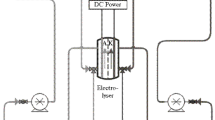
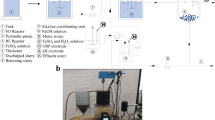
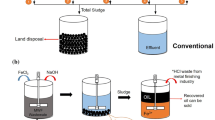
Abstract
Ferrite treatment of wastewater is a promising approach, since it allows to efficiently treat the polluted water containing at the same time dissolved heavy metal complexes within a broad pH range, and organic compounds. Application of ferrite treatment makes it possible to resolve a series of environmental and economic challenges due to the high degree of water purification and final formation of poorly soluble sediments with crystal oxide structure and ferromagnetic properties. This paper contains a review of ferritization treatment methods of multi-component wastewaters. It was shown that the main factor ensuring the formation of ferrites in the studied electrocoagulation treatment process is temperature, which needs to be not less than 70 °C and up to 85 °C. To carry out electrolysis with converting iron (III) hydroxide into Fe3O4, an optimal power consumption of 3–5 Coulombs per 1 mg of iron (III) contained in the sediment is required. The optimal conditions for this process are: ia (current density)—up to 2.5–3.0 A/dm2, temperature 70–80 °C, and pH values 4.0–8.0. Application of high-frequency currents for studied processes was tested, and the ferrite sludge with electric susceptibility of 2000–2200 Oersted was obtained, with higher index of hydraulic fineness of sludge particles which reached 0.86 mm/s. The value of the specific magnetic susceptibility of sediment in the dry state is 2.0–3.0 cm3/g. At the same time, the removal of heavy metal ions within 0.5 h treatment time reached 98–100%. It was shown that despite the high temperatures needed for this type of treatment, the resulted sludge can be readily separated from liquid phase both in gravitational and magnetic fields, which implies a series of advantages, including shorter time for sludge sedimentation and separation and lesser volumes of sedimentation tanks, prevention of environmental pollution with heavy metals, and a variety of possible applications of magnetic sludge.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ishikawa, Y., & Miura, N. (Eds.), 1991. Physics and engineering applications of magnetism. Springer Series in Solid State Sciences.
Polunin, V. M., Ryapolov, P. A., Zhakin, A. I., Shel’deshova, E. V., & Karpova, G. V. (2019). “Wall viscosity” of magnetic fluid oscillations in a strong magnetic field. Russian Physical Journal, 62, 589–597.
Odenbach, S. (Ed.). (2009). Colloidal magneric fluids: basics, development and application of ferrofluids. Lecture notes in physics series (p. 763). Springer.
Charles, S. W. (1992). Magnetic fluids (Ferrofluids), magnetic properties of fine particles. In J. L. Dormann, D. Fiorani (Eds.), North-Holland delta series (pp. 267–276).
Taketomi, S., Tikadzumi, S. (1993). Magnetic fluids [in Russian, trans. from Japanese] (p. 272). Mir.
Thomas, G. P. (2012). A guide to the theory, properties and applications of magnetic fluid.
R Rosensweig 1987 Magnetic fluids Annual Review of Fluid Mechanics 19 437 463
C Vasilescu M Latikka KD Knudsen VM Garamus V Socoliuc R Turcu E Tombacz D Susan-Resiga RHA Ras L Vekas 2018 High concentration aqueous magnetic fluids: Structure, colloidal stability, magnetic and flow properties Soft Matter 14 6648 6666
H Shokrollahi 2013 Structure, synthetic methods, magnetic properties and biomedical applications of ferrofluids Materials Science and Engineering: C 33 5 2476 2487
R Chaniyilparampu P Pande P Kopčanský R Mehta 2001 Application of magnetic fluids in medicine and biotechnology Indian Journal of Pure and Applied Physics 39 683 686
HE Horng C-Y Hong SY Yang HC Yang 2001 Novel properties and applications in magnetic fluids Journal of Physics and Chemistry of Solids 62 9–10 1749 1764
Scherer, C., Figueiredo Neto, A. M. (2005). Ferrofluids: properties and applications. Brazilian Journal of Physics, 35(3A), 718–727.
