Abstract
The concentrations of a number of organochlorine pesticides and related chemicals and two organochlorine industrial chemicals were determined in 163 pooled human milk samples from 82 countries from all United Nations regions. These countries participated in one or more of the five exposure studies on persistent organic pollutants coordinated by the World Health Organization and the United Nations Environment Programme between 2000 and 2019. The compounds included were aldrin, chlordane, chlordecone, DDT, dicofol, dieldrin, endosulfan, endrin, heptachlor, hexachlorobenzene, hexachlorobutadiene, hexachlorocyclohexanes, mirex, pentachlorobenzene, pentachlorophenol/pentachloroanisole, and toxaphene.
Large differences were found for DDT with the highest concentrations found in Africa. However, the median levels of the DDT concentrations of all samples show a decrease of 72% from the 2000–2003 period to the 2016–2019 period, with considerable differences between regions. Due to metabolization of hexachlorocyclohexanes (HCH) in humans, the concentrations of alpha-HCH and gamma-HCH were below the limit of quantification in most human milk samples. The ranges of beta-HCH found in the five periods varied considerably among UN regions, with a maximum found in 2002 in the Asia subgroup of the Asia-Pacific region. A decrease of the median concentrations of all samples of 91% was found from the 2000–2003 period to the 2016–2019 period. In comparison with DDT and beta-HCH, the ranges for hexachlorobenzene (HCB) were much lower with a maximum found in the samples from Eastern Europe. Other organochlorine pesticides and contaminants and their metabolites were found mostly in ranges of low background contamination; some were below the limits of quantification.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Human milk biomonitoring
- Stockholm Convention on Persistent Organic Pollutants
- Legacy and emerging POPs
- Organochlorine pesticides
- DDT
- Hexachlorocyclohexanes (HCH)
- Hexachlorobenzene (HCB)
- Organochlorine industrial chemicals
- Global WHO/UNEP human milk studies
- UN regions
- Time trends
1 Introduction
A number of organochlorine pesticides used in agricultural or public health applications and industrial chemicals used in various applications are chemically and physiologically stable, and thus persist in the environmental and biological systems. They are also highly lipophilic, which results in their biomagnification in the food chain and bioaccumulation in fatty tissues of animals and humans. For these reasons, they are classified as persistent organic pollutants (POPs) (UNEP 2017).
To protect human health and the environment, the Stockholm Convention on POPs has identified selected POPs to reduce or eliminate their release into the environment (UNEP 2001). The number of POPs initially covered by the convention has expanded considerably since its adoption in 2001 (UNEP 2020). As of 2019, a total of 30 POPs were listed in the Stockholm Convention and subject to Article 16, which requires that they be monitored to evaluate the effectiveness of the convention. The analysis of those POPs in human milk has been recommended as one of the core matrices within the framework of the Global Monitoring Plan on POPs (GMP) with air being the other recommended matrix (UNEP 2019). Of these 30 POPs, 18 are covered in this article.
Human milk surveys are reviewed in this compendium from various aspects. As background information for this article, the general introduction (Part I) gives a review of human milk surveys on POPs from a historical perspective (Fürst 2023), an overview of the World Health Organization (WHO)- and United Nations Environment Programme (UNEP)-coordinated exposure studies performed between 1987 and 2019 (Malisch et al. 2023a), and a review on the Stockholm Convention on POPs and its implementation summarized by regional and global monitoring reports (Šebková 2023). The analytical methods used for determination of chlorinated pesticides and industrial chemicals in samples of the WHO/UNEP-coordinated exposure studies and their validation are presented in Part II (Hardebusch et al. 2023).
In this paper, the concentrations of the pesticides aldrin, chlordane (comprising cis-, trans- and oxychlordane and nonachlor), chlordecone, dicofol, dichlorodiphenyltrichloroethane (DDT, comprising the p,p′- and o,p′-isomers of DDT and its metabolites DDE and DDD), dieldrin, endosulfan (comprising alpha- and beta-endosulfan and endosulfan sulfate), endrin (including endrin ketone), heptachlor (including heptachlor epoxide), hexachlorobenzene (HCB), gamma-hexachlorocyclohexane (γ-HCH, lindane) and by-products alpha-HCH and beta-HCH, mirex, pentachlorophenol and its metabolite pentachloroanisole and toxaphene and of the industrial chemicals pentachlorobenzene and hexachlorobutadiene are reported for the five WHO/UNEP exposure studies between 2000 and 2019.
Results of the WHO/UNEP-coordinated exposure studies for the 2000–2008 period (Malisch et al. 2008) and the 2008–2009 round (Malisch et al. 2010) showed that in comparison with DDT, the levels of other chlorinated pesticides were low. Human milk data of these studies in the period 2008–2010 demonstrated the worldwide presence of POPs in human tissues in 32 less developed countries (Fiedler et al. 2013). A comprehensive report for the 6th Conference of the Parties to the Stockholm Convention in 2013 provided an overview on all samples of the three studies spanning the period 2000–2012. It revealed large global differences among various POPs (UNEP 2013). Also, aspects of a risk-benefit assessment of breastfeeding were addressed that were published later in more detail (van den Berg et al. 2016).
Worldwide trends in DDT concentrations in human breast milk were assessed compiling data since 1951 until the end of the 1990s (Smith 1999). A global overview on the spatial and temporal trends of Stockholm Convention POPs in breast milk reviews scientific publications between 1995 and 2011 (Fång et al. 2015). The regional and global monitoring reports for the GMP assess datasets in the core media—ambient air, human tissues (human breast milk or blood), and water for hydrophilic POPs, but also other media such as soil, biota, plants are used to support interpretation of observed levels and their trends (UNEP 2022a).
All substance-specific data of the WHO/UNEP-coordinated exposure studies are deposited at the Global Monitoring Plan Data Warehouse (GMP DWH), which can be publicly accessed (Global Monitoring Plan Data Warehouse 2020).
A total of 82 countries participated in one or more of the five studies conducted between 2000 and 2019 and submitted pooled human milk samples, which are considered to represent a country or, in some cases, a subgroup of a country. The results for 163 pooled samples are discussed from various perspectives in the following sections, namely: Sect. 2 General aspects; then Results and Discussions in Sect. 3.1 for DDT; in Sect. 4 for hexachlorocyclohexanes (alpha-HCH, beta-HCH, and gamma-HCH); in Sect. 5 for HCB; in Sect. 6 for other organochlorine pesticides; and, in Sect. 7 for organochlorine industrial chemicals. Section 8 summarizes the findings.
2 General Aspects
2.1 Countries, UN Regions, Protocol, and Analytes Selected
An overview of the scope, protocols for collection of samples and participation of countries with classification in UN regions and temporal differentiation is given in the general introduction in Part I (Malisch et al. 2023a). In brief, the collection of a number of individual samples and preparation of representative pooled samples in all rounds were supervised by a national coordinator in each country following the WHO/UNEP-standardized protocols. Equal aliquots of individual samples were combined to give a composite sample, which was considered to represent the average levels of POPs for a country at the time of sampling. The pooled samples were sent to WHO/UNEP Reference Laboratories for analysis.
