Abstract
The relationship between sexual selection and the diversity and rapid evolution of male genitalia has been well-documented across many animal taxa, while the morphological variability of female genitalia has received comparatively little attention. Female whales, dolphins, and porpoises possess unusual flaps, folds, and blind sacs in their vaginas, which vary among taxa and may serve several functions. We review the relationship between form and function of these unusual vaginal structures in cetaceans and discuss evidence that supports or refutes various functional hypotheses. A compilation of three-dimensional vaginal endocast models, contemporary high-resolution photographs of dissected reproductive tracts, and detailed anatomical illustrations ranging over 175 years are used to highlight the diversity of forms and fill in gaps in taxonomic knowledge. We discuss the complementary nature of anatomical illustrations and modern analytical and visual tools and how they can help us better understand the evolution of such unusual morphological structures. We identify opportunities for future studies in cetacean genital evolution and discuss the insights they may provide into mating strategies of cetaceans.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Anatomical illustrations
- Cetacean
- Female
- Functional anatomy
- Morphology
- Sexual selection
- Vagina
- Vaginal fold
5.1 Introduction
Across vertebrates, there is a paucity of research on female genital morphology compared to male reproductive organs (Hosken and Stockley 2004; Sloan and Simmons 2019). Male intromittent organs are varied and are described as the most rapidly diverging anatomical structure among species with internal fertilization (Eberhard 1985; Arnqvist 1998). Among insects, male reproductive structures are often used to determine species designation (Tuxen 1970; Eberhard 1985). Female genital morphology, in contrast, has been relatively understudied and undervalued. Compared to external and rigid male intromittent organs, female genitals were thought to be invariable (Eberhard 1996; Eberhard and Ramirez 2004) and more challenging to manipulate as they are soft and located internally within the body (Eberhard 1985; Córdoba-Aguilar 2010; Simmons 2014). Additionally, males were hypothesized to have the more dominant role in mating compared to females (Darwin 1871), and the field of genital evolution was biased with predominantly male researchers (Ah-King et al. 2014). While empirical evidence has refuted the validity of these reasons for preferential investigation of male reproductive morphology over female reproductive morphology, the field of genital evolution continues to explore male reproductive structures more frequently than female genital organs from the 1980s through the present day (Ah-King et al. 2014; Orbach 2022).
Part of the challenge in comparing morphological structures across taxa and exploring diversity and functionality is that ubiquitous defined landmarks are usually essential. A geometric morphometric approach is often used to characterize the shape of an anatomical structure, in which consistent morphological landmarks are present in all samples (Adams et al. 2004). Yet when assessing soft tissues such as female genitalia, morphological landmarks can be difficult to definitively identify. Alternative approaches can be used instead, sometimes supported by three-dimensional visualization. For example, the complexity of the vaginas of cetaceans (whales, dolphins, and porpoises) was explored using linear measurements (Orbach et al. 2017b), two-dimensional geometric morphometrics (Orbach et al. 2018), and alpha shape complexity scores of three-dimensional models (Orbach et al. 2021), which all relied on high-quality images.
Another major challenge in comparing anatomy within and across clades is that inconsistent terminology may be used to characterize unusual features. For example, the unique vaginal structures occurring among cetaceans have been identified for over 230 years (Hunter 1787). These vaginal structures have been termed circular folds (Ommanney 1932; Green 1977; Tarpley and Hillmann 1999), pseudo-cervices (Pycraft 1932; Schroeder 1990), rings of transverse folds (Chen et al. 1984), spermathecal folds (Meek 1918), transverse rugae (Jackson 1845), vaginal folds (Morejohn and Baltz 1972; Clarke et al. 1994; Orbach et al. 2016), and vulvular folds (Murie 1873). From the diversity of these terms, it is unclear if all authors refer to the same anatomical structures. Inclusion of supporting illustrations and images that complement anatomical descriptions can reduce ambiguity and incongruence. In this chapter, we explore the diversity of female cetacean reproductive morphology and emphasize the value of integrating art (e.g., illustrations, three-dimensional graphics) with science.
5.2 Unusual Genital Morphology of Cetaceans
Unlike most mammals (excluding sirenians), cetaceans are fully aquatic with no time spent on land. Over evolutionary time, natural selection pressures have driven many anatomical adaptations that facilitate high-energy-efficient lives in marine environments. In addition to sexual selection pressures that may drive sexually dimorphic traits (i.e., beaked whale dentition, Alves et al. 2023, this book; sperm whale (Physeter macrocephalus) body size, Eguiguren et al. 2023, this book; killer whale (Orcinus orca) dorsal fins, Wright et al. 2023, this book), natural selection pressures can enable and/or constrain mating. For example, female dusky dolphins (Lagenorhynchus obscurus) use their three-dimensional environment to evade males by diving within the water column (Markowitz et al. 2023, this book). Yet male cetaceans generally do not have large ornamental displays that would increase hydrodynamic drag, as found in many terrestrial mammals (Würsig et al. 2023, reviewed in this book). Phylogenetic history can also constrain anatomy. Male cetaceans have a fibroelastic penis, like all closely related even-toed ungulates, yet unlike most mammals that have a vascular penis (Slijper 1966). The fibroelastic penis possesses erectile tissue filled with elastin fibers and collagen that can further engorge with blood during arousal (Slijper 1966). As the penis of cetaceans is in a semi-turgid state, it is held within the body cavity, likely to reduce drag while swimming. However, the penis is everted prior to intromission and sometimes while swimming rapidly (dusky dolphins, Orbach et al. 2015), suggesting it is built to withstand drag forces without damage.
