Abstract
Bottlenose dolphins (Tursiops spp.) live in complex societies with high fission-fusion dynamics and exhibit a polygynandrous mating system in which both sexes mate with multiple partners. The benefits of polygynandry vary between the sexes; males likely increase their reproductive success by maximizing the number of mating partners, whereas females may reduce infanticide risk and/or increase the genetic quality of offspring by mating with multiple males. Socio-ecological theory states that mating strategies are dictated by the distribution of females and the ability of males to monopolize them. However, the tactics that males use to achieve reproductive success vary within and across populations. Although some male bottlenose dolphins appear to use a solitary approach to gain mating access, males in several populations demonstrate a relatively rare mating tactic: cooperative mate guarding within alliances. Male alliances generally consist of a pair or trio of males that work together to sequester a fertile female. However, nested or multilevel alliances have been documented in two populations to date (i.e., Shark Bay, Australia, and Jacksonville, Florida). The complexity of male alliances may vary in response to a suite of specific ecological, demographic, and/or morphological variables that promote male-male cooperation and reduce intrasexual competition. In this chapter, we review population-specific examples of male bottlenose dolphin mating tactics and examine several hypotheses that may explain inter- and intrapopulation variation in alliance complexity. We also explore the sociosexual behavior and potential countertactics used by females.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Bottlenose dolphins (Tursiops spp.) live in complex societies with high fission-fusion dynamics including fluid changes in group size and composition (Connor et al. 2000b). Within this fluid structure, preferences for same-sex associates are common (Wells 2014; Ermak et al. 2017; Galezo et al. 2018; Ham et al. 2023, this book; but see Lusseau et al. 2003 and Wiszniewski et al. 2010) and likely reflect sex-specific reproductive strategies. Due to long gestation and lactation periods (Whitehead and Mann 2000), individual female bottlenose dolphins are unavailable to breed for several years at a time. The resulting male-biased operational sex ratio can lead to high variation in male reproductive success and intense male-male competition (Connor et al. 2000b; Karniski et al. 2018; Gerber et al. 2022; Würsig et al. 2023, this book). Male mating strategies are then constrained by the ability of males to monopolize either females or the resources that are valuable to them (Emlen and Oring 1977). Dolphins’ food resources are often patchily distributed and highly mobile, making territorial defense difficult (Connor et al. 2000b). Females, however, are defensible resources, and mate guarding can be effective at ensuring paternity and increasing reproductive success (Wells 1991; Connor et al. 1992). Although some male bottlenose dolphins appear to use a solitary approach to gain mating access, in several populations, males demonstrate a relatively rare mating tactic – cooperative mate guarding within long-term alliances (Table 12.1). Male alliances generally consist of a pair or trio of males that cooperate to sequester a fertile female (Connor et al. 1992; Owen et al. 2002). While mating can be shared, fertilization is an indivisible resource, making intrasexual reproductive cooperation paradoxical and recurring cooperation among the same individuals uncommon among animals (Díaz-Muñoz et al. 2014). Yet, cooperative mate guarding likely increases male reproductive success as it improves the odds of winning contests against other males and of successfully sequestering fertile females (Connor et al. 1992).
Despite mate guarding attempts by males, bottlenose dolphins have a polygynandrous mating system; both sexes mate with multiple partners in a given breeding season (Connor et al. 1996; Boness et al. 2002). The benefits of polygynandry vary between the sexes; males likely increase their reproductive success by maximizing the number of mating partners (Bateman 1948), whereas females may reduce infanticide risk (Wolff and Macdonald 2004) and/or increase the genetic quality of offspring by mating with multiple males (Stockley 2003). Populations vary with respect to the seasonality of reproduction. Conceptions and births may occur year-round, but diffuse seasonal peaks corresponding with warm water temperatures are common (e.g., Urian et al. 1996; Mann et al. 2000). Mating can occur in a variety of positions, but males mounting along the side (lateral-ventral) or dorsum (dorsoventral) of the female are more commonly documented than ventrum-ventrum (Tavolga and Essapian 1957; Connor et al. 2000b). Mounting and goosing (rostrum to genital area contact) are the most conspicuous sociosexual behaviors, with intromission more difficult to observe and not necessarily indicative of reproduction (Connor et al. 2000b; Connor and Vollmer 2009; Furuichi et al. 2014). Nonreproductive sociosexual behaviors also occur throughout the year and may facilitate pleasure, learning, and establishing or mediating relationships (Connor et al. 2000b; Brennan et al. 2022; da Silva and Spinelli 2023, this book; Ham et al. 2023, this book).
Both sexes likely use conditional, rather than fixed, strategies with alternative mating tactics to optimize reproductive success. Conditional mating tactics are shaped by a combination of morphological, demographic, environmental, and social variables (Gross 1996), which vary greatly among populations of Tursiops spp. The following sections review population-specific examples of male bottlenose dolphin mating tactics and hypotheses that may explain variation in alliance complexity. The sociosexual behavior and potential countertactics used by females are also reviewed.
12.2 Male Mating Tactics
Male bottlenose dolphins engage in agonistic endurance competitions in which they compete for mating opportunities by roving among females; they either depart soon after mating or follow/herd the female to prevent other males from mating with her (i.e., mate guarding; Wells 1991; Connor et al. 1992). Copulation does not guarantee fertilization nor siring offspring if females have multiple mating partners. The degree of sperm competition in a species is typically correlated with testis size relative to body size and sperm count per ejaculate (Harvey and Harcourt 1984; Connor et al. 2000a). Bottlenose dolphins have relatively small testis mass and a moderate degree of sexual size dimorphism compared to other delphinids (Connor et al. 2000a), suggesting sperm competition may not be important, especially if males mate guard (Perrin and Reilly 1984). Mate guarding duration ranges from a few minutes to several months to competitively exclude rival males from copulation during the female’s estrus (Connor et al. 1992, 1996). The predicted number of receptive females and male competitors may influence the length of time males spend guarding individual females (Magnusson and Kasuya 1997). This mate guarding tactic can be temporally costly to males as ensuring paternity with one female may reduce the time available to mate guard others. However, if males do not guard a female for long enough, the likelihood of paternity may be greatly reduced. Connor et al. (1996) observed that females in Shark Bay, Australia, were guarded (and presumably mated) by up to 13 males in a single breeding season. Mate guarding and male-female associations may also be longer than the typical estrus period and/or begin prior to the breeding season as males may be preemptively mate guarding before a female reaches peak attractiveness (Connor et al. 1996; Owen et al. 2002; Robeck et al. 2005).
When cooperatively mate guarding, allied male dolphins frequently travel abreast behind the female or flank her on either side and slightly behind (i.e., a consortship; Fig. 12.1, Connor et al. 1992). Males pursue a female by angling out on either side of her, a feat more difficult to accomplish alone or in deep waters where a female has depth as an escape route (Connor et al. 2000b). The term herding describes coercively maintained consortships (Connor et al. 1996). Mate coercion is a common component of polygynandrous mating systems without strong or long-term intersexual bonds (Smuts and Smuts 1993). To constrain a female’s movements and prevent extra-pair copulations, males threaten females through posture, vocalizations, and charges or by aggressively biting or colliding into females (Connor et al. 1992, 2000b; Connor and Smolker 1996). Intersexual aggression has also been documented through analysis of conspecific tooth-rake marks. In Shark Bay, cycling females have more new rake marks than non-cycling females (Scott et al. 2005), and younger females may receive more aggression from males than do older females, suggesting male preferences for females with high calving success (Watson 2005; Karniski et al. 2018). There is currently no evidence of forced copulation, as females have been observed rolling away from mounting males; however, males may use intimidation tactics to coerce females into copulating (Connor and Vollmer 2009). In Sarasota, Florida, mate coercion occurs less frequently, and allied and non-allied males increase associations with females in the nonbreeding season compared to the breeding season, suggesting males may attempt to form affiliative relationships to influence future mating success through female choice (Owen et al. 2002).
Intrasexual (male-male) aggression is also evident from tooth-rake marks and opportunistic sightings of violent interactions (Connor et al. 1992, 2000b; Scott et al. 2005; Hamilton et al. 2019). However, the rates and severity of aggression may be underestimated as internal wounds from body slamming may not be externally visible and tooth-rake mark scars typically regain pigmentation within 20 months (Lockyer and Morris 1990; Ross and Wilson 1996). Several studies have found a significant sex difference in the prevalence of conspecific tooth-rake marks on bottlenose dolphins; more males have rake marks than females (Scott et al. 2005; Marley et al. 2013; Lee et al. 2019). This consistent sex-specific pattern suggests that aggression occurs in the context of male-male competition for access to mates. Patterns of rake mark coverage appear to vary among populations. In Sarasota, there was no observed sex difference in rake mark coverage (Tolley et al. 1995), whereas in Scotland, males had greater rake mark coverage than females (Marley et al. 2013). This sex difference may reflect the lack of male-male cooperation (i.e., alliances) in Scotland, resulting in increased competition and aggression (Marley et al. 2013).