WHO was initially focused on polychlorinated biphenyls (PCB), polychlorinated dibenzo-p-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF) and had conducted two studies prior to 2000 (WHO 1989; WHO 1996). A third study was performed from 2000 to 2003 with participation of 26 countries/areas. With the adoption of the Stockholm Convention on Persistent Organic Pollutants in 2001 and in anticipation of its ratification in 2004, a pilot study was conducted in 2003 by WHO and the WHO Reference Laboratory for POPs located at the State Institute for Chemical and Veterinary Analysis of Food (CVUA) in Freiburg, Germany. The study aimed to assess the feasibility of measuring in human milk the initial twelve POPs listed by the convention. In addition, 9 more POPs were included in the study, which were later added to the convention, resulting in the analysis of a total of 21 chemicals. For the pilot study, the fat component extracted from human milk samples from the third round (2000–2003) was used, if sufficient sample amount was retained, which was the case for 16 countries. The results confirmed the advantages of using human milk as a matrix to monitor these POPs.
Similarly, when the 2016 round started, a total of 23 chemicals were required to be analyzed but an additional 7 POPs were about to be added to the convention. Therefore, the Reference Laboratory for chlorinated and brominated substances at CVUA Freiburg, Germany, and for perfluorinated compounds at Örebro University, Sweden, analyzed the seven additional POPs in order to have data on the complete picture of the then listed 30 chemicals for samples of the 2016–2019 period as baseline data for future effectiveness evaluations. Of the total of 30 chemicals as listed by the convention up until 2019, 18 are covered in this article, including hexachlorobutadiene, pentachlorophenol and dicofol from the 7 newly added POPs (for expansion of the analytes of interest over time by the Stockholm Convention and the Reference Laboratories, see Malisch et al. 2023a).
In accordance with the implementation of the GMP, parties report flexibly through one of the five United Nations regional organization groups. Therefore, countries participating in UNEP projects for the GMP are classified according to one of these five UN geopolitical groups: (1) African Group, (2) Asia-Pacific Group, (3) Eastern European Group, (4) Group of Latin American and Caribbean Countries (GRULAC), and (5) Western European and Others Group (WEOG). Note that Australia, Israel, New Zealand, and the USA (being informally a member) are included as “Others” in WEOG countries, whereas Cyprus belongs to the Asia-Pacific Group (for participating countries and regional distribution, see Section 6 in Malisch et al. 2023a). It should, therefore, be noted that these results are not intended to be used for the ranking of individual countries.
2.2 Number of Samples and Aggregation of Data
A total of 82 countries submitted pooled samples during one or more of the five studies conducted from 2000 to 2019. Due to the particular scope at beginning of a study with regard to the expansion of analytes of interest over time, 163 pooled samples were analyzed for various organochlorine pesticides and industrial chemicals.
In the 2000–2003 study, countries were encouraged to submit at least two pooled samples, whereas in the following rounds, usually one pooled sample was submitted by a country. If a country had sent two or more pooled samples in a certain round, the median of the individual results was used for aggregation purposes. This yielded 14 median results that are used to represent the country for that round, namely from Hong Kong in 2002 and 2009; Philippines in 2002; Brazil in 2002 and 2012; Fiji in 2002 and 2006; Australia in 2013; Finland in 2007; Germany in 2002 and 2019; Luxembourg in 2002; Spain in 2002; and the USA in 2003. In contrast, a total of 120 results were obtained from the single pooled sample submitted by countries in a certain round. Thus, 134 country results are available for the “one country—one result for a certain period” approach. The detailed data for all 163 pooled samples is contained at the POPs Global Monitoring Plan Data Warehouse and can be publicly retrieved (Global Monitoring Plan Data Warehouse 2020).
2.3 Analysis and Complexes of DDT, Chlordane, Heptachlor, Endrin, and Endosulfan
The analytical methods for determination of organochlorine pesticides and industrial chemical complexes and their validation are presented in Part II (Hardebusch et al. 2023). All concentrations are reported on lipid weight basis.
For many chemicals, it is recommended to determine not only concentrations of the parent molecule but also to include certain metabolites, degradation products, and/or by-products during manufacture. For this reason, the Stockholm Convention provides guidance on the analytes to be included under each parent POP (UNEP 2019; Malisch et al. 2023a). The sum of the parent POP and its other compounds of concern can be calculated based on the determined levels with two options: (1) without correction for molecular weight (mass basis), (2) after correction for molecular weight (molar basis).
The EU regulation on maximum residue levels in feed and food defines the residues for DDT, chlordane, heptachlor, and endosulfan with the addition of “expressed as …”, e.g., “DDT (sum of p,p′-DDT, o,p′-DDT, p-p′-DDE and p,p′-TDE (DDD) expressed as DDT)” (Regulation (EC) No 396/2005). Therefore, according to the “Pesticide Guidelines,” the sum of the components is calculated by adjusting for different molecular weights (“correction factors” or “conversion factors”) (DG-SANTE 2021) This regulatory approach is harmonized internationally with that of the OECD (OECD 2011).
The sum parameters in this article referred to as “complexes” were calculated following the principles of the EU regulation by use of the correction factors as listed in Table 1 and applying the “lower bound approach,” which uses only quantifiable results (Malisch et al. 2008).
2.4 Background Concentrations
Background concentrations are defined as that portion of the measured human milk levels that is found in the absence of specific sources and therefore is not attributable to a known exposure, e.g., to use of the chemical of interest or to emissions within the study area. In contrast to findings of high concentrations, e.g., after use of chemicals, after a sufficient long withdrawal period for many POPs the levels are described as “low background levels.” However, the term “background level” does not imply per se any level of safety. With respect to potential adverse effects, risk assessments need to consider many factors, including the toxicity of the chemical of interest and the found concentration range. For human milk, potential adverse effects have to be balanced against positive health aspects for (breastfed) infants (van den Berg et al. 2016).
3 Results and Discussion
3.1 DDT
Dichlorodiphenyltrichloroethane (DDT) belongs to the group of the “12 Initial POPs” listed in Annex B (Restriction) to the Stockholm Convention (UNEP 2020). UNEP’s DDT web section covers various aspects on DDT (e.g., overview; COP decisions; technical assistance to Parties; guidance; DDT register; DDT toolkit) and information on the Global Alliance for promoting a global partnership on the development and deployment of alternative products, methods, and strategies to DDT for disease vector control (UNEP 2022b).
Commercial DDT is a mixture mainly of the desired para-para′ substituted isomer (p,p′-DDT = 4,4′-DDT) as major component and the ortho-para′ substituted isomeric impurity (o,p′-DDT = 2,4′-DDT). Due to degradation and metabolization, in humans the transformation products 4,4′-DDE (dichlorodiphenyldichloroethylene) and 2,4-DDE, respectively, and 4,4′-DDD (dichlorodiphenyldichloroethane) and 2,4′-DDD, respectively, are of interest (UNEP 2007; Fürst 2023).