Female cetaceans also have unusual genital features, such as a comparatively small uterus, since the fetus develops in a uterine horn instead (Slijper 1966). The ovaries of cetaceans retain corpora luteum scars after ovulation, which are instrumental in many life history studies of cetaceans such as counting ovulation events (Dabin et al. 2008; Chivers and Danil 2023, this book). Perhaps most intriguing is the presence of diverse vaginal folds across cetacean species (Orbach et al. 2017b, 2018). Although these vaginal folds are a shared characteristic with even-toed ungulates (Pabst et al. 1998), we have not found any literature on artiodactyls describing comparable structures. Pigs (Sus domesticus) have several ring-like structures within their cervices (Dyce et al. 2010), yet the tissues of the vaginal folds in cetaceans have been histologically confirmed as non-cervical and of similar structural composition to other vaginal tissues (Orbach et al. 2016). The vaginal folds of cetaceans represent an unparalleled level of diversity in reproductive structures among vertebrates (Fig. 5.1; Orbach et al. 2017b).
Comparison of female cetacean genital morphology. The excised reproductive tracts are positioned ventral up with an incision down the midline. The uterine horns are at the top of each specimen, and the vaginal opening is at the bottom. The species are (1) Balaenoptera acutorostrata, (2) Delphinapterus leucas, (3) Delphinus capensis, (4) Delphinus delphis, (5) Eschrichtius robustus, (6) Globicephala macrorhynchus, (7) Globicephala melas, (8) Kogia breviceps, (9) Kogia sima, (10) Lagenorhynchus acutus, (11) Lagenorhynchus albirostris, (12) Lagenorhynchus obliquidens, (13) Lagenorhynchus obscurus, (14) Megaptera novaeangliae, (15) Mesoplodon bidens, (16) Mesoplodon europaeus, (17) Mesoplodon peruvianus, (18) Mesoplodon stejnegeri, (19) Orcinus orca, (20) Phocoena phocoena, (21) Phocoena sinus, (22) Physeter macrocephalus, (23) Sousa plumbea, (24) Stenella attenuata, (25) Stenella coeruleoalba, (26) Stenella frontalis, (27) Tursiops aduncus, and (28) Tursiops truncatus (figure modified from Orbach et al. (2018). Additional details about the specimens are provided in Supplemental Table 5.1)
5.2.1 Functions of Vaginal Folds
Several alternative and non-mutually exclusive hypotheses have been proposed for the function(s) of vaginal folds in cetaceans (Table 5.1; Clarke et al. 1994; Orbach et al. 2016).
5.2.1.1 Natural Selection Functions of Vaginal Folds
Among the hypotheses supporting natural selection factors, vaginal folds may provide a physical barrier to prevent birth of the underdeveloped fetus during pressure changes while diving (Kellogg 1938). It has also been proposed that vaginal folds could aid in parturition as they funnel caudally and could thus provide a passageway for the fetus (Meek 1918; Slijper 1962). The extensive diversity in the number, shape, size, and positioning of vaginal folds across species does not support either of these hypotheses as convergence in vaginal fold form and location are expected if they function to interact with the fetus (Orbach et al. 2017b, 2018, 2021).
Vaginal folds could function like a “squeegee” during copulation that wipes off seawater from the distal end of the penis and prevents the incursion of saltwater into the upper reproductive tract (Slijper 1962; Green 1972, 1977; Chen et al. 1984; Schroeder 1990; Robeck et al. 1994) as saltwater may be lethal to at least common bottlenose dolphin (Tursiops truncatus) sperm (Schroeder and Keller 1989) and beluga whale sperm (O’Brien et al. 2008). The orientation of the vaginal folds toward the caudal vaginal opening support the “squeegee” hypothesis, as do the often ring or funnel shapes of the vaginal folds (Orbach et al. 2016, 2017b). However, if vaginal folds were to “squeegee” the penis, the caudal fold that first contacts the external environment should be largest and the cranial fold the smallest, yet the opposite pattern occurs (Orbach et al. 2017b). Vaginal folds are also present in a freshwater river dolphin (baiji, Lipotes vexillifer, Chen et al. 1984). Seawater effects on dolphin sperm are not ubiquitous, with mortality rates varying depending on osmolality and exposure duration (unpublished data).
5.2.1.2 Sexual Selection Functions of Vaginal Folds
Most hypotheses related to the function(s) of vaginal folds in cetaceans suggest that sexual selection likely plays an important role, both during and after copulation (Table 5.1). Older hypotheses tended to focus on ways vaginal folds could aid or assist sperm in reaching the ova (“cooperation”), while more recent hypotheses focus on the possibility that the folds exert control over access to the ova and “conflict” between the sexes over which sperm reach the ova (Table 5.1). Vaginal folds were found to have a higher stiffness than other reproductive tract tissues in female common bottlenose dolphins, potentially indicating that the folds function to dampen the forces and damage to the vagina and cervix during rapid intromission (Orbach et al. 2019a).