12.2.1 Variation in Male Mating Tactics
Significant variation in male cooperation exists as not all populations of bottlenose dolphins exhibit reproductive cooperation (i.e., no alliances; Wilson 1995; Lusseau et al. 2003), males within a population may utilize different tactics (i.e., solitary vs. allied; Owen et al. 2002; Wiszniewski et al. 2012a), and alliances may be multilevel (i.e., first-order vs. second-order; Ermak et al. 2017; Connor et al. 2022; Table 12.1). Quantitative measures used to identify alliances differ among researchers (Table S1), which likely influences some of this variation. Qualitatively, first-order alliances are consistently defined as enduring relationships among males with repeated instances of cooperation within a reproductive context (i.e., jointly sequestering and coercing reproductive females; de Waal and Harcourt 1992). In contrast to more opportunistic coalitionary relationships, alliance associations occur year-round during all behavioral states, can last over seasons or years, and are more stable than other ephemeral relationships within dolphin societies (Wells et al. 1987; Connor et al. 1992, 1996; van Hooff and van Schaik 1994). This complex behavior is distinct in that individuals exhibit mutual tolerance, cooperation, and partner preferences to reduce intrasexual competition (Díaz-Muñoz et al. 2014). To mediate social bonds and potentially reduce tensions during consortships, allied males regularly engage in synchronous surfacing (Fig. 12.2; Connor et al. 2006), with the degree of synchrony increasing between partners with weaker bonds (McCue et al. 2020).
12.2.1.1 Populations Without Confirmed Male Alliances
To our knowledge, there is currently no published evidence of confirmed male alliances in populations at the northern and southern limits of bottlenose dolphins’ range (e.g., Scotland, Wilson 1995; New Zealand, Lusseau et al. 2003). Table 12.1 details populations where male alliances have been noted as absent. In Doubtful Sound, New Zealand, no direct mating competition or mate guarding has been observed; Lusseau (2007) hypothesized that mate guarding may be too costly due to both increased female maneuverability in the fjord’s depths and difficulties excluding rivals in the large group sizes (x̄ = 17.2). Male-male aggression, however, is regularly documented; males with higher intrasexual associations were less likely to suffer from aggression (i.e., headbutting) from other males, and they maintained bonds with potential coalition partners through affiliative behavior (i.e., mirroring; Lusseau 2007). While these coalitions function in a non-mate guarding context, coalitions had heterogenous association rates with receptive females and new mothers, suggesting that the maintenance of intrasexual relationships may still be important in this population (Lusseau 2007).
Solitary male mating tactics may not be as conspicuous as the cooperative mate guarding behavior of allied males, so less is known about the variation in solitary tactics across populations (Connor et al. 2000b). It is currently unknown whether individual males consort or attempt to mate guard females, but it is likely that solitary males employ similar tactics to allied males (e.g., roving, mate following/guarding, aggression, and/or displaying to influence female choice). In Sarasota, Florida, “roving” non-allied males have secured paternities, albeit fewer than allied males (Wells 2000; Duffield and Wells 2002; Owen et al. 2002). Stable associations with females may allow a male to be selected as a preferred mate during the breeding season (i.e., female choice; Owen et al. 2002). Although uncommon across populations, preferred male-female associations are a prominent feature of social structures in Ireland (Baker et al. 2020), the Gulf of Trieste, Slovenia (Genov et al. 2019), and Doubtful Sound (Lusseau et al. 2003), where alliance formation has not been documented. Intersex affiliation may play a strong role in determining reproductive success in small populations where alliances are absent and where strong male-female bonds occur (Lusseau et al. 2003; Augusto et al. 2012; Blasi and Boitani 2014; Louis et al. 2017; Baker et al. 2020).
12.2.1.2 Populations with Probable First-Order Male Alliances
Several study sites have indicated probable alliance occurrence based on strong male-male associations but are pending further behavioral analyses or longer study durations to determine the nature of these male bonds (Cedar Key, Florida: Quintana-Rizzo and Wells 2001; Moreton Bay, Australia: Chilvers and Corkeron 2001; San Luis Pass, Texas: Maze-Foley and Würsig 2002; Normano-Breton Gulf, France: Louis et al. 2015; Golfo Dulce, Costa Rica: Moreno and Acevedo-Gutiérrez 2016; Cres-Lošinj archipelago, Croatia: Rako-Gospić et al. 2017). Researchers in Coffin Bay, Australia, identified interconnected male social clusters ranging in size from two to five males resemblant of second-order alliances (Diaz-Aguirre et al. 2018). These preferred associates likely function as alliances, although neither mate guarding nor coercion was documented and male-male aggression appeared to be absent (Diaz-Aguirre et al. 2018). Similarly in Alvarado, Mexico, male dyads and trios had moderate bonds between them; however, researchers noted that detailed behavioral observations to determine the nature of these associations were limited (Morteo et al. 2014).
12.2.1.3 Populations with Confirmed First-Order Male Alliances
The presence and complexity of male alliances vary considerably within and among populations depending on their socio-ecological environments. Males in several nearshore populations cooperatively mate guard through an alliance to decrease intrasexual competition and increase reproductive success (Wells et al. 1987; Connor et al. 1992; Wiszniewski et al. 2012b). To our knowledge, first-order alliances have been reported in Florida (Owen et al. 2002; Bouveroux and Mallet 2010; Ermak et al. 2017; Brightwell et al. 2020), the Bahamas (Parsons et al. 2003; Elliser and Herzing 2011), Ecuador (Félix et al. 2019), Japan (Nishita et al. 2017), and Australia (Smolker et al. 1992; Möller et al. 2001; Chabanne et al. 2022). Table 12.1 provides a list of bottlenose dolphin populations with confirmed alliances.
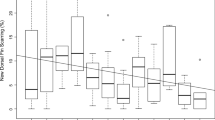
The size and stability of first-order alliances vary. Across populations, pairs are the most commonly documented alliance size (Owen et al. 2002; Parsons et al. 2003; Elliser and Herzing 2011; Nishita et al. 2017; Félix et al. 2019; Brightwell et al. 2020). In Shark Bay, Australia, trio formation is the preferred alliance size (Connor et al. 1999), but the number of partners participating in a consortship is influenced by the habitat’s ecological variation (Connor et al. 2017). Greater intrapopulation variation has been observed in Port Stephens, Australia, and the St. Johns River, Florida, where alliances ranged from pairs to quads, with pairs most common (Wiszniewski et al. 2012a; Ermak et al. 2017; Fig. 12.3). Wiszniewski et al. (2012a) documented considerable variation among Port Stephens alliances that encapsulates the continuum of alliance tactics across populations: males in strong highly stable alliances, males in weaker and more labile alliances, and males that were allied for a short duration. At the longest running behavioral study sites, Sarasota, Florida, and Shark Bay, Australia, researchers have documented alliances ranging in duration from labile (e.g., changing each season or consortship) to stable partnerships lasting decades (Wells 1991; Connor et al. 1999, 2001; Connor and Krützen 2015). Disappearances can cause partner changes on shortened timescales, and males may form new alliances with unallied males whose partners may have also disappeared (Connor et al. 2000b). However, partner switches also occur when a previous alliance partner remains present in the same geographic area, indicating changes in association preferences (Wiszniewski et al. 2012a, Karle 2016; Brightwell et al. 2020).
Social network of 23 dyadic and 2 triadic alliances in bottlenose dolphins (Tursiops truncatus) in the St. Johns River, Jacksonville, Florida, from April 2011 to March 2018. Edge weights correspond to association strength calculated using the simple ratio index (SRI). Associations less than twice the nonzero male mean (SRI = 0.114) were removed. Node colors denote first-order alliance membership with second-order alliances sharing similar colors: yellows are the 6 dyads and 1 trio that form only first-order alliances; pinks, oranges, and reds are the 6 dyads that only form 1 second-order alliance each; and purples, blues, and greens are the 11 dyads and 1 trio that are part of larger second-order complexes wherein some (but not all) of the first-order alliances form multiple second-order alliances. SRIs were calculated in SOCPROG 2.9 (Whitehead 2009) and nodes arranged using the Force Atlas 2 algorithm in GEPHI (Bastian et al. 2009)
In populations where alliances have been documented, solitary (unallied) males are also present. The relative percentage of allied vs. unallied males varies; in some populations, solitary males are as prevalent, or more so, than allied males (≥50% unallied males in Little Bahama Bank, Bahamas, Elliser and Herzing 2011; St. Johns River, Ermak et al. 2017; Indian River Lagoon, Florida, Brightwell et al. 2020). In other populations, most males form alliances (<30% unallied males in Shark Bay, Smolker et al. 1992; Port Stephens, Möller et al. 2001; Sarasota, Owen et al. 2002). It is possible that solitary males are successfully using an alternative mating tactic. For example, Krützen et al. (2004) found that unallied juvenile males sired offspring.