For calculation of the summarizing parameter “DDT complex,” correction factors for molecular weight as shown in Table 1 were applied (see Sect. 2.3). The detailed results for DDT complex (including the isomers of parent molecules and metabolites) are publicly available (Global Monitoring Plan Data Warehouse 2020). As a general conclusion, in most human milk samples concentrations of o,p′-DDD, p,p′-DDD and o,p′-DDE were below the limit of quantification (0.5 μg/kg lipid) and their contribution to DDT complex was below 1%. In most human milk samples, p,p′-DDE contributes about 95% while p,p′-DDT contributes about 5% and o,p′-DDT 0.5%. However, in cases of more recent use or contamination, considerably higher contributions of p,p′-DDT (maximum found: 46%) and slightly increased concentrations of o,p′-DDT (maximum found: 4.2%) can be found.
3.1.1 General Comparison of Ranges
A suitable starting point for the discussion is the comparison of the ranges among UN regions found in the five studies performed over 20 years. Large differences covering three orders of magnitude were found, with a minimum of 17 μg DDT complex/kg lipid found in 2019 in a country from Africa and a maximum of 23,500 μg DDT complex/kg lipid found in 2012 in another country from Africa. The median of 134 country results (see Sect. 2.2) was 255 μg DDT complex/kg lipid.
As the five studies had slightly different time lengths, the comparison of results for the regions is presented in five equal periods of 4 years each, namely: 2000–2003, 2004–2007, 2008–2011, 2012–2015, and 2016–2019 (Malisch et al. 2023a). Table 2 compiles the most important statistical data (number of samples, minimum, median, mean, and maximum) for the 134 country results for the five UN regions (here with split of the of the Asia-Pacific Group into two subgroups, namely the Asian and the Pacific Island countries).
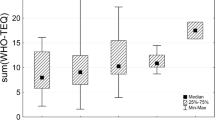
As general estimation of time trends, the median of the DDT complex concentrations of all country results shows a decrease of 72% from the 2000–2003 period (median for 16 countries: 445 μg/kg lipid) to the 2016–2019 period (median for 43 countries: 125 μg/kg lipid). The downward trend between these two end periods was found in all regions, but with considerable differences among regions and a great variation among the three rounds in the middle, as illustrated by Fig. 1. In all groups, the median of the DDT complex concentrations was higher in the 2000–2003 period than in the 2016–2019 period. However, in the three rounds in-between, a considerable variation within UN regions was observed; obviously with a substantial maximum in Latin American and Caribbean countries in the 2004–2007 period and elevated levels also in Africa at that time period. Therefore, conclusions on time trends in the different groups cannot easily be drawn.
Time trends for median concentrations of DDT in human milk (expressed as μg DDT complex/kg lipid) in the five UN regions (with split of the Asia-Pacific Group [APG] into the subgroups Asia and Pacific Islands; GRULAC, Group of Latin American and Caribbean Countries; EEG, Eastern European Group; WEOG, Western European and Others Group) in five periods between 2000 and 2019
If calculated as the mean, the DDT complex concentration was higher in the 2000–2003 period than in the 2016–2019 period in all regions except Africa. Again, in the three rounds in-between, a considerable variation was observed, with a substantial maximum in Africa in the 2012–2015 period (Fig. 2).
Time trends for mean concentrations of DDT in human milk (expressed as μg DDT complex/kg lipid) in the five UN regions (with split of the Asia-Pacific Group [APG] into the subgroups Asia and Pacific Islands; GRULAC, Group of Latin American and Caribbean Countries; EEG, Eastern European Group; WEOG, Western European and Others Group) in five periods between 2000 and 2019
As a result, the median, mean, and ranges of DDT complex concentrations found in the UN regions in five periods over these 20 years cannot be used directly to derive continuous time trends. A closer look into the details of Table 2 reveals the reason: The number of countries participating from a certain UN region in a certain period varied considerably. As example, from 26 countries participating in the 2000–2003 period, 21 were from the Eastern European Group and the Western European and Others Group, whereas only five countries from the other groups (African; Asia-Pacific that is split into two subgroups Asia and Pacific Islands; Latin American and Caribbean) had participated. The proportion of countries from the UN regions changed in the 2008–2011 period, when 37 of the 45 participating countries were from Africa, Asia, Latin America and the Caribbean, and similarly in 2016–2018, when 36 of the 43 countries were from these other groups (Malisch et al. 2023a). It is observed that it is difficult to draw general conclusions on time trends, especially when three or less countries in a region have participated in a certain period, and in particular if a country with a very high concentration participated in single period: This could have a considerable effect on median or mean concentrations for this group during the period.
The assessment of time trends based only on country-specific results of countries with repeated participation allows more certainty in drawing of conclusions, and therefore is optimal for the evaluation of the effectiveness for the purpose of Article 16. This evaluation (comprising also DDT) is published separately in Part IV (Malisch et al. 2023b).
3.1.2 African Group
Of all UN regions, Africa had the widest variation in contamination of human milk with DDT complex. Figure 3 illustrates these results for country results (with the five 4-year periods between 2000 and 2019 shown in different colors; for “country results” see Sect. 2.2). The lowest concentration (17 μg DDT complex/kg lipid) was found in Egypt in 2019; the highest concentration of 23,500 μg DDT complex/kg lipid (or 23.5 mg DDT complex/kg lipid) found in Ethiopia in 2012. In this sample, about 50% of the DDT complex came from p,p′-DDE, 46% from p,p′-DDT and 4% from o,p′-DDT. This is indicative of more recent use and contamination probably due to public health use of DDT to combat mosquitos for malaria control, which is permitted under the Stockholm Convention with some constraints. These conclusions are in line with findings of high mean levels of DDT complex of 12,680 μg/kg lipid (calculated without correction factors) in human milk from Ethiopia collected in 2010 in three cities in areas where malaria is prevalent, and where annual spraying for malaria control was common. Between 55 and 71% of DDT complex was attributed to p,p′-DDT which was indicative of the continued use of DDT at that time. A number of measures were recommended to reduce the levels of DDT exposure (Gebremichael et al. 2013). As a result of the implementation of these measures, the 2019 sample from Ethiopia showed a considerable downward trend with 7100 μg DDT complex/kg lipid. Importantly, in the 2019 sample p,p′-DDE contributed nearly 80% and p,p′-DDT 20% to DDT complex.
3.1.3 Asia-Pacific Group
3.1.3.1 Asia Subgroup
Figure 4 illustrates the DDT results with the period of participation between 2000 and 2019 indicated for the Asian countries of the Asia-Pacific Group. A wide range of DDT concentrations was found, with the lowest concentration in Mongolia in 2018 (45 μg DDT complex/kg lipid) and the highest concentration in Tajikistan in 2009 (8490 μg DDT complex/kg lipid). In this sample with (in other units) about 8.5 mg DDT complex/kg lipid, nearly 98% of the contribution to the sum parameter “DDT complex” came from p,p′-DDE, which suggests legacy contamination through past exposure.