Computed tomography (CT) scans revealed that the depth of penile penetration during copulation appears to be curtailed by large vaginal folds that present a physical barrier to the penis (Orbach et al. 2017a). The pattern is particularly obvious in harbor porpoises (Phocoena phocoena; Orbach et al. 2017a, 2020). Harbor porpoises have comparatively complex vaginal fold patterns among cetaceans, with one caudal vaginal fold that is especially prominent, thick, deep, and asymmetrically positioned (Orbach et al. 2017a, 2020, 2021). Male harbor porpoises exclusively sexually approach a mate on her left side (Keener et al. 2018; Webber et al. 2023, this book), which appears to be the only orientation in which the penis can bypass the vaginal fold labyrinth (Orbach et al. 2020). Thus, vaginal fold complexity and asymmetry appear to have coevolved with laterality of male (and possibly female) sexual behaviors in an evolutionary arms race of adaptations and counter-adaptations to control paternity (Arnqvist and Rowe 1995; Orbach et al. 2019b, 2020).
During copulation, physical contact from the vaginal folds could stimulate the penis and induce ejaculation (Meek 1918; Harrison 1969). The clitoris of the common bottlenose dolphin is more innervated than any other animal known to experience pleasure during copulation (Brennan et al. 2022). As the penis is homologous to the clitoris (Brennan 2016), and the bottlenose dolphin penis is also highly innervated (unpublished data), physical contact is likely important in inducing ejaculation. The extensive coevolution in shape between female and male reproductive morphologies among cetaceans (Orbach et al. 2017a) supports the ejaculation stimulation hypothesis. Future studies that explore contact points of the penis with vaginal folds during intromission will be valuable in discerning potential stimulatory functions.
Vaginal folds could assist with sperm storage and transport after copulation. Seminal vesicles and bulbourethral glands (i.e., Cowper’s gland, present in most mammals but absent in marine mammals) aid in semen coagulation (Williams-Ashman 1984). As male cetaceans lack both these anatomical features, the aperture of the vaginal folds with a tight seal would help prevent the loss of semen (Meek 1918; Slijper 1966; Harrison 1969). Vaginal folds may provide a pathway for semen to travel toward the ovaries (Orbach et al. 2017b). The vaginal folds of bottlenose dolphins are composed of fine longitudinal bands (Orbach et al. 2016). Longitudinal bands on the cervical mucosa of bovines and goats aid in sperm transport (Mattner 1968; Mullins and Saacke 1989). Vaginal folds may produce a pump-like action to uptake semen (Bonner 1980). Alternatively, vaginal folds may act antagonistically in sperm transport. Although some species can shunt sperm within their reproductive tracts away from sperm storage organs or ova (arctiid moths, Utetheisa ornatrix, Curril and LaMunyon 2006; domestic fowl, Gallus gallus domesticus, Pizzari and Birkhead 2000), the vaginal folds of cetaceans are composed of smooth muscle and are not under somatic control, suggesting females cannot selectively expel sperm from particular mates (Orbach et al. 2016). Further research is needed to explore innervation patterns and mechanisms of vaginal peristalsis.
The interspecific diversity in vaginal folding suggests that female genitalia are under strong selective forces. While research on vaginal fold functionality has expanded substantially in the past decade, there are still many unknowns that preclude a definitive role of vaginal folds. Studies are needed that further investigate the interactions of vaginal folds and surrounding tissues with penises, semen, and seawater to test functional hypotheses. Research using in vivo animals will be particularly valuable and may provide insights not evident using ex vivo samples. Molecular and biochemical studies will be essential to explore physiological mechanisms related to vaginal fold functionality. Characterization of differences between species, age classes, and individuals will also assist (Orbach et al. 2017b). Such quantitative characterizations of anatomical structures are aided when augmented by detailed visuals including illustrations, three-dimensional models, and photographs.
5.3 Art Augments Science
We compiled female reproductive tract images to illustrate the diversity in genital form within cetaceans. We include contemporary photographs from dissections and three-dimensional visualization, as well as historic and current illustrations to demonstrate how different tools can complement each other and provide perspectives that aid in the understanding of functionality and evolution of anatomical structures.
5.3.1 Dissection, Three-Dimensional Models, and Photographs
Excised reproductive tracts of female cetaceans were collected opportunistically by marine mammal stranding networks across the USA and occasionally in other countries. The specimens were provided to authors DNO or SLM under the National Oceanic and Atmospheric Administration National Marine Fisheries Services parts authorization letters or permits from the Convention on International Trade in Endangered Species of Wild Fauna and Flora. We requested whole reproductive tracts from cetaceans of any age class (calf, immature, mature) or reproductive state (resting, pregnant, lactating) that were less than 48 hours postmortem. The specimens were immediately frozen (−20°C) upon removal from the postmortem animals. When possible, the excised reproductive tracts included the intact genital opening, vagina, cervix, uterine horns, and ovaries. Specimens were shipped and stored frozen (−20°C) until thawed for making endocasts and/or dissection.
For select females, we made silicone molds of the vaginal lumen and caudal os (opening) cervix. The reproductive tracts were suspended with the vaginal openings facing up. The vaginal lumen was filled with Mold Star® 16 FAST or Elite HDTM light body dental silicone (Orbach et al. 2021). Once solidified, the silicone endocasts were carefully extracted to prevent tearing the reproductive tract tissues. The endocasts were digitized with a Canon EOS Rebel T5i camera with 100 mm lens. A photogrammetric technique was applied; overlapping photographs of the endocasts were used to build three-dimensional models that were reconstructed and scaled in 3DF Zephyr lite (3Dflow SRL) photogrammetry software (Supplemental Video 5.1; Orbach et al. 2021).