12.2.1.4 Populations with Documented Multilevel Male Alliances
Second-order alliances consist of multiple first-order alliances that cooperate in contests over females (e.g., attempted thefts or defense of females from rival males; Connor et al. 1992). Quantitatively, the social bonds among members of second-order alliances are more moderate in strength than those among first-order alliance partners (Connor et al. 1992, 1999; Ermak et al. 2017; Table S1). This level of male social complexity is extremely rare; multilevel bottlenose dolphin alliances have been documented in only two populations to date: Shark Bay, Australia, and the St. Johns River, Florida. The majority of Shark Bay males are members of second-order alliances ranging in size from 4 to 14 members, with alliance size potentially related to the members’ foraging tactics (Connor and Krützen 2015; Bizzozzero et al. 2019; O’Brien et al. 2020). Second-order alliances are believed to be the core male social unit in the Shark Bay population (Connor and Krützen 2015), as males choose their first-order (herding) partner(s) from within their second-order alliances (Connor et al. 2011; King et al. 2021). While the identity of some first-order pairs and trios is stable (i.e., high partner fidelity), many second-order alliances demonstrate much greater flexibility in the formation of pairs and trios (Connor and Krützen 2015). This frequent partner switching is believed to maintain cooperative relationships within a larger group (Connor et al. 1999). Second-order alliances can endure for 20 years, ending due to gradual attrition more often than relationship changes (Connor and Krützen 2015). Surviving members of second-order alliances that have dissolved to the size of a first-order alliance (“lone trios”) still form relationships with other alliances, but at the association level of third-order alliances (Connor et al. 2011).
In contrast, second-order alliances do not appear to be the core male social unit in the St. Johns River, as males in this community exhibit a variety of mating tactics. As shown in Fig. 12.3, males may be unallied, form only a first-order alliance, form only one second-order alliance, or form multiple second-order alliances (Ermak et al. 2017). Among allied males, partner fidelity is high with most alliances dissolving due to a partner’s death or disappearance (Brightwell and Gibson, unpublished data). However, some alliances have reduced associations despite partners remaining in the area (Karle 2016). Switching herding partners between consortships, as observed in Shark Bay, has not been documented in the St. Johns River. Second-order alliance duration also appears to be more variable within the St. Johns River than in Shark Bay.
A third level of alliance formation, cooperation among multiple second-order alliances, has also been reported (Connor and Krützen 2015). Although the functions of first- and second-order alliances differ (i.e., consortships vs. female theft/defense), Connor and Krützen (2015) proposed that second- and third-order alliances are functionally similar. Third-order alliances in Shark Bay increased consortship duration by increasing the likelihood that allies were nearby (Connor et al. 2022). While there have been observations of groups containing multiple second-order alliances in the St. Johns River (Fig. 12.3), it is not yet clear if third-order alliances are present.
12.2.2 Cooperation Benefits
Populations with bisexual philopatry (i.e., both sexes remain in the same area postweaning) allow for association and affiliative bonding with kin postweaning (van Hooff and van Schaik 1994; Tsai and Mann 2013; Wells 2014; Wallen et al. 2017). As fertilizations cannot be shared, cooperation among individuals can provide direct (i.e., increased reproductive success) and/or indirect (e.g., kin selection) fitness benefits (Hamilton 1964; Würsig et al. 2023, this book). Although relatedness is not yet documented for many populations, where it has been studied, there does not appear to be a clear pattern across populations. Mean genetic relatedness was higher within than between alliances off Abaco, Bahamas (Parsons et al. 2003). Similarly, in Coffin Bay, Australia, preferential associates were more likely to be related than by chance (i.e., probable alliances; Diaz-Aguirre et al. 2018). In contrast, alliance members in Port Stephens, Australia (Möller et al. 2001), and Sarasota (Duffield and Wells 2002), were primarily unrelated, despite the presence of male relatives in the population. Findings from Shark Bay are mixed; early reports indicated that males in small, stable first- and second-order alliances were more related than those in a large second-order alliance with more labile herding partners (i.e., first-orders within a “super-alliance,” Krützen et al. 2003). Recent Shark Bay analyses evaluated individual male relatedness, as opposed to average group relatedness, and found that while kinship explained adolescent associations, similar ages between males were a better predictor of adult associations (Gerber et al. 2021), similar to the patterns observed among allied pairs in Sarasota (Wells 2014).
Alliance partner preferences for close relatives may not be a successful tactic given differences in sexual and social maturity among siblings due to demographic constraints (i.e., single births, extended interbirth intervals), and joint skill may be a more important driver than relatedness in partner selection (Möller 2012; Diaz-Aguirre et al. 2018; Gerber et al. 2021). However, depending on the population, partner choice is likely influenced to varying degrees by a mixture of kin selection and a form of reciprocity or by-product mutualism based on the availability of similar sexually and socially mature individuals (Trivers 1971; West-Eberhard 1975; Díaz-Muñoz et al. 2014). The adaptive benefits of reproductive cooperation, in the shape of increased reproductive success, likely offsets any incurred costs due to sharing copulations with unrelated allies.
Alliance membership is believed to be advantageous to reproductive success in populations that exhibit this male mating tactic. In Sarasota, both solitary and allied males sired offspring, with allied males siring disproportionately more calves despite appearing to associate equally with females (Wells 2000; Duffield and Wells 2002; Owen et al. 2002). In Shark Bay, non-allied males sired few, if any, offspring (Krützen et al. 2004), as males with more homogenous social bonds with second-order partners obtained the most paternities (Gerber et al. 2022). In Port Stephens, paternities were positively correlated with the number of males in an alliance and evenly distributed among members (Wiszniewski et al. 2012b), yet alliance social bond strengths were not predictors of success. Wiszniewski et al. (2012b) hypothesized that the variance in male reproductive success was attributed to mate guarding within a diffuse breeding season.
While alliances function in a reproductive context, they may also provide additional advantages through protection (e.g., reduced predation risk; Wells 1991; Hill and Lee 1998). Allied males in Sarasota, Florida, had larger home ranges, and although they acquired more shark bite scars, they lived longer than solitary males (Wells 1991). This pattern suggests that alliance partners may provide increased predator detection or enable cooperative defense (Wells 1991). Predation risk can be approximated through documentation of shark bite scars in field observations or through postmortem reports, as relatively few predation attempts have been directly observed by researchers (e.g., Gibson 2006). However, predation risk is likely underestimated in all areas; typically only survivors of predation attempts are observed by researchers in the field, and carcass recovery may not be feasible. Males may also guard a partner during recovery from an injury (Wells 1991). This hypothesis was supported by observations that alliances remained stable after anthropogenic injuries were incurred, with the exception of a male that died post-injury (Greenfield et al. 2021). In contrast, two Gulf of Guayaquil, Ecuador, alliances dissolved during a partner’s entanglement in fishing gear, and did not resume alliance status with their original partner after disentanglement (Félix 2021).
12.3 Hypotheses on Differences
We examine several socio-ecological factors that may help explain the variation in male bottlenose dolphin alliance complexity among populations. Encounter rates, in concert with the operational sex ratio and sexual size dimorphism, likely affect a male’s choice of mating tactic. In populations with a high rival encounter rate, limited availability of breeding partners, and minimal intersexual size differences, alliance formation should be favored if it leads to increased mating opportunities for allied males that can outcompete lone males or smaller alliances (Whitehead and Connor 2005). In contrast, in populations with low encounter rates, with stable availability of receptive females, and where males are large enough to effectively monopolize a female, alliance formation may not confer any significant reproductive benefits (Connor et al. 2000b; Möller 2012).
12.3.1 Sexual Size Dimorphism
Without a large degree of sexual size dimorphism (SSD), it may be difficult for a lone male to sequester and monopolize a female in a three-dimensional environment. Alliances are likely beneficial in that males can coordinate their spatial positions to effectively restrict female movements, while more robust males may be able to intimidate females on their own and not need assistance in mate guarding (Connor et al. 2000a). Bottlenose dolphin SSD is constrained, particularly with respect to body shape and size variations, possibly due to the energetic costs associated with increasing drag (Connor et al. 2000a). If SSD is present, the most pronounced differences are in robustness and modes of propulsion (males 11–47% heavier than females; Tolley et al. 1995; McFee et al. 2010); however, differences in mass (kg) are less often reported. Population differences in the degree of SSD and alliance formation tend to follow this predicted pattern (Connor et al. 2000a). There is minimal SSD in Shark Bay, Australia (van Aswegen et al. 2019), where multilevel alliances are present; slight-to-moderate SSD in Florida (Tolley et al. 1995; McFee et al. 2010), which has first-order alliances; and more moderate SSD in Scotland (Cheney et al. 2018a) and Brazil (Fruet et al. 2012) where alliances are absent. Although this comparison may be confounded by species-specific (T. truncatus vs. T. aduncus) differences in morphology, second-order alliances have been documented in both T. aduncus (Shark Bay; Connor et al. 1992) and T. truncatus (St. Johns River, Florida; Ermak et al. 2017). In the Bahamas, bottlenose dolphins are much larger than the sympatric spotted dolphins (Stenella frontalis), and bottlenose dolphin males attempt interspecific matings without the assistance of alliance partners (Elliser and Herzing 2016).
12.3.2 Operational Sex Ratio
Alliance formation would be expected in populations with a strongly male-biased operational sex ratio (OSR) as a tactic to reduce male-male competition (Daly and Wilson 1983; Whitehead and Connor 2005). Although the ratio of reproductively available males to females can be difficult to assess directly, the average interbirth interval (IBI) of females in a population can serve as a proxy for the OSR. Due to long gestation and lactation periods (Mann et al. 2000; Henderson et al. 2014), individual female bottlenose dolphins are unavailable to breed for several years at a time which can influence the degree of male-male competition. Few studies have reported a mean IBl of <3 yr for surviving calves, with most documented IBIs ranging between 3 and 4 years (Table 12.1). Among the populations with mean IBIs >4 yr, which suggests high levels of male-male competition, the full continuum of male social complexity (from no alliances to multilevel alliances) is observed. Thus, a male-biased OSR (and longer IBIs) may be a contributing factor for alliance formation, but it is unlikely to be the primary driver. However, calf mortality rates should also be considered due to their impacts on IBIs and the OSR (Mann et al. 2000; Karniski et al. 2018).