J Aswathy M Suresh 2014 Ferrofluids: Synthetic strategies, stabilization, physicochemical features, characterization, and applications ChemPlusChem—A Multidisciplinal Journal Centering on Chemistry 79 10 1382
M Erdem F Tumen 2004 Chromium removal from aqueous solution by the ferrite process Journal of Hazardous Materials 109 1–3 71 77
SJ Keny AG Kumbhar A Sanjukta S Pandey 2014 Antimony sorption and removal on carbon steel/magnetite surfaces in relation to pressurized heavy water reactors Current Science 106 8 1094 1100
Chamoli, P., Shukla, R., Bezbaruah, A., Kar, K., & Raina, K. (2021). Ferrites for water purification and wastewater treatment. In Ferrites and multiferroics. Engineering Materials Series (117-127). Springer.
RR Kefeni BB Mamba TAM Msagati 2017 Application of spinel ferrite nanoparticles in water and wastewater treatment: A review Separation and Purification Technology 188 399 422
V Sharma H Singh S Guleria N Bhardwaj S Puri SK Arya M Khatri 2022 Application of superparamagnetic iron oxide nanoparticles (SPIONs) for heavy metal adsorption: A 10-year meta-analysis Environmental Nanotechnology, Monitoring & Management 18 100716
D Demirel O Yenigün M Bekbölet 1999 Removal of Cu, Ni and Zn from wastewaters by the ferrite process Environmental Technology 20 9 963 970
N Masunga O Kelebogile KK Kefeni BB Mamba 2019 Recent advances in copper ferrite nanoparticles and nanocomposites synthesis, magnetic properties and application in water treatment: Review Environmental Chemical Engineering 7 3 103179
H Singh N Bhardwaj SK Arya M Khatri 2020 Environmental impacts of oil spills and their remediation by magnetic nanomaterials Environmental Nanotechnology, Monitoring & Management 14 100305
Qiao, R., Tian, W., Bai, J., Wang, L., Zhao, J., Du, Zh., & Gong, X. (2019). Application of magnetic adsorbents based on iron oxide nanoparticles for oil spill remediation: A review. Journal of the Taiwan Institute of Chemical Engineers, 97, 227–236.
SK Dutta M Akhter J Ahmed MK Amin PK Dhar 2022 Synthesis and catalytic activity of spinel ferrites: a brief review Biointerface Research in Applied Chemistry 12 4 4399 4416
M Sugimoto 2004 The past, present, and future of ferrites Journal of American Ceramic Society 82 269 280
M Hua SJ Zhang BC Pan WM Zhang L Lv QX Zhang 2012 Heavy metal removal from water/wastewater by nanosized metal oxides: A review Journal of Hazardous Materials 211–212 317 331
Covaliov, V. V., Caraghiaur, V. G. (1985). Intensification of electrocoagulation wastewater treatment/Electromagnetite treatment. Chisinau, VINITI, 85(18).
E Barrado F Prieto M Vega F Fernandez-Polanco 1998 Optimization of the operational variables of a medium-scale reactor for metal-containing wastewater purification by ferrite formation Water Resources 32 3055
Covaliov, V. V., & Covaliova, O. V. (2003). Theoretical and practical aspects of electrochemical treatment of water (p. 415) (Rus.). MSU Publ.House.
Birdi, K. S. (Ed.) (2016). Handbook on surface and collois chemistry (4th ed., p. 708). CRC Press.
Covaliova, O. (2008). Method of electrochemical treatment of wastewater containing the heavy metal ions (Patent MD No. 3912).
Covaliova, O., Covaliov, V., Lavrikova, O., & Gabova, I. (1991). Method of the wastewater sludge conditioning (Patent RF, No. 1673528).
T Yutaka C Gyong K Takashi 1979 The Fe3O4 formation by the ferrite processes Water Reasearch 13 21 31
Kytaogorodsky, A. I. (1952). Roentgenostructural analysis (p. 270). (Rus.), M.-L.
Mihiv, V. I. (1957). Roentgenometric determinator of minerals (Vol. 2). (Rus.). M., Gosgeotechizdat.
AV Pynaev 2006 Study of leaching of heavy metal ions from ferrite plating sludge under the field conditions. (Rus.) Proceedings of Universities from North Caucasus Region 4 46 48
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Duca, G., Covaliov, V., Covaliova, O., Romanciuc, L. (2023). Combined Ferrite Treatment of Multi-component Wastewaters Under the Elevated Temperature. In: Debik, E., Bahadir, M., Haarstrick, A. (eds) Wastewater Management and Technologies. Water and Wastewater Management. Springer, Cham. https://doi.org/10.1007/978-3-031-36298-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-36298-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36297-2
Online ISBN: 978-3-031-36298-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)