The genitals were cleaned to remove excess ligament and muscle tissues not part of the reproductive tract. The specimens were oriented in a dorsal recumbency on a dissecting table. To open the reproductive tracts for visualization and measurements, a single incision was made down the ventral midline from the bifurcation in the uterine horns to just cranial to the clitoris (Orbach et al. 2016). Care was taken not to cut through the clitoris so that its functionality could be subsequently investigated (Brennan et al. 2022). The uterine horns were opened by incisions down the midline on the ventral plane to search for fetuses. Mucus was gently scraped out of the reproductive tracts. High-resolution digital photographs were collected from a bird’s-eye view using different models of Nikon and Canon cameras concurrently with linear measurements (Orbach et al. 2016). A single representative photograph of each species was selected to display diversity. When possible, the representative photograph was from a sexually mature animal in early stages of decomposition, with the photograph in clear focus and depicting the entire genital organ. Photographs were edited in Adobe Photoshop 2023 to delete excess tissues.
As depicted in Figs. 5.1 and 5.2, there is extensive interspecific variation in vaginal shapes among cetacean species. Shape complexity of the vagina is driven by the diversity of vaginal folds (Orbach et al. 2021). Patterns were similar when using alpha shape complexity scores of three-dimensional vaginal endocasts (Fig. 5.2), linear measurements of dissected organs, or geometric morphometric analysis of two-dimensional photographs from dissections (Fig. 5.1). There does not seem to be a strong phylogenetic signal; female genital shape evolves rapidly even among closely related taxa (Orbach et al. 2017b, 2018, 2021). Factors associated with sexual selection including relative testes size, and with natural selection including relative neonate size, do not explain the extensive genital shape variation and complexity among female cetaceans (Orbach et al. 2018, 2021). The question of what drives vaginal fold diversity in cetaceans remains unanswered. Perhaps statistical patterns are not yet apparent as the available data of cetacean reproductive tracts is missing representative specimens from many of the about 96 extant species.
Three-dimensional reconstruction of silicone endocasts of the vaginal lumen and cervix of 13 cetacean species. The cervix is at the top of each model, and vaginal opening is at the bottom. The invaginations in the models are regions where vaginal folds protrude into the vaginal lumen. The species are (1) Eschrichtius robustus, (2) Megaptera novaeangliae, (3) Mesoplodon densirostris, (4) Kogia breviceps, (5) Stenella attenuata, (6) Stenella coeruleoalba, (7) Delphinus delphis, (8) Lagenorhynchus albirostris, (9) Lagenorhynchus obscurus, (10) Lagenorhynchus obliquidens, (11) Orcinus orca, (12) Tursiops truncatus, and (13) Phocoena phocoena (figure modified from Orbach et al. (2021). Additional details about the specimens are provided in Supplemental Table 5.2)
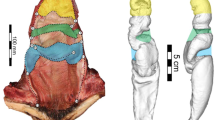
There is also extensive intraspecific variation in vaginal shapes among cetaceans, which was only partly explained by allometry and ontogeny (Fig. 5.3; Orbach et al. 2018, 2020, 2021). Visual aids help highlight the breadth of gross morphological variation among female reproductive organs. We use the harbor porpoise to further demonstrate how art can augment science. In the assessment of vaginal endocast complexity and shape, substantial differences were found between individual harbor porpoises (Fig. 5.3a; Orbach et al. 2021). Similarly, individual variation accounted for 52.6% of total reproductive tract shape variation using a two-dimensional geometric morphometric approach (Orbach et al. 2020). Sexually mature harbor porpoises had wider overall reproductive tracts with prominent cranial vaginas compared to sexually immature individuals (Fig. 5.3b; Orbach et al. 2018). Overall variation in the reproductive tract shape of harbor porpoises was mostly driven by the relative size of the caudal vagina followed by a bias in the right or left curvature of the reproductive tract (Orbach et al. 2020). The vaginal folds formed a spiral pattern in harbor porpoises (Fig. 5.3c; Supplemental Video 5.1). The chirality (“handedness”) of the largest vaginal fold (Fig. 5.3c), which is captured with the endocasts (Fig. 5.3a), is visually absent when the reproductive tract is fully opened (Fig. 5.3d). Scientific illustrations (e.g., Figure 5.3e) can help fill in gaps and provide insights into the evolutionary drivers of cetacean vaginal diversity. Illustrations are particularly helpful in showing these structures intact through cutaways and cross-sections.