12.3.3 Encounter Rates
The encounter rate with rival males, which is often estimated using population density (dolphins/km2; Connor et al. 2000b), likely impacts alliance formation; however, density can vary among and within study sites as it may be influenced by demographics, predation pressure, resource availability, and habitat (Heithaus and Dill 2002; Wiszniewski et al. 2012a; Connor et al. 2017). Theoretically, given a set population density within a community, an increase in daily travel distance could increase the male-male encounter rate with adjacent communities, and a more open habitat would increase the detectability of rivals through better sound propagation (reviewed in Connor et al. 2000b). When the likelihood of encountering potential rivals is high, males may reduce competition and increase reproductive success via cooperative mating tactics (i.e., alliance formation; Connor and Whitehead 2005). The costs of sharing mating opportunities would be lower than the accrued benefits of gaining and maintaining access to fertile females. As population density and thus competition increase, cooperation benefits and alliance sizes should increase as well. However, the spatiotemporal distribution of male-male competition varies; clusters of increased competition may lead to the formation of clusters in the distribution of alliance sizes (e.g., pairs and trios; Whitehead and Connor 2005). An alternative explanation for this potential correlation between population density and alliance formation is that social complexity is easier for researchers to document in populations with high density. Table 12.1 summarizes male alliance complexity with respect to population density across populations.
The two locations with the greatest alliance complexity (i.e., multilevel alliances and multiple/shifting tactics [intrapopulation variation]) also have some of the highest reported population densities (Shark Bay, Australia, Bejder et al. 2006; St. Johns River, Florida, Ermak et al. 2017 and Mazzoil et al. 2020). In Shark Bay, alliance range overlap increases during the breeding season, and consortship size (male pairs vs. trios) and aggression (new tooth-rake marks) increase at the study site’s transition from shallow banks to open habitat, suggesting alliance size is being driven by both encounter rate and rival detection (Whitehead and Connor 2005; Connor et al. 2017; Hamilton et al. 2019). St. Johns River dolphins also demonstrate seasonal shifts in habitat use during the breeding season, which coincides with a large influx of transients and seasonal residents whose core areas are concentrated near the mouth of the river (Mazzoil et al. 2020; Szott et al. 2022). Upriver range expansion is limited due to low salinity levels which compacts dolphin density within the river despite seasonal habitat shifts (Ermak et al. 2017; Mazzoil et al. 2020).
Encounter rates in Sarasota, Florida, may be affected by home ranges in a shallow and fragmented habitat, where rival detectability can be restricted. Allied males have larger ranges compared to unallied males (Wells et al. 1987; Owen et al. 2002), and males occasionally leave the study area for months at a time, thereby increasing their encounters with males in adjacent communities (Wells 1991; Urian et al. 2009). Further, range overlap among communities can increase competition in those areas (Wells et al. 1987). In Port Stephens, Australia, males with more labile alliance partners, larger group sizes, and larger social networks relative to the population’s averages concentrated their spatial use close to the entrance of the embayment where they would encounter males from the coastal population (Wiszniewski et al. 2010, 2012a). As with the fluid first-order alliances within Shark Bay’s larger second-order alliances, Port Stephens males with a large social network likely have reduced costs of partner switching due to maintenance of social bonds among potential partners (Connor et al. 1999; Whitehead and Connor 2005; Wiszniewski et al. 2012a). In the Gulf of Guayaquil, Ecuador, the two communities with the most survey effort demonstrate a male-biased OSR (3:1 and 2:1; Félix and Burneo 2020) with sightings concentrated at channel mouths (Félix et al. 2017). Alliances with low male-female associations had wider home ranges than the alliance with stronger male-female bonds (Félix et al. 2019), suggesting these males may be forced to rove between communities for mating opportunities.
Bottlenose dolphin alliance formation on the Little Bahama Bank, Bahamas, is likely influenced by both intra- and interspecific encounter rates with the sympatric spotted dolphin (Stenella frontalis) population. Cross-species mating and suspected hybridization have been reported in the Bahamas (Herzing et al. 2003; Herzing and Elliser 2013), effectively doubling the population density and increasing male-male competition in this area (average 100 individuals of each species per season; Volker and Herzing 2021). Bottlenose dolphin alliance members are often observed alone during mixed-species encounters (Elliser and Herzing 2016), as their larger size allows them to outcompete the small spotted dolphin males for mating opportunities. Herzing and Johnson (1997) found that it takes six spotted males to chase away one bottlenose dolphin.
In populations with relatively stable population density and low encounter rates, alliance formation is less commonly reported (Table 12.1; Connor and Whitehead 2005). Doubtful Sound, New Zealand, is a small, closed population wherein density remains relatively stable and there is no need to restrict resident females from accessing males from other communities (Lusseau et al. 2003). Moray Firth, Scotland, is also composed of a small population that has increased in abundance from approximately 100 to 200 individuals since the 1980s. Yet dolphins have also expanded their range along the east coast of Scotland (Wilson et al. 2004), keeping encounter rates low.
12.4 Female Mating Tactics
The mating tactics of female bottlenose dolphins have received relatively little research attention compared to those of males; in many cases, female mating tactics can be masked by male-male competition and sexual coercion (Clutton-Brock and McAuliffe 2009), and there are likely more female tactics than are currently reported. The cost of poor mate choice is higher for females than males given the discrepancy in parental investment; female bottlenose dolphins have a yearlong gestation period, produce a single offspring per reproductive event, and exhibit extended interbirth intervals due to long lactation periods (Table 12.1; Whitehead and Mann 2000). Firstborn offspring of young adult females tend to have low survival rates, potentially due to inexperience in parenting, mate choice, or toxic offload (Wells 2000; Schwacke et al. 2002). Calf survival also decreases with maternal age due to reproductive senescence (Karniski et al. 2018). Although physiological factors play a strong role in female reproductive success, social factors such as associations with kin and other females in the same reproductive state can influence fitness as well (Mann et al. 2000; Möller and Harcourt 2008). Mate guarding by males can be costly to females by altering their foraging patterns and energetic budgets due to range and habitat shifts during consortships (Wallen et al. 2016), and it likely also limits their ability to select a preferred mate, at least outside those consorting her. Non-mutually exclusive female countertactics to mate guarding involve polygynandrous mating, preferential association with potential mates, and male avoidance.
Paired with polygynandry, repeated estrus cycles can counteract conception monopolization and reduce harassment, obscure paternity, and improve the genetic quality of offspring (Robeck et al. 2005; Watson 2005; Furuichi et al. 2014). Mate fidelity is uncommon (Duffield and Wells 2002; Wiszniewski et al. 2012b), and the risk of rejecting males can increase harassment, aggression, and injury during herding (Scott et al. 2005; Watson 2005). Mothers with calves may also attempt to avoid adult and juvenile males to reduce the threat of infanticide or aggression; in Shark Bay, sexual segregation is driven by female avoidance of aggressive males (Gibson and Mann 2008; Galezo et al. 2018). Calf-directed aggression and infanticide are favored in species with seasonal breeding, where lactation duration exceeds gestation duration, and year-round intersexual association occurs (Connor et al. 2000a). Males may be less likely to commit infanticide when there is a possibility that calves may be their offspring; thus, it is in a female’s best interest to mate with multiple males and not exhibit mate or alliance fidelity (van Schaik and Kappeler 1997; Wiszniewski et al. 2012b; Chap. 11) and in a male’s best interest to exert paternity control through mate guarding.
Multiple estrus cycles may enable females to mate with non-preferred males during one cycle and a preferred male during the next (Connor et al. 1996; Robeck et al. 2005). Males may have imperfect fertility detection, as suggested by the finding that male habitat use and ranging patterns shift during consortships, regardless of the female’s cycling status (Wallen et al. 2016). Consortships occur year-round, even though there can be seasonal peaks in reproduction (Connor et al. 1996; Mann et al. 2000; Karle 2016). However, both sexes may use these opportunities to strengthen bonds and utilize countertactics. Males may consort non-cycling females to strengthen male-male bonds and provide consortship practice prior to the mating season; females may be attempting to confuse paternity and/or evaluate males’ fitness (Connor et al. 1996; Furuichi et al. 2014). Connor et al. (1996) proposed that females may attempt escapes during consortships to test a male’s physical fitness.
Preferentially seeking out or associating with preferred males can facilitate female mate choice (Watson 2005). Females’ associations with males were high during breeding seasons in which the female was cycling (Wells et al. 1987; Smolker et al. 1992), and both non-agonistic and preferred female-male associations have been observed (Connor et al. 1996; Owen et al. 2002; Lusseau 2007; Wiszniewski et al. 2010). Sarasota alliances begin associating with females in the middle of the nonbreeding season, potentially to create affiliative relationships to influence female choice (Owen et al. 2002); this is further supported by the relatively low observations of mate coercion in this population (Tolley et al. 1995). Synchronous surfacing and displays by males facilitate social bonding among males (Fig. 12.4) but may also indicate mate quality to females (Connor et al. 2006; Sakai et al. 2010). Australian alliances (i.e., Shark Bay and Swan Canning Riverpark) have been observed conducting displays near female consorts, suggesting females may utilize these displays as a choice criterion among alliance members (Connor et al. 1992, 2000b; Chabanne et al. 2022).
