagnosis. Cancer Causes Control. 2005;16:295–9.
Jorgensen N, Vierula M, Jacobsen R, Pukkala E, Perheentupa A, Virtanen HE, Skakkebaek NE, Toppari J. Recent adverse trends in semen quality and testis cancer incidence among Finnish men. Int J Androl. 2011;34:e37–48.
Rives N, Perdrix A, Hennebicq S, Saias-Magnan J, Melin MC, Berthaut I, Barthélémy C, Daudin M, Szerman E, Bresson JL, Brugnon F, Bujan L. The semen quality of 1158 men with testicular cancer at the time of cryopreservation: results of the French national CECOS network. J Androl. 2012;33:1394–401.
Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertil Steril. 2015;103:66–71.
Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol. 2009;170:559–6.
Montano L. Reproductive biomarkers as early indicators for assessing environmental health risk. In: Marfe G, Di Stefano C, editors. Toxic waste management and health risk. Bentham Science Publishers; 2020. https://doi.org/10.2174/97898114547451200101. eBook eISBN 978-981-14-5474-5. https.//www.eurekaselect.com/185279/chapter.
Gennaro Lettieri G, Marra F, Moriello C, Prisco P, Notari T, Trifuoggi M, Giarra A, Bosco L, Montano L, Piscopo M. Molecular alterations in spermatozoa of a family case living in the land of fires—a first look at possible transgenerational effects of pollutants. Int J Mol Sci. 2020;21(18):6710.
Raimondo S, Gentile M, Esposito G, Gentile T, Ferrara I, Crescenzo C, Palmieri M, Cuomo F, De Filippo S, Lettieri G, Piscopo M, Montano L. Could Kallikrein-Related Serine Peptidase 3 Be an Early Biomarker of Environmental Exposure in Young Women? Int J Environ Res Public Health. 2021;18:8833. https://doi.org/10.3390/ijerph1816883.
Suzuki K. The developing world of DOHaD. Dev Orig Health Dis. 2018;9(3):266–9.
Bianco-Miotto T, Craig JM, Gasser YP, van Dijk SJ, Ozanne SE. Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis. 2017;8(5):513–9.
Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. BioEssays. 2014;36(4):359–71. https://doi.org/10.1002/bies.201300113. Epub 2014 Jan 16.
Soubry A. POHaD: why we should study future fathers. Environ Epigenet. 2018;4(2):dvy007.
Soubry A. Epigenetics as a driver of developmental origins of health and disease: did we forget the fathers? BioEssays 2018; 40(1).
Curley JP, Mashoodh R, Champagne FA. Epigenetics and the origins of paternal effects. Horm Behav. 2011;59(3):306–14.
Braun K, Champagne FA. Paternal influences on offspring development: behavioural and epigenetic pathways. Neuroendocrinology. 2014;26(10):697–706.
Zhao ZH, Schatten H, Sun QY. Environmentally induced paternal epigenetic inheritance and its effects on offspring health. Reprod Dev Med. 2017;1(2):89–99.
Xavier MJ, Roman SD, Aitken RJ, Nixon B. Transgenerational inheritance: how impacts to the epigenetic and genetic information of parents affect offspring health. Hum Reprod Update. 2019;25:dmz017.
Bergman A, Brandt I, Brouwer A, Harrison P, Holmes P, Humfrey C, Keiding N, Randall G, Sharpe R and Skakkebaek N. European workshop on the impact of endocrine disrupters on human health and wildlife. 2–4 December 1996. Report of Proceedings, Weybridge, UK, 1997.
European Commission. Communication from the Commission to the Council and the European Parliament—Community strategy for endocrine disrupters—A range of substances suspected of interfering with the hormone systems of humans and wildlife. 1999. COM/99/0706 final. https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:51999DC0706.
European Parliament, Council of the European Union. Decision No 1386/ 2013/EU of the European Parliament and of the Council of 20 November 2013 on a General Union Environment Action Programme to 2020 Living well, within the limits of our planet. 2014. http://data.europa.eu/eli/dec/2013/1386/oj.
Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC,93/105/EC and 2000/21/EC.
Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC.
Mantovani A, Fucic A. Challenges in endocrine disruptor toxicology and risk Assessment. London: The Royal Society of Chemistry; 2021. https://doi.org/10.1039/9781839160738.
Commission Regulation (EU) 2018/605 of 19 April 2018 setting out scientific criteria for the determination of endocrine-disrupting and amending Annex II to Regulation (EC) 1107/2009. 2018. http://data.europa.eu/eli/reg/2018/605/oj.
Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products. https://echa.europa.eu/regulations/biocidal-products-regulation/understanding-bpr.
European Commission, Commission Delegated Regulation (EU) 2017/2100 of 4 September 2017 setting out scientific criteria for the determination of endocrine-disrupting properties pursuant to Regulation (EU) No 528/2012 of the European Parliament and Council. 2018. http://data.europa.eu/eli/reg_del/2017/2100/oj.
Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA relevance). http://data.europa.eu/eli/reg/2017/745/2020-04-24.
Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 On in vitro diagnostic medical devices and repealing directive 98/79/EC and commission decision 2010/227/EU (text with EEA relevance). ELI: http://data.europa.eu/eli/reg/2017/746/2022-01-28.
Commission Regulation (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food). Bundesinstitut fu¨r Risikobewertung (BfR), Database BfR Recommendations on Food Contact Materials. https://bfr.ble.de/kse/faces/DBEmpfehlung_en.jsp.
UN Environment Programme. Strategic approach to international chemicals management. Endocrine-disrupting chemicals. 2020. http://www.saicm.org/Implementation/EmergingPolicyIssues/EndocrineDisruptingChemicals/tabid/5476/language/en-US/Default.aspx. Accessed 31 Mar 2020.
UN Environment Programme. Scientific knowledge of endocrine disrupting chemicals. 2019. https://www.unenvironment.org/explore-topics/chemicals-waste/what-we-do/emerging-issues/scientific-knowledge-endocrine-disrupting. Accessed 30 Mar 2020.
Schmidt CW. TSCA 2.0: a new era in chemical risk management. Environ Health Perspect. 2016;124:A182–6.
Trasande L, Massey RI, DiGangi J, Geiser K, Olanipekun AI, Gallagher L. How developing nations can protect children from hazardous chemical exposures while sustaining economic growth. Health Aff (Millwood). 2011;30:2400–9.
Bellanger M, Demeneix B, Grandjean P, Zoeller RT, Trasande L. Neurobehavioral deficits, diseases, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015;100:1256–66.
Hauser R, Skakkebaek NE, Hass U, et al. Male reproductive disorders, diseases, and costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015;100:1267–77.
Legler J, Fletcher T, Govarts E, et al. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015;100:1278–88.
Attina TM, Malits J, Naidu M, Trasande L. Racial/ethnic disparities in disease burden and costs related to exposure to endocrine-disrupting chemicals in the United States: an exploratory analysis. J Clin Epidemiol. 2019;108:34–43.
Forouzanfar MH, Alexander L, Anderson HR, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386:2287–323.
Grosse SD, Teutsch SM, Haddix AC. Lessons from cost-effectiveness research for United States public health policy. Annu Rev Public Health. 2007;28:365–91.
Salkever DS. Assessing the IQ-earnings link in environmental lead impacts on children: have hazard effects been overstated? Environ Res. 2014;131:219–30.
Trasande L, Zoeller RT, Hass U, et al. Estimating burden and disease costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015;100:1245–55.
Attina TM, Hauser R, Sathyanarayana S, et al. Exposure to endocrine-disrupting chemicals in the USA: a population-based disease burden and cost analysis. Lancet Diabetes Endocrinol. 2016;4:996–1003.
Trasande L, Zoeller RT, Hass U, et al. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology. 2016;4:565–72.
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342.
Zoeller RT, Bergman A, Becher G, et al. A path forward in the debate over health impacts of endocrine disrupting chemicals. Environ Health. 2014;13:118.
Vandenberg LN, Colborn T, Hayes TB, et al. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod Toxicol. 2013;38:1–15.
Shaffer RM, Sellers SP, Baker MG, et al. Improving and expanding estimates of the global burden of disease due to environmental health risk factors. Environ Health Perspect. 2019;127:105001.
Pumarega J, Gasull M, Lee DH, López T, Porta M. Number of persistent organic pollutants detected at high concentrations in blood samples of the United States population. PLoS One. 2016;11:e0160432.
Solecki R, Kortenkamp A, Bergman Å, et al. Scientific principles for the identification of endocrine-disrupting chemicals: a consensus statement. Arch Toxicol. 2017;91:1001–6.
Danish Centre on Endocrine Disrupters. Report on Interpretation of knowledge on endocrine disrupting substances (EDs) – what is the risk? 2019. http://www.cend.dk/files/ED_Risk_report-final-2019.pdf.
US Environmental Protection Agency. Endocrine Disruptor Screening and Testing Advisory Committee (EDSTAC) final report. Washington, DC: US Environmental Protection Agency; 2021.
Markey CM, Michaelson CL, Veson EC, Sonnenschein C, Soto AM. The mouse uterotrophic assay: a reevaluation of its validity in assessing the estrogenicity of bisphenol A. Environ Health Perspect. 2001;109:55–60.
Ceccatelli R, Faass O, Schlumpf M, Lichtensteiger W. Gene expression and estrogen sensitivity in rat uterus after developmental exposure to the polybrominated diphenylether PBDE 99 and PCB. Toxicology. 2006;220:104–16.
La Merrill MA, Vandenberg LN, Smith MT, et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol. 2020;16:45–57.
Schug TT, Abagyan R, Blumberg B, et al. Designing endocrine disruption out of the next generation of chemicals. Green Chem. 2013;15:181–98.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Montano, L., Guglielmino, A. (2023). Policy Implication and Community Interventions to Reduce EDCs Exposure. In: Marci, R. (eds) Environment Impact on Reproductive Health. Springer, Cham. https://doi.org/10.1007/978-3-031-36494-5_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-36494-5_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36493-8
Online ISBN: 978-3-031-36494-5
eBook Packages: MedicineMedicine (R0)