Samples from earlier rounds (UNEP 2013) had higher levels than countries participating in the 2016–2019 round. Hong Kong SAR of China participated twice and showed a decrease to the 2009 level of 1040 μg DDT complex/kg lipid (median of four samples from different subgroups) from the 2002 level of 1580 μg DDT complex/kg lipid (median of ten samples from different population subgroups [Hui et al. 2008]).
3.1.3.2 Pacific Islands Subgroup
Figure 5 illustrates the DDT results with the period of participation between 2000 and 2019 indicated for the Pacific Islands countries of the Asia-Pacific Group. A wide range of DDT concentrations was found, with the lowest concentration in the Marshall Islands in 2019 (31 μg DDT complex/kg lipid) and the highest concentration in the Solomon Islands in 2011 (4760 μg DDT complex/kg lipid). In this sample with (in other units) 4.8 mg DDT complex/kg lipid, 91% of the contribution to the sum parameter “DDT complex” came from p,p′-DDE. With 1390 μg DDT complex/kg lipid, the sample of 2019 from the Solomon Islands had considerably lower DDT levels, with a contribution of 95% from p,p′-DDE.
Results of four participations of Fiji (in 2001, 2006, 2011, and 2019) show a considerable downwards trend from initially 1340 μg DDT complex/kg lipid to 105 μg/kg lipid, with a contribution of p,p′-DDE to DDT complex increasing gradually from 82% in 2001 to 96% in 2019 as indication of an old contamination (with decreasing absolute p,p′-DDE levels from 990 to 91 μg/kg lipid).
3.1.4 Group of Latin American and Caribbean Countries (GRULAC)
Figure 6 illustrates the DDT results with the period of participation between 2000 and 2019 indicated for Latin American and Caribbean countries. A wide range of DDT concentrations was found, with the lowest concentration in Uruguay in 2019 (46 μg DDT complex/kg lipid). The highest concentration was observed in Haiti in 2005 (2830 μg DDT complex/kg lipid), but more recent samples showed a considerable downward trend to 574 μg DDT complex/kg lipid in 2011 and 263 μg DDT complex/kg in 2015.
Due to its large size and population of over 200 million, Brazil submitted 10 pooled samples from various regions in 2001 and 2002 and three pooled national samples in 2012. Brazil is an example of the need for flexible criteria in collecting a representative sample for a country. According to the protocol, one pooled sample for countries with populations of fewer than 50 million is requested. Countries with populations well over 50 million (or with sufficient resources) were encouraged to prepare a second pooled sample (or more) if feasible.
3.1.5 Eastern European Group
Figure 7 illustrates the DDT results for the period of 2000–2019 for countries from the Eastern European Group. The DDT concentrations ranged from 64 μg DDT complex/kg lipid (Croatia, 2014) to 1820 μg DDT complex/kg lipid (Moldova, 2009), which showed a decrease to 1180 μg DDT complex/kg lipid in 2015. Results for the Czech Republic in 2001, 2006, 2014, and 2019 show a downward trend from initially 461 μg DDT complex/kg lipid to 192 μg DDT complex/kg lipid.
3.1.6 Western European and Others Group (WEOG)
On average, countries of the Western European and Others Group (WEOG), which includes Australia, Israel, New Zealand, and the USA (being informally a member) as “Others,” had the lowest DDT concentrations, with a range between 29 μg DDT complex/kg lipid (Finland, 2007) and 615 μg DDT complex/kg (Australia, 2010) and a downward trend in Australia to 227 μg DDT complex/kg lipid in 2013 (Fig. 8). The comparably low concentrations in WEOG countries are likely due to early bans on the use of DDT in agriculture implemented in most of these countries.
The gradual downward trend of the background contamination over the whole-time span between 2000 and 2019 can be illustrated by Germany. After the ban of DDT in the Federal Republic of Germany in 1972 and in the German Democratic Republic at the end of the 1980s (Umweltbundesamt 2021), background levels were at 161 μg DDT complex/kg in 2002 and went further down to 57 μg DDT complex/kg lipid in 2019.
4 Hexachlorocyclohexanes (Alpha-HCH, Beta-HCH, and Gamma-HCH)
Technical-grade hexachlorocyclohexane (HCH) is a mixture mainly of three isomers comprising about 65–70% alpha-HCH, 7–20% beta-HCH, and 14–15% gamma-HCH. Note that only gamma-HCH (lindane) has insecticidal properties. Due to metabolization, mainly beta-HCH accumulates in humans (Fürst 2023; Fång et al. 2015). Alpha-HCH, beta-HCH, and gamma-HCH are the most important isomers and were listed in 2009 in Annex A (for elimination) by the Stockholm Convention (UNEP 2009). In contrast, the minor components delta-HCH (6–10%) and epsilon-HCH (1–2%) were not listed. Therefore, in this article, HCH complex is defined as the sum of alpha-HCH, beta-HCH, and gamma-HCH.
As a result of the metabolization of hexachlorocyclohexanes in humans, the concentrations of alpha-HCH and gamma-HCH were below the limit of quantification in most human milk samples (<0.5 μg/kg lipid) with a median of quantifiable residues of about 1 μg/kg lipid and maxima of 10.5 μg/kg for alpha-HCH and 16 μg/kg for gamma-HCH. In most cases with HCH complex concentrations above 10 μg/kg lipid, about 95–100% of this sum parameter are attributed to beta-HCH. Therefore, only the ranges found for beta-HCH are discussed in more detail in the following subsection. The detailed results for alpha-HCH, beta-HCH, and gamma-HCH are available at the POPs Global Monitoring Plan Data Warehouse (Global Monitoring Plan Data Warehouse 2020).
4.1 General Comparison of Beta-HCH Ranges
The comparison of the ranges of beta-HCH among UN regions found in the five periods over 20 years is compiled in Table 3. Great differences were found, with a minimum of <0.5 μg beta-HCH /kg lipid found in few countries and a maximum of 1020 μg beta-HCH /kg lipid found in 2002 in the Asia subregion of the Asia-Pacific Group. The median of 134 country results (see Sect. 2.2) was 5.9 μg beta-HCH /kg lipid.
The wide range of concentrations raised the question whether this reflects different patterns of usage or production. Historical production of technical HCH and lindane occurred in many European countries, including the Czech Republic, Spain, France, Germany, United Kingdom, Italy, Romania, Bulgaria, Poland, and Turkey, and took place mainly from 1950 or earlier and stopped in 1970 to the 1990s. According to a research by IHPA, technical HCH and lindane have also been produced in other countries including Albania, Argentina, Austria, Azerbaijan, Brazil, China, Ghana, Hungary, India, Japan, Russia, Slovakia and the United States. Exact information is difficult to obtain, as many countries do not keep records of historical pesticides production, sales and usage or the industry considers this to be proprietary information (UNEP 2006a). In its risk profile on beta-HCH, POPRC reviewed concentrations in breast milk and found reports of very high concentrations (up to 800 ng/g) in Russia, Ukraine, and Romania (UNEP 2006b). In 2009, alpha-HCH, beta-HCH, and gamma-HCH were listed to the Stockholm Convention (UNEP 2009). The global overview of POPs data in human milk compiled in a review of scientific publications between 1995 and 2011 shows a wide range of beta-HCH concentrations, with ∑HCH concentrations up to 22,000 ng/g fat (Fång et al. 2015).