In bisexually philopatric populations, evasion of related males can reduce the cost of inbreeding. Inbreeding can reduce fitness through lower calving success and extended weaning age (Frère et al. 2010). Mating with unrelated males can increase the chances of better genetic compatibility by obtaining genetically diverse sperm (Jennions and Petrie 2000). Shark Bay females almost never associate with their sons while cycling but do preferentially associate with sons compared to non-sons during anestrous periods, suggesting they may be mitigating for inbreeding risk during estrous (Wallen et al. 2017).
When sexual coercion occurs, modified genitalia may provide females with a mechanism for cryptic female choice and the ability to evade fertilization (Eberhard 1996). While ventrum-ventrum mating has been observed (Tavolga and Essapian 1957), males attempting mating alongside females at the surface or by lateral-ventral or dorsoventral mounting may have better fertilization success (Connor et al. 2000b). Optimal copulatory fit corresponds to a dorsoventral positioning, and penile penetration may be curtailed by a vaginal fold (Orbach et al. 2016, 2017); females can subtly shift position and may obstruct a male’s fertilization success by redirecting sperm or the penis from non-preferred partners into vaginal recesses.
12.5 Conclusions and Future Directions
Within the polygynandrous mating systems of bottlenose dolphins, each sex exhibits conditional mating tactics to optimize sex-specific reproductive success. In estuarine or nearshore coastal populations, males rove between receptive females, solitarily or cooperatively mate guard females, or form preferential intersex bonds (Wilson 1995; Lusseau et al. 2003; Connor and Krützen 2015). Females counteract mate guarding through multi-male mating, evasive behaviors, and preferential intersex bonds (Boness et al. 2002; Galezo et al. 2018; Baker et al. 2020). Less is known about the sexual strategies of offshore dolphins. Offshore studies provide logistical challenges, and larger group sizes can make it difficult to maintain proximity to specific individuals; it is hypothesized that larger groups and deeper depths make it difficult for males to sequester and monopolize a female (Gowans et al. 2008).
Further research on contiguous study sites using similar methodological approaches to each other would be beneficial for modeling predictive parameters of alliance formation, while filling in data gaps on morphological, genetic, demographic, and socio-environmental differences (e.g., SSD, OSR/IBI, population density) would enhance a global comparison. Technological advances can reduce some of these data gaps. For example, laser photogrammetry can be used to assess sexual size dimorphism, and drones can provide greater context during behavioral studies (Cheney et al. 2018a; King et al. 2021). Where possible, focal follows should be conducted on individuals of both sexes to provide a more thorough understanding of the context in which alliances form and insight into both solitary male and female mating tactics.
References
Ansmann IC, Lanyon JM, Seddon JM, Parra GJ (2013) Monitoring dolphins in an urban marine system: total and effective population size estimates of Indo-pacific bottlenose dolphins in Moreton Bay. Australia. PLoS one 8(6):e65239
Arso Civil M, Cheney B, Quick NJ, Thompson PM, Hammond PS (2017) A new approach to estimate fecundity rate from inter-birth intervals. Ecosphere 8(4):e01796
Augusto JF, Rachinas-Lopes P, Dos Santos ME (2012) Social structure of the declining resident community of common bottlenose dolphins in the Sado estuary, Portugal. J Mar Biol Assoc UK 92:1773–1782
Baker I, O’Brien J, McHugh K, Berrow S (2018) Female reproductive parameters and population demographics of bottlenose dolphins (Tursiops truncatus) in the Shannon Estuary, Ireland. Mar Biol 165:1–19
Baker I, O’Brien J, McHugh K, Berrow S (2020) Fine-scale sociality reveals female–male affiliations and absence of male alliances in bottlenose dolphins (Tursiops truncatus) in the Shannon Estuary, Ireland. Mar Mamm Sci 36:66–88
Bastian M, Heymann S, Jacomy M (2009) Gephi: an open source software for exploring and manipulating networks. Proc Int AAAI Conf Web Soc Media 3:361–362
Bateman AJ (1948) Intra-sexual selection in drosophila. Heredity 2:349–368
Bejder L, Samuels A, Whitehead H, Gales N, Mann J, Connor R, Heithaus M, Watson-Capps J, Flaherty C, Krützen M (2006) Decline in relative abundance of bottlenose dolphins exposed to long-term disturbance. Cons Biol 20:1791–1798
Bizzozzero MR, Allen SJ, Gerber L, Wild S, King SL, Connor RC, Friedman WR, Wittwer S, Krützen M (2019) Tool use and social homophily among male bottlenose dolphins. Proc R Soc Lond B 286:20190898
Blasi MF, Boitani L (2014) Complex social structure of an endangered population of bottlenose dolphins (Tursiops truncatus) in the Aeolian Archipelago (Italy). PLoS one 9(12):e114849
Blasi MF, Bruno C, Boitani L (2020) Female reproductive output in a Mediterranean bottlenose dolphin Tursiops truncatus population. Aqua Biol 29:123–136
Bolaños-Jiménez J, Morteo E, Delfín-Alfonso CA, Fruet PF, Secchi ER, Bello-Pineda J (2021) Population dynamics reveal a core community of the common bottlenose dolphin (Tursiops truncatus) in open waters of the South-Western Gulf of Mexico. Front Mar Sci 8:753484
Boness DJ, Clapham PJ, Mesnick SL (2002) Life history and reproductive strategies. In: Hoelzel AR (ed) Marine mammal biology: an evolutionary approach. Blackwell Science, Oxford, pp 278–324
Bouveroux TH, Mallefet J (2010) Social structure of bottlenose dolphins, Tursiops truncatus, in Panama City, Florida. J Mar Biol Assoc UK 90:1685–1692
Bouveroux T, Tyson RB, Nowacek DP (2014) Abundance and site fidelity of bottlenose dolphins in coastal waters near Panama City, Florida. J Cetacean Res Manag 14:37–42
Brennan PLR, Cowart JR, Orbach DN (2022) Evidence of a functional clitoris in dolphins. Curr Biol 32:R24–R26
Brightwell K, Titcomb EM, Mazzoil M, Gibson Q (2020) Common bottlenose dolphin (Tursiops truncatus) social structure and distribution changes following the 2008 unusual mortality event in the Indian River lagoon, Florida. Mar Mamm Sci 36:1271–1290
Brusa JL, Young RF, Swanson T (2016) Abundance, ranging patterns, and social behavior of bottlenose dolphins (Tursiops truncatus) in an estuarine terminus. Aqua Mamm 42(1):109
Castro MDPB (2021) Parámetros reproductivos de la población residente de Tursiops truncatus (Cetartiodactyla: Delphinidae) en el Golfo Dulce, Costa Rica. PhD thesis, Universidad Estatal a Distancia
Chabanne DBH, Pollock KH, Finn H, Bejder L (2017) Applying the multistate capture-recapture robust design to characterize metapopulation structure. Methods Ecol Evol 8:1547–1557
Chabanne DB, Krützen M, Finn H, Allen SJ (2022) Evidence of male alliance formation within a small dolphin community. Mamm Biol 102(4):1285–1298
Cheney B, Wells RS, Barton TR, Thompson PM (2018a) Laser photogrammetry reveals variation in growth and early survival in free-ranging bottlenose dolphins. Anim Cons 21(3):252–261
Cheney B, Graham IM, Barton TR, Hammond, PS, Thompson PM (2018b) Site condition monitoring of bottlenose dolphins within the Moray Firth special area of conservation: 2014–2016. Scottish Natural Heritage Research Report No. 1021
Chilvers BL, Corkeron PJ (2001) Trawling and bottlenose dolphins’ social structure. Proc R Soc Lond B 268:1901–1905
Clutton-Brock TH, McAuliffe K (2009) Female mate choice in mammals. Q Rev Biol 84:3–27
Connor RC, Krützen M (2015) Male dolphin alliances in Shark Bay: changing perspectives in a 30-year study. Anim Behav 103:223–235
Connor RC, Smolker RA (1996) “Pop” goes the dolphin: a vocalization male bottlenose dolphins produce during consortships. Behaviour 133:643–662
Connor RC, Vollmer N (2009) Sexual coercion in dolphin consortships: a comparison with chimpanzees. In: Muller MN, Wrangham RW (eds) Sexual coercion in primates: an evolutionary perspective on male aggression against females. Harvard University Press, Cambridge, MA, pp 218–243
Connor RC, Whitehead H (2005) Alliances II: rates of encounter during resource utilization: a general model of intrasexual alliance formation in fission-fusion societies. Anim Behav 69:127–132