Visual tools used to explore intraspecific reproductive tract variation in female harbor porpoises (Phocoena phocoena). (a) Three-dimensional reconstruction of silicone vaginal endocasts of three animals. The cervix is at the top of each model, and vaginal opening is at the bottom (modified from Orbach et al. 2021). (b) Principal component analysis showing the morphospace of reproductive tract images subjected to two-dimensional geometric morphometric analysis. The gray-shaded area denotes sexually immature females, and the red-shaded area identifies sexually mature females (modified from Orbach et al. 2018). (c) Image of partially dissected vagina in cross-sectional view. The prominent vaginal fold forms a tongue-like thick flap that spirals into cranial vaginal folds. (d) Images of two dissected reproductive tracts of sexually mature females. The animals are positioned in a dorsal recumbency with the uterine horns at the top. An incision was made down the midline to highlight the vaginal folds. (e) Modern illustration of the opened reproductive tract of a sexually mature female positioned in a dorsal recumbency. The illustration emphasizes the asymmetric vaginal folds and their relative size
5.3.2 Historical Illustrations of Female Cetacean Reproductive Tracts
Historical illustrations, created in an era before digital or high-resolution digital photography, provide a snapshot of current knowledge at that time. Historical illustrations can highlight key aspects of anatomical structures, eliminating unnecessary elements that are often byproducts of dissections. Illustrations can emphasize specific anatomical structures by using dark shading or muting to remove surrounding tissues, unlike photographs. Illustration can also conceptually and visually communicate through cutaways, sections, and transparencies. The illustrations were created by working directly with the anatomist(s) performing the dissections or, in some cases, by the scientists.
Figures 5.4 and 5.5 include historical illustrations of cetacean genitalia from ten species dating from 1848 (Fig. 5.4c) through 1949 (Fig. 5.5d). The cranial vaginas of the three species of baleen whales (blue whale, Fig. 5.4a; fin whale, Fig. 5.4b; sei whale, Fig. 5.4c) illustrated between 1848 and 1882 fill data gaps; our dissections of female baleen whale genitalia have been limited to minke whales (Fig. 1.1) and humpback whales (Fig. 1.14). The blue whale is the largest animal to have ever lived and has a proportionately large reproductive tract. Logistical constraints of shipping and storing such sizable frozen specimens limit access to baleen whale reproductive tracts, particularly of sexually mature animals. Some frozen excised specimens we dissected were in early stages of tissue atrophy, which may yield spurious characterizations.
Compilation of historical illustrations of female baleen whale (mysticete) reproductive tracts. The species are (a) Balaenoptera musculus (Beauregard and Boulart 1882), (b) Balaenoptera physalus (Beauregard and Boulart 1882), and (c) Balaenoptera borealis (Vrolik 1848). The illustrations highlight the cervix (top) and cranial vagina (bottom)
Compilation of historical illustrations of female toothed whale (odontocete) reproductive tracts. The species are (a) Ziphius sp. (Scott and Parker 1889), (b) Platanista gangetica (Anderson 1878), (c) Phocoena phocoena (Pycraft 1932), (d) Globicephala melas (Harrison 1969), (e) Tursiops truncatus (Pycraft 1932), (f) Delphinus delphis (Pycraft 1932), and (g) Lagenorhynchus albirostris (Van Beneden 1861)
The historical illustrations further complement the dissection images by detailing features of functional importance. For example, Fig. 5.5c and g portray a harbor porpoise and white-beaked dolphin female reproductive tract, respectively. These two illustrations emphasize longitudinal pleats in the cranial vagina and vaginal folds that may function as channels for semen; these pleats are less overt in dissection photographs (Figs. 1.20 and 1.11, respectively). Figure 5.4c captures the fine leaflike vaginal folds of the sei whale, an attribute we confirmed in the beluga whale yet did not adequately capture with a photograph (Fig. 1.2). In contrast, Fig. 5.5c depicts the thick rounded vaginal folds of the harbor porpoise; the largest vaginal fold can be over 5 cm in depth and 16 mm in thickness, which is conveyed in the three-dimensional endocast (Fig. 5.3a) and cross-sectional photograph (Fig. 5.3c), but not in the bird’s-eye view photograph (Fig. 5.3d). Revisiting historical illustrations has been enlightening and crucial to our current understanding of cetacean genital morphology.
5.4 Conclusions and Future Directions
The field of female genital evolution remains a rich and vastly underexplored area of basic anatomical research across all taxa (Ah-King et al. 2014; Orbach 2022). Female cetaceans have the most diverse vaginal morphologies within a vertebrate clade due to the presence of vaginal folds that vary in number, shape, size, and positioning across species (Orbach et al. 2017b). Several functional hypotheses for vaginal folds related to sexual and natural selection pressures have been proposed. Further research is needed to empirically test hypotheses. Experiments exploring the genitals of live animals will be of particular utility in ascertaining if there is “cooperation” or “conflict” between the sexes to control paternity (e.g., Arnqvist and Rowe 2005). For example, experiments could track the movement of fluorescently labeled sperm that are artificially inseminated into the vagina of a live female to assess if vaginal folds assist with sperm uptake, retention, or rejection. Research is needed to validate the hypothesis that seawater is lethal to cetacean sperm (Schroeder and Keller 1989; O’Brien et al. 2008), as the duration of exposure and salinity levels may vary results. The benign or hostile nature of the vagina can be explored by testing the concentration of leukocytes or the community composition of the microbiome. The potential role of cervical mucus as a semen plug warrants investigation. Further research is needed to understand if the longitudinal bands within the vagina provide protected channels for sperm transport and how these channels may vary with estrous state.