As for DDT, conclusions on time trends for beta-HCH in the different regions cannot easily be drawn. As illustrated by Fig. 9, the time trends of the median concentrations in the UN regions are not consistent. For the median of the samples in all UN regions, a decrease of 91% was found between the 2000–2003 period and the 2016–2019 period. A considerable decrease is also seen in the individual UN regions if the median of the beta-HCH concentrations of the 2000–2003 period is compared to the 2016–2019 period. However, in the three rounds in-between, considerable variations were observed, notably in Asian and in the Eastern European countries starting with an initial downward trend from 2000–2003 to 2004–2007. This, however, is followed by an increase in the period 2008–2011 before the concentrations decrease again to 2016–2019.
Time trends for median concentrations of beta-HCH in human milk (expressed as μg beta-HCH /kg lipid) in the five UN regions (with split of the Asia-Pacific Group [APG] into the subgroups Asia and Pacific Islands; GRULAC, Group of Latin American and Caribbean Countries; EEG, Eastern European Group; WEOG, Western European and Others Group) in five periods between 2000 and 2019
The picture using the mean concentrations instead of median looks quite comparable with no significant maxima in other periods (Fig. 10). As explained for DDT, a more precise method to derive time trends is the evaluation only of country-specific results of countries with repeated participation. This allows more certainty in drawing of conclusions on temporal trends. Therefore, this assessment of these more precise time trends for beta-HCH is part of a special chapter in Part IV (Malisch et al. 2023b).
Time trends for mean concentrations of beta-HCH in human milk (expressed as μg beta-HCH /kg lipid) in the five UN regions (with split of the Asia-Pacific Group [APG] into the subgroups Asia and Pacific Islands; GRULAC, Group of Latin American and Caribbean Countries; EEG, Eastern European Group; WEOG, Western European and Others Group) in five periods between 2000 and 2019
4.2 African Group
The range of beta-HCH in samples from African countries varied between <0.5 μg/kg lipid in three countries and 51.3 μg/kg lipid found in Egypt in 2002 (Fig. 11). In Egypt, a considerable downward trend was observed when 19.7 μg/kg lipid was found in 2019. In both samples from this country, 94% of the HCH complex was from beta-HCH.
In contrast, 16 μg gamma-HCH /kg lipid was found in the sample from Senegal of 2009, corresponding to 25% of HCH complex, with 48.3 μg beta-HCH /kg lipid. Here, the contribution of gamma-HCH to HCH complex fell to 13% until 2018, and the beta-HCH concentration fell to 11.7 μg/kg.
4.3 Asia-Pacific Group
4.3.1 Asia Subgroup
Figure 12 illustrates the beta-HCH results for the period 2000–2019 for the Asian countries of the Asia-Pacific Group. A wide range of concentrations was found. Samples from previous rounds (UNEP 2013) had higher levels than samples from countries participating in the 2016–2019 round. The lowest concentration was found in the sample from Cambodia collected in 2019 (0.6 μg beta-HCH /kg lipid) and the highest concentration was in the sample from Hong Kong SAR of China in 2002 (1020 μg beta-HCH /kg lipid as the median of ten pooled samples [Hedley et al. 2010]). Hong Kong participated twice, with a considerable decrease found in the 2009 sample, where the concentration was 290 μg beta-HCH/kg lipid (median of four samples from different population groups).
4.3.2 Pacific Islands Subgroup
Figure 13 illustrates the beta-HCH results for the Pacific Islands countries of the Asia-Pacific Group. All countries in all periods had beta-HCH concentrations in the range of low background contamination between <0.5 μg/kg lipid and 6.5 μg/kg lipid. Overall, countries of this region had the lowest beta-HCH concentrations in human milk.
4.4 Group of Latin American and Caribbean Countries (GRULAC)
Figure 14 illustrates the beta-HCH results for Latin American and Caribbean countries. The results ranged between <0.5 μg beta-HCH /kg lipid (Haiti, 2004 and 2015) and 29.7 μg beta-HCH /kg lipid (Uruguay, 2009). In countries with beta-HCH concentrations above 5 μg/kg as found in samples submitted before 2011 (Uruguay, 2009; Brazil, 2001 and 2002; Peru, 2011; Chile, 2008; Barbados, 2010), a considerable downward trend was observed until 2019.
4.5 Eastern European Group
Figure 15 illustrates the beta-HCH results for countries of the Eastern European Group. A wide range of concentrations was observed between the low background contamination as found in 2019 in the Slovak Republic (2.3 μg beta-HCH /kg lipid) and the Czech Republic (2.5 μg beta-HCH /kg lipid), and the maximum of 476 μg beta-HCH /kg lipid found in 2009 in Moldova. In Moldova, these concentrations decreased considerably to 150 μg beta-HCH/kg lipid in 2015. The Czech Republic participated four times between 2000 and 2019; here, a continuous downward trend was observed from 26 μg beta-HCH /kg lipid in 2001 to 2.5 μg beta-HCH /kg lipid in 2019.
4.6 Western European and Others Group (WEOG)
Figure 16 illustrates the beta-HCH results for countries of the Western European and Others Group. The concentrations ranged from 1.9 μg beta-HCH /kg lipid (Sweden, 2019) to 58.8 μg beta-HCH /kg lipid (Spain, 2002, median of three pooled samples). The three highest concentrations were found in countries participating in the 2000–2003 period and the three lowest concentrations in the 2016–2019 period.
The gradual downward trend of the background contamination over the whole span of time between 2000 and 2019 is illustrated by Ireland: Background levels of beta-HCH were at 24 μg/kg in 2002 and went down 17.2 μg/kg lipid in 2010 and finally to 3.6 μg/kg lipid in 2019.
5 Hexachlorobenzene (HCB)
Hexachlorobenzene (HCB) belongs to the group of the “12 Initial POPs” under the Stockholm Convention. Production and use of HCB, e.g., formerly used as a fungicide for seed treatment or for various technical purposes, is prohibited by listing HCB in Annex A (Elimination) of the convention. Furthermore, HCB can be formed as an unintentional by-product, e.g., in certain thermal processes. Therefore, HCB is also listed in Annex C (Unintentional production) for minimization and, where feasible, ultimate elimination of unintentional releases (UNEP 2001). HCB is chemically and physiologically stable, and thus persists in the environment and in biological systems. It is also highly lipophilic, which results in its biomagnification in the food chain and bioaccumulation in fatty tissues of animals and humans (Fürst 2023). The global overview of POPs data in human milk compiled in a review of scientific publications between 1995 and 2011 shows a wide range of HCB concentrations up to the range of about 1000 ng/g fat (Fång et al. 2015).