Connor RC, Smolker RA, Richards AF (1992) Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc Natl Acad Sci U S A 89:987–990
Connor RC, Richards A, Smolker R, Mann J (1996) Patterns of female attractiveness in Indian Ocean bottlenose dolphins. Behaviour 133:37–69
Connor RC, Heithaus MR, Barre LM (1999) Superalliance of bottlenose dolphins. Nature 371:571–572
Connor RC, Read AJ, Wrangham R (2000a) Male reproductive strategies and social bonds. In: Mann J, Connor R, Tyack P, Whitehead H (eds) Cetacean societies: field studies of whales and dolphins. University of Chicago Press, Chicago, IL, pp 247–269
Connor RC, Mann J, Read A, Wells RJ (2000b) The bottlenose dolphin: social relationships in a fission-fusion society. In: Mann J, Connor R, Tyack P, Whitehead H (eds) Cetacean societies: field studies of whales and dolphins. University of Chicago Press, Chicago, IL, pp 91–126
Connor RC, Heithaus MR, Barre LM (2001) Complex social structure, alliance stability and mating access in a bottlenose dolphin ‘super-alliance’. Proc R Soc Lond B 268(1464):263–267
Connor RC, Smolker R, Bejder L (2006) Synchrony, social behaviour and alliance affiliation in Indian Ocean bottlenose dolphins, Tursiops aduncus. Anim Behav 72:1371–1378
Connor RC, Watson-Capps JJ, Sherwin WB, Krützen M (2011) A new level of complexity in the male alliance networks of Indian Ocean bottlenose dolphins (Tursiops sp). Biol Lett 7:623–626
Connor RC, Cioffi WR, Randić S, Allen SJ, Watson-Capps J, Krützen M (2017) Male alliance behaviour and mating access varies with habitat in a dolphin social network. Sci Rep 7:1–9
Connor RC, Krützen M, Allen SJ, Sherwin WB, King SL (2022) Strategic intergroup alliances increase access to a contested resource in male bottlenose dolphins. Proc Natl Acad Sci U S A 119:e2121723119
Coxon J, Arso Civil M, Claridge D, Dunn C, Hammond PS (2022) Investigating local population dynamics of bottlenose dolphins in the northern Bahamas and the impact of hurricanes on survival. Mamm Biol 102:1–16
Currey RJ, Dawson SM, Slooten E (2007) New abundance estimates suggest doubtful sound bottlenose dolphins are declining Pac Cons Biol 13(4):274–282
da Silva VMF, Spinelli LG (2023) Play, sexual display, or just boredom relief? In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Daly M, Wilson M (1983) Sex, evolution, and behavior, 2nd edn. Wadsworth Publishing, Belmont, CA
de Waal FBM, Harcourt AH (1992) Coalitions and alliances: a history of ethological research. In: Harcourt A, de Waal FBM (eds) Coalitions and alliances in humans and other animals. Oxford University Press, New York, NY, pp 1–19
Diaz-Aguirre F, Parra GJ, Passadore C, Möller L (2018) Kinship influences social bonds among male southern Australian bottlenose dolphins (Tursiops cf. australis). Behav Ecol Sociobiol 72:1–13
Díaz-Muñoz SL, Du Val EH, Krakauer AH, Lacey EA (2014) Cooperating to compete: altruism, sexual selection and causes of male reproductive cooperation. Anim Behav 88:67–78
Duffield DA, Wells RS (2002) The molecular profile of a resident community of bottlenose dolphins, Tursiops truncatus. In: Pfeiffer CJ (ed) Molecular and cell biology of marine mammals. Krieger, Melbourne, pp 3–11
Durden WN, Stolen ED, Jablonski T, Moreland L, Howells E, Sleeman A, Denny M, Biedenbach G, Mazzoil M (2021) Abundance and demography of common bottlenose dolphins (Tursiops truncatus truncatus) in the Indian River Lagoon, Florida: a robust design capture-recapture analysis. PLoS One 16(4):e0250657
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton, NJ
Elliser CR, Herzing DL (2011) Replacement dolphins? Social restructuring of a resident pod of Atlantic bottlenose dolphins, Tursiops truncatus, after two major hurricanes. Mar Mamm Sci 27:39–59
Elliser CR, Herzing DL (2016) Changes in interspecies association patterns of Atlantic bottlenose dolphins, Tursiops truncatus, and Atlantic spotted dolphins, Stenella frontalis, after demographic changes related to environmental disturbance. Mar Mamm Sci 32(2):602–618
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Ermak J, Brightwell K, Gibson Q (2017) Multi-level dolphin alliances in northeastern Florida offer comparative insight into pressures shaping alliance formation. J Mamm 98:1096–1104
Fearnbach H, Durban J, Parsons K, Claridge D (2012) Photographic mark–recapture analysis of local dynamics within an open population of dolphins. Ecol Appl 28:402–411
Félix F (2021) Impacts of fishing entanglement on the bottlenose dolphin society in the Gulf of Guayaquil. Ecuador. Aqua Mamm 47(2):127–134
Félix F, Burneo SF (2020) Imminent risk of extirpation for two bottlenose dolphin communities in the Gulf of Guayaquil. Ecuador. Front Mar Sci 7:734
Félix F, Calderón A, Vintimilla M, Bayas-Rea RA (2017) Decreasing population trend in coastal bottlenose dolphin (Tursiops truncatus) from the Gulf of Guayaquil, Ecuador. Aqua Cons 27:856–866
Félix F, Van Bressem MF, Van Waerebeek K (2019) Role of social behaviour in the epidemiology of lobomycosis-like disease in estuarine common bottlenose dolphins from Ecuador. Dis Aqua Org 134:75–87
Frau S, Ronchetti F, Perretti F, Addis A, Ceccherelli G, La Manna G (2021) The influence of fish farm activity on the social structure of the common bottlenose dolphin in Sardinia (Italy). PeerJ 9:e10960
Frère CH, Krützen M, Kopps AM, Ward P, Mann J, Sherwin WB (2010) Inbreeding tolerance and fitness costs in wild bottlenose dolphins. Proc R Soc Lond B 277:2667–2673
Fruet PF, Kinas PG, Silva KG, Di Tullio JC, Monteiro DS, Dalla Rosa L, Estima SC, Secchi ER (2012) Temporal trends in mortality and effects of by-catch on common bottlenose dolphins, Tursiops truncatus, in southern Brazil. J Mar Biol Assoc UK 92:1865–1876
Fruet PF, Daura-Jorge FG, Möller LM, Genoves RC, Secchi ER (2015a) Abundance and demography of bottlenose dolphins inhabiting a subtropical estuary in the southwestern Atlantic Ocean. J Mamm 96:332–343
Fruet PF, Genoves RC, Möller LM, Botta S, Secchi ER (2015b) Using mark-recapture and stranding data to estimate reproductive traits in female bottlenose dolphins (Tursiops truncatus) of the southwestern Atlantic Ocean. Mar Biol 162(3):661–673
Furuichi T, Connor R, Hashimoto C (2014) Non-conceptive sexual interactions in monkeys, apes, and dolphins. In: Yamagiwa J, Karczmarski L (eds) Primates and cetaceans: field research and conservation of complex mammalian societies. Springer, Tokyo, pp 385–408
Galezo AA, Krzyszczyk E, Mann J (2018) Sexual segregation in Indo-Pacific bottlenose dolphins is driven by female avoidance of males. Behav Ecol 29(2):377–386
Genov T, Kotnjek P, Lesjak J, Hace A, Fortuna CM (2008) Bottlenose dolphins (Tursiops truncatus) in Slovenian and adjacent waters (northern Adriatic Sea). Ann Ser Hist Nat 18(2):227–244
Genov T, Centrih T, Kotnjek P, Hace A (2019) Behavioural and temporal partitioning of dolphin social groups in the northern Adriatic Sea. Mar Biol 166:1–16
Genoves RC, Fruet PF, Di Tullio JC, Möller LM, Secchi ER (2018) Spatiotemporal use predicts social partitioning of bottlenose dolphins with strong home range overlap. Ecol Evol 8(24):12597–12614
Gerber L, Wittwer S, Allen SJ, Holmes KG, King SL, Sherwin WB, Wild S, Willems EP, Connor RC, Krützen M (2021) Cooperative partner choice in multi-level male dolphin alliances. Sci Rep 11(1):1–10
Gerber L, Connor RC, Allen SJ, Horlacher K, King SL, Sherwin WB, Willems EP, Wittwer S, Krützen M (2022) Social integration influences fitness in allied male dolphins. Curr Biol 32(7):1664–1669
Gibson QA (2006) Non-lethal shark attack on a bottlenose dolphin (Tursiops sp.) calf. Mar Mamm Sci 22:190–197
Gibson QA, Mann J (2008) The size, composition and function of wild bottlenose dolphin (Tursiops sp.) mother–calf groups in Shark Bay, Australia. Anim Behav 76(2):389–405
Gowans S, Würsig B, Karczmarski L (2008) The social structure and strategies of delphinds: predictions based on an ecological framework. Adv Mar Biol 53:195–294
Greenfield MR, McHugh KA, Wells RS, Rubenstein DI (2021) Anthropogenic injuries disrupt social associations of common bottlenose dolphins (Tursiops truncatus) in Sarasota Bay, Florida. Mar Mamm Sci 37:29–44
Gross MR (1996) Alternative reproductive strategies and tactics: diversity within sexes. Trends Ecol Evol 11:92–98