As copulation is a direct mechanical interaction between females and males, sexual selection likely acts concurrently on the genitalia of both sexes (Brennan 2016). Although this chapter focuses on the genital morphology of female cetaceans, studies on intromittent organs are needed, especially those that integrate gross morphology, microanatomy, and varied visual tools to underscore diversity and functionality. Comparative studies of cetacean male genitalia have been limited to the relative testes sizes and penis lengths of baleen whales (Brownell and Ralls 1986). There are historic illustrations that depict broad interspecific variations in cetacean penis morphology and showcase extraordinary examples of unusual reproductive structures. For example, the Indus river dolphin (Platanista minor) lacks the pelvic bones that anchor the muscle that erect the penis in other cetacean species (Pilleri 1976; Dines et al. 2014). The Indus river dolphin also has erectile side lobes and a fibrous septum between the corpora cavernosa, which are anomalous traits among cetacean genitalia (Pilleri 1976). Future research on cetacean reproductive tracts, particularly when complemented with graphical imagery, will facilitate improved understanding of sexual and mating systems.
Knowledge of sexual anatomy can inform our understanding of cetacean mating systems and provide clues into the mating strategies of the sexes. For example, testes mass (relative to body size) is positively correlated with the intensity of sperm competition across many taxa, including cetaceans, and provides insights into the relative strength of pre- or post-copulatory sexual selection (Kenagy and Trombulak 1986; Dines et al. 2015). Similarly, many of the hypotheses related to the functions of vaginal folds in cetaceans support a role in sexual selection. The number, size, and complexity of vaginal folds vary widely across cetacean species. While the mechanism remains unknown, the diversity likely reflects opportunities for cryptic female choice or other forms of post-copulatory sexual selection. The harbor porpoise stands out as a species with extravagant genitalia in both sexes. Females have thick, complex, and spiralized vaginal folding (Fig. 5.3, Orbach et al. 2020). Males have some of the largest testes relative to body size of any mammal (Kenagy and Trombulak 1986). The vaginal folds and deep recesses may curtail the depth or direction of penile penetration and/or semen movement (Orbach et al. 2017a, 2020). While the order of development of complex vaginal labyrinths, large relative testes sizes, long penises, and lateralized mating behavior remain unknown, it is clear that the genitalia of both sexes of harbor porpoises have coevolved (Orbach et al. 2020). Further exploration of the reproductive anatomy, mechanics of copulation, and mating behavior of both sexes are warranted.
References
Adams DC, Rohlf FJ, Slice DE (2004) Geometric morphometrics: ten years of progress following the ‘revolution’. Ital J Zool 71:5–16
Ah-King M, Barron AB, Herberstein ME (2014) Genital evolution: why are females still understudied? PLoS Biol 12:e1001851
Alves F, Badenas A, Mesnick S, Pitman R, Rosso M (2023) Beaked whale sexual dimorphism, mating strategies, and diversification. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Anderson J (1878) Anatomical and zoological researches: comprising an account of the zoological results of the two expeditions to western Yunnan in 1868 and 1875 and a monograph of the two cetacean genera, Platanista and Orcella. B. Quaritch, London. v.1 [text] v. 2 [atlas]
Arnqvist G (1998) Comparative evidence for the evolution of genitalia by sexual selection. Nature 393:784–786
Arnqvist G, Rowe L (1995) Sexual conflict and arms races between the sexes: a morphological adaptation for control of mating in a female insect. Proc R Soc Lond B 261:123–127
Arnqvist G, Rowe L (2005) Sexual conflict. Princeton University Press, Princeton, NJ
Beauregard H, Boulart R (1882) Recherches sur les appareils génito-urinaires de Balaenides. Journ de l’anat et de la phys 18:158–201
Bonner WN (1980) Whales. Blandford Press, Poole and Dorset
Brennan PLR (2016) Studying genital coevolution to understand intromittent organ morphology. Integr Comp Biol 56:669–681
Brennan PLR, Cowart J, Orbach DN (2022) Evidence of a functional clitoris in dolphins. Curr Biol 32:R24–R26
Brownell RL, Ralls K (1986) Potential for sperm competition in baleen whales. Rep Int Whal Commn Spec Iss 8:97–112
Chen PX, Liu RJ, Lin KJ (1984) Reproduction and the reproductive system in the Beiji, Lipotes vexillifer. In: Perrin WF, Brownell RL Jr, DeMaster DP (eds) Reproduction of whales, dolphins, and porpoises. Rep Int Whal Commn Spec Iss 6:445–450
Chivers S, Danil K (2023) Interspecific comparison of reproductive systems. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Clarke R, Paliza O, Aguayo AL (1994) Sperm whales of the Southeast Pacific. Part VI. Growth and breeding in the male. In: Pilleri G (ed) Investigations on cetacea, vol 25. Paciano, Museum of Natural History, pp 93–224
Córdoba-Aguilar A (2010) The evolution of primary sexual characters in animals: a summary. In: Leonard J, Córdoba-Aguilar A (eds) The evolution of primary sexual characters in animals. Oxford University Press, Oxford, pp 494–497