5.1 General Comparison of HCB Ranges
The comparison of the ranges of HCB among UN regions found in the five periods over 20 years is compiled in Table 4. The maximum levels and therefore the ranges were much lower than found for DDT and beta-HCH, with a minimum of about 1–2 μg/kg lipid found in some countries and a maximum of 154 μg/kg lipid found in 2009 in an Eastern European country. The median of 134 country results (see Sect. 2.2) was 5.1 μg/kg lipid. Samples from all countries from Africa were at all times in this low background range below 5 μg/kg lipid, and samples from many countries from the Pacific Islands and Latin America and the Caribbean were in the same low range. The highest HCB concentrations were found in the Eastern European Group.
In general, concentrations of HCB show a downward trend globally: The median of all samples decreases from the 2000–2003 period (16 countries, 16.4 μg/kg lipid) to the 2016–2019 period (43 countries, 3.3 μg/kg lipid) by about 80%. Of particular interest are time trends in regions with higher initial concentrations. Figure 17 illustrates the decline of the median HCB concentrations in various groups. In the Eastern European Group, the median HCB concentration falls from the 2000–2003 period to the 2012–2015 period and then levels out around 13 μg/kg lipid. In countries of the Western European and Others Group, a downward trend is also observed from the 2000–2003 period to the 2008–2011 period and then leveling out at about 10 μg/kg lipid. In other groups, the background contamination levels out at concentrations up to 5 μg HCB /kg lipid (limit of quantification: 0.5 μg/kg lipid).
Time trends for median concentrations of HCB in human milk (μg/kg lipid) in the five UN regions (with split of the Asia-Pacific Group [APG] into the subgroups Asia and Pacific Islands; GRULAC, Group of Latin American and Caribbean Countries; EEG, Eastern European Group; WEOG, Western European and Others Group) in five periods between 2000 and 2019
The picture using the mean concentrations instead of median looks as if there was a maximum in the Eastern European Group in the 2008–2011 period (Fig. 18). However, this is caused by a high HCB concentration found in the pooled sample of one country submitted at that time. As explained before, a more precise method to derive time trends is the evaluation of results of countries only with repeated participation. This allows more certainty in drawing of conclusions on temporal trends, which are not potentially influenced by single results of a country submitted for a single round. Therefore, the assessment of time trends for HCB of countries with repeated participation is part of the special chapter in Part IV (Malisch et al. 2023b).
Time trends for mean concentrations of HCB in human milk (μg/kg lipid) in the five UN regions (with split of the Asia-Pacific Group [APG] into the subgroups Asia and Pacific Islands; GRULAC, Group of Latin American and Caribbean Countries; EEG, Eastern European Group; WEOG, Western European and Others Group) in five periods between 2000 and 2019
5.2 African Group
HCB concentrations in all African countries in the whole period 2000–2019 were in the range of low background concentrations and varied between 1 and 5 μg/kg lipid (Fig. 19). A downward trend at the upper range of this background contamination is observed when results of Nigeria and Mauritius (in the 2008–2011 period) and Egypt (in the 2000–2003 period) are compared with results of these countries in the 2016–2019 period. In most other countries with repeated participation, the concentrations are quite stable at low concentrations of approximately 2–3 μg/kg lipid.
5.3 Asia-Pacific Group
5.3.1 Asia Subgroup
Figure 20 illustrates the HCB results for the period 2000–2019 for the Asian countries of the Asia-Pacific Group. A wider range of concentrations was found with the lowest concentration in the sample from Cambodia, 2019 (2.5 μg/kg lipid) and the highest concentration in the one from Mongolia, 2018 (34 μg/kg lipid). Hong Kong SAR of China participated twice, with an increase from 2002 (19.3 μg/kg lipid as median of ten pooled samples [Hedley et al. 2010]) to the 2009 level of 25.8 μg HCB /kg lipid (median of four samples from different population subgroups). Two subgroups of 2009 with residents who had been living in Hong Kong for 10 years or more had comparable concentrations (20.5–21.0 μg/kg lipid) to 2002, whereas two subgroups of 2009 who had been living in Hong Kong for less than 10 years had concentrations of 30–35 μg/kg lipid.
5.3.2 Pacific Islands Subgroup
Figure 21 illustrates the HCB results for the Pacific Islands countries of the Asia-Pacific Group. A downward trend is observed from the 2008–2011 period to the 2016–2019 period: Whereas the average background contamination in the 2008–2011 period was about 6 μg HCB /kg lipid, this dropped to about 3 μg/kg lipid in the 2016–2019 period. Four participations of Fiji between 2000 and 2019 showed quite stable background concentrations between about 3 and 5 μg/kg lipid.
5.4 Group of Latin American and Caribbean Countries (GRULAC)
Figure 22 illustrates the HCB results for Latin American and Caribbean countries. The results ranged from 1.0 μg/kg lipid (Haiti, 2015) to 14 μg/kg lipid (Uruguay, 2009). Three countries found at the upper end of this distribution all participated in the 2008–2011 period. From here, downward trends were observed in Uruguay from 2009 to 2019, in Mexico from 2011 to 2017, and in Chile from 2008 to 2011.
5.5 Eastern European Group
Figure 23 illustrates the HCB results for countries of the Eastern European Group. A wide range of concentrations was observed between the background contamination as found in Croatia, 2014 (7.6 μg/kg lipid) and Bulgaria, 2014 (7.7 μg/kg lipid), in comparison with 154 μg/kg found in Moldova, 2009. In Moldova, these concentrations decreased considerably to 46.7 μg HCB/kg lipid in 2015. The Czech Republic participated four times between 2000 and 2019; here, a continuous downtrend is found from 76 μg/kg lipid in 2001 to 12.5 μg/kg lipid in 2019. The intentional production in the Czech Republic ceased in 1968. The use as a pesticide was banned in Czechoslovakia in 1977, in Ukraine since 1997 (UNEP 2014).
5.6 Western European and Others Group (WEOG)
Figure 24 illustrates the HCB results for countries of the Western European and Others Group. The concentrations ranged from 2.8 μg/kg lipid (Finland, 2007, median of three pooled samples) to 71.5 μg/kg lipid (Spain, 2002, median of three pooled samples). The highest concentrations were found in countries participating in the 2000–2003 period.
The gradual downward trend of the background contamination over the whole span of time between 2000 and 2019 can be illustrated by three participations of Ireland where background levels of HCB were at 13.5 μg/kg in 2002 and went down to 8.5 μg/kg lipid in 2019.
6 Other Organochlorine Pesticides
6.1 Aldrin, Dieldrin
Aldrin and dieldrin belong to the group of the “12 initial POPs” under the Stockholm Convention. As the review on findings in humans in the introduction part shows, aldrin is rapidly metabolized to dieldrin, and therefore aldrin is not usually found in humans (Fürst 2023). Rather high dieldrin concentrations were reported in some countries (Fång et al. 2015).