Ham JR, Lilley MK, Manitzas Hill H (2023) Non-conceptive sexual behavior in cetaceans: comparison of form and function. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Hamilton WD (1964) The genetical evolution of social behaviour. J Theor Biol 7:17–52
Hamilton RA, Borcuch T, Allen SJ, Cioffi WR, Bucci V, Krützen M, Connor RC (2019) Aggression varies with consortship rate and habitat in a dolphin social network. Behav Ecol Sociobiol 73:141
Harvey PH, Harcourt AH (1984) Sperm competition, testes size, and breeding systems in primates. In: Smith RJ (ed) Sperm competition and the evolution of animal mating systems. Academic Press, Orlando, FL, pp 589–600
Heithaus MR, Dill LM (2002) Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology 83:480–491
Henderson SD, Dawson SM, Currey RJC, Lusseau D, Schneider K (2014) Reproduction, birth seasonality, and calf survival of bottlenose dolphins in doubtful sound, New Zealand. Mar Mamm Sci 30(3):1067–1080
Herzing DL, Elliser CR (2013) Directionality of sexual activities during mixed-species encounters between Atlantic spotted dolphins (Stenella frontalis) and bottlenose dolphins (Tursiops truncatus). Int J Comp Psych 26:124–134
Herzing DL, Johnson CM (1997) Interspecific interactions between Atlantic spotted dolphins (Stenella frontalis) and bottlenose dolphins (Tursiops truncatus) in The Bahamas, 1985–1995. Aqua Mamm 23:85–99
Herzing DL, Moewe K, Brunnick BJ (2003) Interspecies interactions between Atlantic spotted dolphins, Stenella frontalis, and bottlenose dolphins, Tursiops truncatus, on great Bahama Bank, Bahamas. Aqua Mamm 29:335–341
Hill RA, Lee PC (1998) Predation risk as an influence on group size in cercopithecoid primates: implications for social structure. J Zool Lond 245:447–456
Jennions MD, Petrie M (2000) Why do females mate multiply? A review of the genetic benefits. Biol Rev 75:21–64
Karle K (2016) Structure and function of male bottlenose dolphin alliances in Northeast Florida. MS thesis, University of North Florida
Karniski C, Krzyszczyk E, Mann J (2018) Senescence impacts reproduction and maternal investment in bottlenose dolphins. Proc R Soc Lond B 285:1123
King SL, Connor RC, Krützen M, Allen SJ (2021) Cooperation-based concept formation in male bottlenose dolphins. Nat Comm 12:2373
Krützen M, Sherwin WB, Connor RC, Barré LM, Van de Casteele T, Mann J, Brooks R (2003) Contrasting relatedness patterns in bottlenose dolphins (Tursiops sp.) with different alliance strategies. Proc R Soc Lond B 270:497–502
Krützen M, Barré LM, Connor RC, Mann J, Sherwin WB (2004) ‘O father: where art thou?’— paternity assessment in an open fission–fusion society of wild bottlenose dolphins (Tursiops sp) in Shark Bay, Western Australia. Mol Ecol 13:1975–1990
Lee HH, Wallen MM, Krzyszczyk E, Mann J (2019) Every scar has a story: age and sex-specific conflict rates in wild bottlenose dolphins. Behav Ecol Sociobiol 73(5):1–11
Lockyer CH, Morris RJ (1990) Some observations on wound healing and persistence of scars in Tursiops truncatus. Rep Int Whal Commn Spec Issue 12:113–118
Lohrengel K, Evans PGH, Lindenbaum CP, Morris CW, Stringell TB (2018) Bottlenose dolphin monitoring in Cardigan Bay 2014–2016. Tech Rep NRW Evid Rep 191
Louis M, Gally F, Barbraud C, Béesau J, Tixier P, Simon-Bouhet B, Le Rest K, Guinet C (2015) Social structure and abundance of coastal bottlenose dolphins, Tursiops truncatus, in the Normano-Breton gulf, English Channel. J Mamm 96:481–493
Louis M, Buanic M, Lefeuvre C, Nilliot PL, Ridoux V, Spitz J (2017) Strong bonds and small home range in a resident bottlenose dolphin community in a marine protected area (Brittany, France, Northeast Atlantic). Mar Mamm Sci 33:1194–1203
Lusseau D (2007) Evidence for social role in a dolphin social network. Evol Ecol 21:357–366
Lusseau D, Schneider K, Boisseau OJ, Haase P, Slooten E, Dawson D (2003) The bottlenose dolphin community of doubtful sound features a large proportion of long-lasting associations: can geographic isolation explain this unique trait? Behav Ecol Sociobiol 54:396–405
Magnusson KG, Kasuya T (1997) Mating strategies in whale populations: searching strategy vs harem strategy. Ecol Model 102:225–242
Mann J, Connor RC, Barre LM, Heithaus MR (2000) Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group-size effects. Behav Ecol 11:210–219
Marley SA, Cheney B, Thompson PM (2013) Using tooth rakes to monitor population and sex differences in aggressive behaviour in bottlenose dolphins (Tursiops truncatus). Aqua Mamm 39(2):107–115
Maze-Foley K, Würsig B (2002) Patterns of social affiliation and group composition for bottlenose dolphins (Tursiops truncatus) in San Luis pass. Texas. GoM Sci 20(2):122–134
Mazzoil M, Gibson Q, Durden WN, Borkowski R, Biedenbach G, McKenna Z, Gordon N, Brightwell K, Denny M, Howells E, Jakush J, Moreland L, Perna A, Pinto G, Caldwell M (2020) Spatiotemporal movements of common bottlenose dolphins (Tursiops truncatus truncatus) in Northeast Florida, USA. Aqua Mamm 46:285–300
McCue LM, Cioffi WR, Heithaus MR, Barrè L, Connor RC (2020) Synchrony, leadership, and association in male Indo-Pacific bottlenose dolphins (Tursiops aduncus). Ethology 126(7):741–750
McFee WE, Schwacke JH, Stolen MK, Mullin KD, Schwacke LH (2010) Investigation of growth phases for bottlenose dolphins using a Bayesian modeling approach. Mar Mamm Sci 26(1):67–85
Möller LM (2012) Sociogenetic structure, kin associations and bonding in delphinids. Mol Ecol 21:745–764
Möller LM, Harcourt RG (2008) Shared reproductive state enhances female associations in dolphins. Res Lett Ecol 2008:498390
Möller LM, Beheregaray LB, Harcourt RG, Krützen M (2001) Alliance membership and kinship in wild male bottlenose dolphins (Tursiops aduncus) of southeastern Australia. Proc R Soc Lond B 268:1941–1947
Möller LM, Allen SJ, Harcourt RG (2002) Group characteristics, site fidelity and seasonal abundance of bottlenose dolphins Tursiops aduncus in Jervis Bay and port Stephens, South-Eastern Australia. Aust Mamm 24:11–21
Moreno K, Acevedo-Gutiérrez A (2016) The social structure of Golfo Dulce bottlenose dolphins (Tursiops truncatus) and the influence of behavioural state. R Soc Open Sci 3:160010
Morteo E, Rocha-Olivares A, Abarca-Arenas L (2014) Sexual segregation of coastal bottlenose dolphins (Tursiops truncatus) in the southwestern Gulf of Mexico. Aqua Mamm 40:375–385
Mourão F (2006) Patterns of association among bottlenose dolphins in the bay of islands, New Zealand. MS thesis, University of Auckland
Nishita M, Shirakihara M, Iwasa N, Amano M (2017) Alliance formation of Indo-Pacific bottlenose dolphins (Tursiops aduncus) off Amakusa, Western Kyushu, Japan. Mamm Study 42:125–130
O’Brien O, Allen SJ, Krützen M, Connor RC (2020) Alliance-specific habitat selection by male Indo-Pacific bottlenose dolphins in Shark Bay, Western Australia. Anim Behav 164:39–49
Orbach DN, Marshall CD, Würsig B, Mesnick SL (2016) Variation in female reproductive tract morphology of the common bottlenose dolphin (Tursiops truncatus). Anat Rec 299(4):520–537
Orbach DN, Kelly DA, Solano M, Brennan PLR (2017) Genital interactions during simulated copulation amongst marine mammals. Proc R Soc Lond B 284(1864):20171265
Owen ECG, Wells RS, Hofman S (2002) Ranging and association patterns of paired and unpaired adult male Atlantic bottlenose dolphins, Tursiops truncatus, in Sarasota, Florida, provide no evidence for alternative male strategies. Can J Zool 80:2072–2089
Parsons KM, Durban JW, Claridge DE (2003) Male-male aggression renders bottlenose dolphin (Tursiops truncatus) unconscious. Aqua Mamm 29:360–362
Passadore C, Möller L, Diaz-Aguirre F, Parra GJ (2017) Demography of southern Australian bottlenose dolphins living in a protected inverse estuary. Aqua Cons Mar Freshw Ecosys 27(6):1186–1197
Perrin WF, Reilly SB (1984) Reproductive parameters of dolphins and small whales of the family delphinidae. In: Perrin WF, Brownell RL Jr, DeMaster DP (eds) Reproduction in whales, dolphins and porpoises, Rep Int Whal Commn Spec Issue, vol 6, pp 97–133
Quintana-Rizzo E, Wells RS (2001) Resighting and association patterns of bottlenose dolphins (Tursiops truncatus) in the cedar keys, Florida: insights into social organization. Can J Zool 79:447–456
Rako-Gospić N, Radulović M, Vučur T, Pleslić G, Holcer D, Mackelworth P (2017) Factor associated variations in the home range of a resident Adriatic common bottlenose dolphin population. Mar Poll Bull 124(1):234–244