Curril IM, LaMunyon CW (2006) Sperm storage and arrangement within females of the arctiid moth Utetheisa ornatrix. J Insect Physiol 52:1182–1188
Dabin W, Cossais F, Pierce GJ, Ridoux V (2008) Do ovarian scars persist with age in all cetaceans: new insight from the shortbeaked common dolphin (Delphinus delphis Linnaeus, 1758)? Mar Biol 156:127–139
Darwin C (1871) The descent of man and selection in relation to sex, 2nd edn. J Murray, London
Dines JP, Otárola-Castillo E, Ralph P, Alas J, Daley T, Smith AD, Dean MD (2014) Sexual selection targets cetacean pelvic bones. Evolution 68(11):3296–3306
Dines JP, Mesnick SL, Ralls K, May-Collado L, Agnarsson I, Dean MD (2015) A trade-off between precopulatory and postcopulatory trait investment in male cetaceans. Evolution 69(6):1560–1572
Dyce KM, Sack WO, Wensing CJG (2010) The pelvis and the reproductive organs of the pig. In: Textbook of veterinary anatomy, 4th edn. Saunders/Elsevier, St. Louis, MO, pp 772–779
Eberhard WG (1985) Sexual selection and animal genitalia. Harvard University Press, Cambridge, MA
Eberhard WG (1996) Females control: sexual selection by cryptic female choice. Princeton University Press, Princeton, NJ
Eberhard WG, Ramirez N (2004) Functional morphology of the male genitalia of four species of drosophila: failure to confirm both lock and key and male-female conflict predictions. Ann Entomol Soc Am 97:1007–1117
Eguiguren A, Konrad Clarke CM, Cantor M (2023) Sperm whale sexual strategies: current knowledge and future directions. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Green RF (1972) Observations on the anatomy of some cetaceans and pinnipeds. In: Ridgway SH (ed) Mammals of the sea: biology and medicine. Charles C. Thomas, Springfield, MA, pp 247–269
Green RF (1977) Anatomy of the reproductive organs in dolphins. In: Ridgway SH, Benirschke K (eds) Breeding dolphins: present status, suggestions for the future. NTIS PB-273-673. U.S. Dept. of Commerce, Washington, DC, pp 185–191
Harrison RJ (1969) Reproduction and reproductive organs. In: Anderson HT (ed) The biology of marine mammals. Academic Press, New York, NY, pp 253–348
Hosken DJ, Stockley P (2004) Sexual selection and genital evolution. Trends Ecol Evol 19:87–93
Hunter J (1787) Observations on the structure and oeconomy of whales. Phil Trans R Soc Lond 77:371–450
Jackson JBS (1845) Dissections of a spermaceti whale and three other cetaceans. Boston J Nat Hist 5:137–171
Keener W, Webber MA, Szczepaniak ID, Markowitz TM, Orbach DN (2018) The sex life of harbor porpoises: male lateralized and aerial behavior. Aqua Mamm 44:620–632
Kellogg R (1938) Adaptation of structure to function in whales. In: Kellogg R (ed) Cooperation in research. Carnegie Institute of Washington, Washington, DC. Publication #501, pp 649–682
Kenagy GJ, Trombulak SC (1986) Size and function of mammalian testes in relation to body size. J Mamm 67(1):1–22
Markowitz T, Markowitz W, Würsig B, Orbach DN (2023) Socio-sexual behavior of nocturnally foraging dusky and spinner dolphins. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Mattner PE (1968) The distribution of spermatozoa and leukocytes in the female genital tract in goats and cattle. J Repro Fertil 17:253–261
Meek A (1918) The reproductive organs of cetacea. J Anat 52:186–210
Morejohn GV, Baltz DM (1972) On the reproductive tract of the female dall porpoise. J Mamm 53:606–608
Mullins KJ, Saacke RG (1989) Study of the functional anatomy of bovine cervical mucosa with special reference to mucus secretion and sperm transport. Anat Rec 225:106–117
Murie J (1873) On the organization of the ca’ain whale (Globicephalus melas). Trans Zool Soc Lond 8:235–301
O’Brien JK, Steinman KJ, Schmitt T, Robeck TR (2008) Semen collection, characterisation and artificial insemination in the beluga (Delphinapterus leucas) using liquid-stored spermatozoa. Repro Fert Devel 20(7):770–783
Ommanney FD (1932) The urino-genital system of the fin whale (Balaenoptera physalus). Discov Rep 5:363–466
Orbach DN (2022) Gender bias in the study of genital evolution: females continue to receive less attention than males. Integr Comp Biol 62:533
Orbach DN, Packard JM, Kirchner T, Würsig B (2015) Evasive behaviours of female dusky dolphins (Lagenorhynchus obscurus) during exploitative scramble competition. Behaviour 152:1953–1977
Orbach DN, Marshall CD, Würsig B, Mesnick SL (2016) Variation in female reproductive tract morphology of the common bottlenose dolphin (Tursiops truncatus). Anat Rec 299:520–537
Orbach DN, Kelly DA, Solano M, Brennan PLR (2017a) Genital interactions during simulated copulation amongst marine mammals. Proc R Soc Lond B 284:20171265
Orbach DN, Marshall CD, Mesnick SL, Würsig B (2017b) Patterns of cetacean vaginal folds yield insights into functionality. PLoS One 12:e0175037
Orbach DN, Hedrick B, Würsig B, Mesnick SL, Brennan PLR (2018) The evolution of genital shape variation in female cetaceans. Evolution 72:261–273
Orbach DN, Rattan S, Hogan M, Crosby AJ, Brennan PLR (2019a) Biomechanical properties of female dolphin reproductive tissue. Acta Biomater 86:117–124