Aldrin was not detected in any sample (<0.5 μg/kg lipid). Based on 134 country results, nearly 90% of the samples had dieldrin levels in a low background range below 5 μg/kg lipid. Figure 25 illustrates the results for countries that are above 5 μg/kg lipid, with a maximum of 37.8 μg/kg lipid found in a sample from Tajikistan in 2009. A downward trend of the background levels for dieldrin from the 2000–2003 period to the 2016–2019 period was observed (Table 5).
6.2 Chlordane
Chlordane belongs also to the group of the “12 initial POPs” under the Stockholm Convention. Depending on the production process, technical chlordane can comprise more than 100 components. According to the residue definition for pesticides in food, the sum parameter “chlordane complex” comprises cis-chlordane (= “alpha-chlordane”) and trans-chlordane (= “gamma-chlordane”) (both more relevant for food of plant origin) and the metabolite oxychlordane (relevant for food of animal origin). These were also the recommended analytes according to the Guidance on the Global Monitoring Plan (GMP) for POPs as of 2007 (UNEP 2007). Later, also cis- and trans-nonachlor, which are impurities in chlordane production, were added to the list of recommended analytes for chlordane in the GMP guidance. Thus, the “chlordane group” comprises cis- and trans-chlordane, cis- and trans-nonachlor, and oxychlordane (UNEP 2019). For definition of the sum parameters and correction factors for oxychlordane and nonachlor, see Sect. 2.3. For reported findings in humans, see the introduction part (Fürst 2023) and Fång et al. 2015.
Based on 134 country results for chlordane complex, determined as sum of cis- and trans-chlordane and oxychlordane, 23% of the samples had levels below the limit of quantification (0.5 μg/kg lipid), 61% in a low background range below 5 μg/kg lipid and 16% between 5 and 17 μg/kg lipid (Fig. 26). A downward trend was observed from the 2000–2003 period (median of 16 samples: 5.36 μg chlordane complex/kg; range 0.97–16.9) to the 2016–2019 period (median of 43 samples: 0.86 μg chlordane complex/kg; range 0.50–4.42).
In 42 of 44 samples, the nonachlor concentrations were below the background range of 5 μg/kg lipid, two samples were slightly above (Hong Kong SAR, 2002, 12.4 μg nonachlor /kg lipid; Barbados, 2018, 11.4 μg nonachlor /kg lipid).
6.3 Endrin
Endrin belongs to the group of the “12 initial POPs,” as well. It is rapidly metabolized to endrin ketone. “Endrin complex” is the sum of endrin and endrin ketone. In contrast to reported findings (Fång et al. 2015), endrin and endrin ketone were not detected in any of 163 pooled samples of the periods 2000–2019 in which the limit of quantification was 0.5 μg/kg lipid.
6.4 Heptachlor
Heptachlor is also one of the “12 initial POPs.” The review in the introduction part shows that it is also rapidly metabolized to heptachlor epoxide and that in humans cis-heptachlor epoxide can be found (Fürst 2023). Heptachlor complex is the sum of heptachlor, cis-heptachlor epoxide, and trans-heptachlor epoxide (for calculation of the sum parameter, see Sect. 2.3). As expected, in none of the 163 pooled samples heptachlor was found, in about half of them, heptachlor epoxide was below the limit of quantification (0.5 μg/kg lipid). In most cases of quantifiable residues, only cis-heptachlor epoxide was found with concentrations below 5 μg/kg lipid. Three samples were slightly above these low background levels, namely Luxembourg, 2002, 8.4 μg cis-heptachlor epoxide /kg lipid; Bulgaria, 2001, 12 μg cis-heptachlor epoxide /kg lipid; and Bulgaria, 2014, 14.1 μg cis-heptachlor epoxide /kg lipid.
6.5 Mirex
Mirex is another chemical that belongs to the group of the “12 initial POPs.” It can be found in humans (Fång et al. 2015) but in about 80% of the samples of human milk of the WHO/UNEP-coordinated exposure studies, no mirex was detected (limit of quantification 0.5 μg/kg lipid). The remaining 20% of samples had concentrations in the range up to 3 μg/kg lipid with the exception of one sample that had a slightly higher level (Uruguay, 2009, 9.8 μg/kg lipid). However, Uruguay subsequently had a downward trend in 2019 to 2.9 μg/kg lipid.
6.6 Toxaphene
Toxaphene also belongs to the group of the “12 initial POPs.” It is a complex mixture of chlorinated bornanes and chlorinated camphenes comprised of about 16,000 congeners/isomers. Marker compounds to be monitored are the congeners Parlar 26 (P26), Parlar 50 (P50), and Parlar 62 (P62) (UNEP 2019) as basis for the sum parameter toxaphene complex. This was calculated with application of the “lower bound approach” where only analytical results above the limit of quantification (0.5 μg/kg lipid) are used. Findings in humans were reported only in a few studies (Fång et al. 2015).
In 67% of the samples (134 country results), neither P26, P50 nor P62 was detected. In 32% of the samples, toxaphene was found in a range between 0.5 μg toxaphene complex/kg lipid and 6 μg toxaphene complex/kg lipid. Two older samples had higher concentrations, namely Norway, 2001, 10.0 μg toxaphene complex/kg lipid and Ukraine, 2001, 16.2 μg toxaphene complex/kg lipid.
6.7 Chlordecone
Chlordecone was listed in the Stockholm Convention in 2009. No studies were found reporting chlordecone concentrations in mother’s milk (Fång et al. 2015). In none of 42 pooled samples submitted in the period 2016–2019 was chlordecone detected (limit of quantification 0.5 μg/kg lipid).
6.8 Endosulfan
Technical-grade endosulfan, which contains a number of related isomers, was listed in the Stockhom Convention in 2011. The sum parameter endosulfan complex comprises the analytes alpha-endosulfan, beta-endosulfan, and endosulfan sulfate (UNEP 2019; see Sect. 2.3). Only some scattered endosulfan mothers’ milk data were found (Fång et al. 2015).
Of the 134 country samples, 98% did not have measurable residues above the limit of quantification (0.5 μg/kg lipid). Two samples had small amounts of about 1 μg endosulfan complex/kg lipid (Sudan, 2006; Brazil, 2012) and one sample had 6.3 μg endosulfan complex/kg lipid (Nigeria, 2009).
6.9 Pentachlorophenol (PCP), Pentachloroanisole (PCA)
Pentachlorophenol (PCP) was listed in the convention in 2015. PCP does not bioaccumulate, whereas its metabolite, pentachloroanisole (PCA), can be found after the use of PCP. Therefore, PCA is the recommended analyte for human milk (UNEP 2019).
As expected, none of 43 pooled samples of the 2016–2019 period had residues of PCP, and 41 of these samples had no residues of PCA (limit of quantification 0.5 μg/kg lipid). One sample had a trace of PCA (Fiji, 2019, 1.1 μg/kg lipid), while another sample had a high level of 33.3 μg/kg lipid (Vanuatu, 2018).