Robeck TR, Steinman KJ, Yoshioka M, Jensen E, O’Brien JK, Katsumata E, Gili C, McBain JF, Sweeney J, Monfort SL (2005) Estrous cycle characterisation and artificial insemination using frozen–thawed spermatozoa in the bottlenose dolphin (Tursiops truncatus). Reproduction 129(5):659–674
Ronje E, Whitehead H, Barry K, Piwetz S, Struve J, Lecours V, Garrison L, Wells RS, Mullin KD (2020) Abundance and occurrence of common bottlenose dolphins (Tursiops truncatus) in three estuaries of the northwestern Gulf of Mexico. Gulf Caribb Res 31(1):18–34
Ross HM, Wilson B (1996) Violent interactions between bottlenose dolphins and harbor porpoises. Proc R Soc Lond B 263(1368):283–286
Sakai M, Morisaka T, Kogi K, Hishii T, Kohshima S (2010) Fine-scale analysis of synchronous breathing in wild Indo-Pacific bottlenose dolphins (Tursiops aduncus). Behav Proc 83(1):48–53
Schwacke LH, Voit EO, Hansen LJ, Wells RS, Mitchum GB, Hohn AA, Fair PA (2002) Probabilistic risk assessment of reproductive effects of polychlorinated biphenyls on bottlenose dolphins (Tursiops truncatus) from the Southeast United States coast. Envir Toxic Chem 21:2752–2764
Scott EM, Mann J, Watson-Capps JJ, Sargeant BL, Connor RC (2005) Aggression in bottlenose dolphins: evidence for sexual coercion, male-male competition, and female tolerance through analysis of tooth-rake marks and behaviour. Behaviour 142:21–44
Smolker RA, Richards AF, Connor RC, Pepper JW (1992) Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour 123:38–69
Smuts BB, Smuts RW (1993) Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv Study Behav 22(22):1–63
Stockley P (2003) Female multiple mating behavior, early reproductive failure and litter size variation in mammals. Proc R Soc B 270(1512):271–278
Szott EA, Brightwell K, Gibson Q (2022) Assessment of social mixing and spatial overlap as a pathway for disease transmission in a Northeast Florida estuarine dolphin community. Mamm Biol 102:1–17
Tavolga MC, Essapian FS (1957) The behavior of bottle-nosed dolphin (Tursiops truncatus): mating, pregnancy, parturition and mother-infant behavior. Zoologica 42:11–31
Tezanos-Pinto G, Constantine R, Brooks L, Jackson JA, Mourão F, Wells S, Scott Baker C (2013) Decline in local abundance of bottlenose dolphins (Tursiops truncatus) in the bay of islands, New Zealand. Mar Mamm Sci 29(4):E390–E410
Tezanos-Pinto G, Constantine R, Mourão F, Berghan J, Scott Baker C (2015) High calf mortality in bottlenose dolphins in the Bay of Islands, New Zealand, a local unit in decline. Mar Mamm Sci 31:540–559
Thomson S (2021) Drivers of change in social networks of bottlenose dolphins (Tursiops truncatus) in Cardigan Bay, Wales. MS thesis, Bangor University
Tolley KA, Read AJ, Wells RS, Urian KW, Scott MD, Irvine AB, Hohn AA (1995) Sexual dimorphism in wild bottlenose dolphins (Tursiops truncatus) from Sarasota, Florida. J Mamm 76(4):1190–1198
Trivers RL (1971) The evolution of reciprocal altruism. Q Rev Biol 46:35–57
Tsai YJJ, Mann J (2013) Dispersal, philopatry, and the role of fission-fusion dynamics in bottlenose dolphins. Mar Mamm Sci 29:261–279
Urian KW, Duffield DA, Read AJ, Wells RS, Shell ED (1996) Seasonality of reproduction in bottlenose dolphins, Tursiops truncatus. J Mamm 77:394–403
Urian KW, Hofmann S, Wells RS, Read AJ (2009) Fine-scale population structure of bottlenose dolphins, Tursiops truncatus, in Tampa Bay, Florida. Mar Mamm Sci 25:619–638
van Aswegen M, Christiansen F, Symons J, Mann J, Nicholson K, Sprogis K, Bejder L (2019) Morphological differences between coastal bottlenose dolphin (Tursiops aduncus) populations identified using non-invasive stereo-laser photogrammetry. Sci Rep 9(1):1–14
van Hooff JA, van Schaik CP (1994) Male bonds: affiliative relationships among nonhuman primate males. Behaviour 130:309–337
van Schaik CP, Kappeler PM (1997) Infanticide risk and the evolution of male–female association in primates. Proc R Soc Lond B 264(1388):1687–1694
Vermeulen E (2018) Association patterns of bottlenose dolphins (Tursiops truncatus) in Bahía San Antonio, Argentina. Mar Mamm Sci 34(3):687–700
Vermeulen E, Bräger S (2015) Demographics of the disappearing bottlenose dolphin in Argentina: a common species on its way out? PLoS One 10:e0119182
Volker CL, Herzing DL (2021) Aggressive behaviors of adult male Atlantic spotted dolphins: making signals count during intraspecific and interspecific conflicts. Anim Behav Cogn 8(1):36–51
Wallen MM, Patterson E, Krzyszczyk E, Mann J (2016) Ecological costs to females in a system with allied sexual coercion. Anim Behav 115:227–236
Wallen MM, Krzyszczyk E, Mann J (2017) Mating in a bisexually philopatric society: bottlenose dolphin females associate with adult males but not adult sons during estrous. Behav Ecol Sociobiol 71:1–12
Watson JJ (2005) Female mating behavior in the context of sexual coercion and female ranging behavior of bottlenose dolphins (Tursiops sp.) in Shark Bay, Western Australia. PhD thesis, Georgetown University
Wells RS (1991) The role of long-term study in understanding the social structure of a bottlenose dolphin community. In: Pryor K, Norris KS (eds) Dolphin societies: discoveries and puzzles. University of California Press, Berkeley, CA, pp 199–225
Wells RS (2000) Reproduction in wild bottlenose dolphins: overview of patterns observed during a long-term study. In: Duffield D, Robeck T (eds) Bottlenose dolphin reproduction workshop report. AZA Marine Mammal Taxon Advisory Group, Silver Spring, MD, pp 57–74
Wells RS (2014) Social structure and life history of common bottlenose dolphins near Sarasota Bay, Florida: insights from four decades and five generations. In: Yamigawa J, Karczmarski L (eds) Primates and cetaceans: field research and conservation of complex mammalian societies. Springer Press, Tokyo, pp 149–172
Wells RS, Scott MD, Irvine AB (1987) The social structure of free-ranging bottlenose dolphins. In: Genoways HH (ed) Current mammalogy. Plenum, New York, NY, pp 247–304
West-Eberhard MJ (1975) The evolution of social behavior by kin selection. Q Rev Biol 50:1–33
Whitehead H (2009) SOCPROG programs: analysing animal social structures. Behav Ecol Sociobiol 63:765–778
Whitehead H, Connor R (2005) Alliances I. how large should alliances be? Anim Behav 69:117–126
Whitehead H, Mann J (2000) Female reproductive strategies of cetaceans: life histories and calf care. In: Mann J, Connor RC, Tyack PL, Whitehead H (eds) Cetacean societies: field studies of dolphins and whales. University of Chicago Press, Chicago, IL, pp 219–246
Wilson DRB (1995) The ecology of bottlenose dolphins in the Moray firth, Scotland: a population at the northern extreme of the species’ range. PhD thesis, Aberdeen University
Wilson B, Reid RJ, Grellier K, Thompson PM, Hammond PS (2004) Considering the temporal when managing the spatial: a population range expansion impacts protected areas-based management for bottlenose dolphins. Anim Cons 7:331–338
Wiszniewski J, Lusseau D, Möller LM (2010) Female bisexual kinship ties maintain social cohesion in a dolphin network. Anim Behav 80:895–904
Wiszniewski J, Brown C, Möller LM (2012a) Complex patterns of male alliance formation in a dolphin social network. J Mamm 93(1):239–250
Wiszniewski J, Corrigan S, Beheregaray LB, Möller LM (2012b) Male reproductive success increases with alliance size in Indo-Pacific bottlenose dolphins (Tursiops aduncus). J Anim Ecol 81(2):423–431
Wolff JO, Macdonald DW (2004) Promiscuous females protect their offspring. Trends Ecol Evol 19(3):127–134
Würsig B, Rich J, Orbach DN (2023) Sex and behavior. In: Würsig B, Orbach DN (eds) Sex in cetaceans. Springer Nature, Cham
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
12.1 Electronic Supplementary Material
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Brightwell, K., Gibson, Q. (2023). Inter- and Intrapopulation Variation in Bottlenose Dolphin Mating Strategies. In: Würsig, B., Orbach, D.N. (eds) Sex in Cetaceans. Springer, Cham. https://doi.org/10.1007/978-3-031-35651-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-35651-3_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35650-6
Online ISBN: 978-3-031-35651-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)