Orbach DN, Keener W, Ziltener A, Packard J, Würsig B (2019b) Testes size, vaginal complexity, and behavior in toothed whales (odontocetes): arms race or tradeoff model for dusky dolphins (Lagenorhynchus obscurus), harbor porpoises (Phocoena phocoena), and bottlenose dolphins (Tursiops spp.)? J Comp Psych 133(3):359–372
Orbach DN, Brennan PLR, Hedrick BP, Keener W, Webber M, Mesnick SL (2020) Asymmetric and spiraled genitalia coevolve with unique lateralized mating behavior. Sci Rep 10:3257
Orbach DN, Brassey CA, Gardiner JD, Brennan PLR (2021) 3D genital shape complexity in female marine mammals. Ecol Evol 11:3210–3218
Pabst DA, Rommel SA, McLellan WA (1998) Evolution of thermoregulatory function in cetacean reproductive systems. In: Thewissem JGM (ed) The emergence of whales: evolutionary patterns in the origins of cetacean. Plenum Press, New York, NY, pp 379–397
Pilleri G (1976) The penis of Platanista indi and remarks on the taxonomy of the genus. In: Pilleri G (ed) Investigations on cetacea, vol 6. Hirnanatomisches Institut der Universität, Berne
Pizzari T, Birkhead TR (2000) Female feral fowl eject sperm of subdominant males. Nature 405:787–789
Pycraft WP (1932) On the genital organs of a female common dolphin (Delphinus delphinus). Proc Zool Soc Lond 102:807–812
Robeck TR, Curry BF, McBain JF, Kraemer DC (1994) Reproductive biology of the bottlenose dolphin (Tursiops truncatus) and the potential application of advanced reproductive technologies. J Zoo Wildl Med 25:321–336
Schroeder JP (1990) Breeding bottlenose dolphins in captivity. In: Leatherwood S, Reeves RR (eds) The bottlenose dolphin. Academic Press, San Diego, CA, pp 435–446
Schroeder JP, Keller KV (1989) Seasonality of serum testosterone levels and sperm density in Tursiops truncatus. J Exp Zool 249:316–321
Scott JH, Parker TH (1889) On a specimen of Ziphius recently obtained near Dunedin. Trans Zool Soc Lond 7:241–248
Simmons LW (2014) Sexual selection and genital evolution. Austr Entom 53:1–17
Slijper EJ (1962) Whales. Hutchinson & Co. Ltd, London
Slijper EJ (1966) Functional morphology of the reproductive system in cetacean. In: Norris NS (ed) Whales, dolphins, and porpoises. University of California Press, Berkeley, CA, pp 277–319
Sloan NS, Simmons LW (2019) The evolution of female genitalia. J Evol Biol 32:882–899
Tarpley RJ, Hillmann DJ (1999) Observations on ovary morphology, fetal size and functional correlates in the bowhead whale Balaena mysticetus. Department of Wildlife Management, Barrow, AK, p 276
Tuxen SL (1970) Taxonomist’s glossary of genitalia in insects. Scandinavian University Press, Copenhagen
Van Beneden P-J (1861) Recherches sur la faune littorale de Belgique: Cétacés. Mém Acad R Sci Lett B-Arts Belg 32:1–38. pls. I & II
Vrolik W (1848) Natuur- en ontleedkundige beschouwing van den Hyperoodon. Natuurlijke Verhandelingen van de Hollandsche Maatschappij der Wetenschappen. Haarlem. pp 128, 15 pls
Webber M, Keener K, Wahlberg M, Elliser CR, MacIver K, Torres Ortiz S, Jakobsen F, Hamel H, Rieger A, Siebert U, Dunn H, Anderson D, Hall AM, Birdsall C, Pielmeier K, Paiu RM, Boege Tobin DD, Orbach DN (2023) Sexual behavior in harbor porpoises (Phocoena phocoena). In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Williams-Ashman HG (1984) Transglutaminases and the clotting of mammalian seminal fluids. Mol Cell Biochem 58:51–61
Wright B, Stedulinsky E, Ford J (2023) Sex in killer whales: behavior, exogamy and the evolution of sexual strategies in the ocean’s apex predator. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Würsig B, Rich J, Orbach DN (2023) Sex and behavior. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Acknowledgments
We thank the many marine mammal stranding networks that provided us with excised female reproductive tracts including Alaska Veterinary Pathology Services, Cascadia Research Collective, Florida Fish and Wildlife Conservation Commission, International Fund for Animal Welfare, Long Marine Lab Stranding Network, Marine Animal Response Society Nova Scotia, New Zealand Common Dolphin Project, North Carolina Division of Marine Fisheries/North Carolina State University Center for Marine Sciences and Technology, Oregon State University, Port Elizabeth Museum, SeaWorld Parks & Entertainment, Southwest and Southeast Fisheries Science Centers National Marine Fisheries Services National Oceanic & Atmospheric Administration, The Marine Mammal Center, the University of North Carolina-Wilmington, and Virginia Aquarium & Marine Science Center. We thank Bernd Würsig and Bill Keener for their insightful edits on this paper.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
5.1 Electronic Supplementary Material
Supplemental Tables 5.1 and 5.2
(XLSX 15 kb)
Video of three-dimensional vaginal endocast of a sexually mature harbor porpoise (Phocoena phocoena). Specimen TMMC C-434 from The Marine Mammal Center (MOV 944 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Orbach, D.N., Gorter, U., Mesnick, S. (2023). Sexual Anatomy of Female Cetaceans: Art and Science Contribute Insights into Functionality. In: Würsig, B., Orbach, D.N. (eds) Sex in Cetaceans. Springer, Cham. https://doi.org/10.1007/978-3-031-35651-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-35651-3_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35650-6
Online ISBN: 978-3-031-35651-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)