6.10 Dicofol
Dicofol was listed in the Stockholm Convention in 2019. Therefore, it was not included in the list of 23 POPs that were requested to be analyzed when the seventh round started in 2016. However, the analysis of dicofol was undertaken to have a complete picture of all 30 POPs currently covered by the convention. Dicofol was detected in human breast milk from China, Korea, and Japan (Fujii et al. 2011).
In 40 samples which were submitted between 2017 and 2019, dicofol was not detected (limit of quantification 0.5 μg/kg lipid). Only one sample had a measurable level of dicofol at 3 μg/kg lipid (Ethiopia, 2019)
7 Organochlorine Industrial Chemicals
7.1 Pentachlorobenzene (PeCB)
Production and use of pentachlorobenzene (PeCB), e.g., formerly for various technical purposes, are prohibited by listing PeCB in the Stockhom Convention in 2009 in Annex A (Elimination). Furthermore, PeCB can also be formed as unintentional by-product during combustion and thermal and industrial processes. Therefore, PeCB is also listed in Annex C (Unintentional production) for minimization and, where feasible, ultimate elimination of unintentional releases (UNEP 2001).
In 90 of 99 pooled samples submitted during 2008 and 2019, no PeCB was found (limit of quantification 0.5 μg/kg lipid). In the nine remaining samples, low levels of between 0.5 and 1.2 μg/kg lipid were found.
7.2 Hexachlorobutadiene (HCBD)
Production and use of hexachlorobutadiene (HCBD), e.g., formerly for various technical purposes, are prohibited by listing HCBD in 2015 in Annex A (Elimination) of the Stockholm Convention. HCBD can also be formed as unintentional by-product and was therefore listed in 2017 also in Annex C (Unintentional production) (UNEP 2001).
HCBD was not detected above the limit of quantification (0.5 μg/kg lipid) in any of the 43 pooled samples submitted in the period 2016–2019.
8 Summary
Between 2000 and 2019, five rounds of human milk studies on persistent organic pollutants (POPs) were coordinated by the World Health Organization (WHO) and the United Nations Environment Programme (UNEP) with 82 countries from all the five United Nations regions. The intent of these studies was to evaluate the effectiveness of the Stockholm Convention on POPs in eliminating or reducing emissions of selected POPs. Countries submitted composite samples prepared by pooling many individual samples. These pooled samples are considered representative of the national average of the analytes of interest at the time of sampling. The concentrations of 30 POPs presently listed in the convention were determined, among them the 18 organochlorine pesticides and industrial contaminants reported in this article, including the parent molecules, certain metabolites, degradation products and/or by-products during manufacture if recommended by the Guidance Document on the Global Monitoring Plan. If a country provided two or more pooled samples for a certain round, the median of results is used to represent the country. Up to 134 country results were available for this evaluation. Note that the results presented here are based on UN regions and are not intended for ranking of individual countries.
For dichlorodiphenyltrichloroethane (DDT), huge differences were found, with a minimum of 17 μg DDT complex/kg lipid found in 2019 in a country from the African Group and 23,500 μg DDT complex/kg lipid found in 2012 in another country from the African Group (median of 134 country results: 255 μg DDT complex/kg lipid). The summarizing parameter (DDT complex) comprises the p,p′- and o,p′-isomers of DDT and their metabolites dichlorodiphenyldichloroethylene (p,p′-DDE; o,p′-DDE) and dichlorodiphenyldichloroethane (p,p′-DDD; o,p′-DDD), respectively. Because p,p′-DDT is extensively metabolized, its transformation product p,p′-DDE contributes on average about 95% to the sum parameter “DDT complex.” Consequently, in most human milk samples, p,p′-DDT only contributes about 5% to DDT complex. However, in cases of a more recent use and contamination, considerably higher contributions of p,p′-DDT can be found. As an example, 46% p,p′-DDT was found in a sample of 2012 from a country in the African Group probably due to public health use of DDT to combat mosquitos for malaria control, which is permitted under the Stockholm Convention with some constraints.
As a general estimation of time trends, the median of the DDT complex concentrations of all samples shows a decrease of 72% from the 2000–2003 period (median for 16 countries: 445 μg DDT complex/kg lipid) to the 2016–2019 period (median for 43 countries: 125 μg DDT complex/kg lipid). The downward trend was found in all UN regions although considerable differences between groups and rounds were noted. The assessment of time trends based only on country-specific results of countries with repeated participation allows more certainty in drawing of conclusions, and therefore is optimal for the evaluation of the effectiveness under of Article 16 of the convention. This evaluation is published separately in Part IV of this compendium.
Due to metabolization of hexachlorocyclohexanes (HCH) in humans, the concentrations of alpha-HCH and gamma-HCH were, in most human milk samples, below the limit of quantification (<0.5 μg/kg lipid) with a median of quantifiable residues of about 1 μg/kg lipid for alpha-HCH and gamma-HCH and with maxima of 10.5 μg/kg for alpha-HCH and 16 μg/kg for gamma-HCH. In most cases when the sum of HCH isomer concentrations was above 10 μg/kg lipid, about 95–100% of this is due to beta-HCH, which is the HCH isomer that mainly bioaccumulates in humans. The ranges of beta-HCH found in the five studies over 20 years varied considerably among UN regions. Residues below the limit of quantification (<0.5 μg/kg lipid) were found in few countries. The lowest beta-HCH concentrations were found in Pacific Islands countries (range: 0.5–6.5 μg beta-HCH /kg lipid). With an overall median of 5.9 μg beta-HCH/kg lipid, the 134 results for all countries were in a similar range. The maximum of 1020 μg beta-HCH/kg lipid was found in 2002 in an Asian country of the Asia-Pacific Group, but the levels have significantly fallen since then.
Based on the median of the beta-HCH concentrations of all samples, a decrease of 91% was found from the 2000–2003 period (median for 16 countries: 25.3 μg beta-HCH /kg lipid) to the 2016–2019 period (median for 43 countries: 2.4 μg beta-HCH /kg lipid). However, in the three rounds in-between, considerable variations in results from single submissions by countries were observed. Therefore, a time trend for beta-HCH is also included in the separate assessment of time trends based only on results from countries with repeated participation in the studies presented separately in Part IV.
In comparison with DDT and HCH, the maximum levels and ranges for hexachlorobenzene (HCB) were much lower, with a minimum of about 1–2 μg/kg lipid found in some countries and maximum of 154 μg HCB /kg lipid found in 2009 in a country of the Eastern European Group. With median levels between 12.8 μg/kg lipid in the 2016–2019 period and 58.3 μg/kg lipid in 2000–2003 period, the Eastern European Group had the highest median concentrations for five periods. The median of 134 country results was 5.1 μg/kg lipid. All samples from the African Group were in the low background range below 5 μg/kg lipid. This was also true for many countries from the Asia-Pacific Group and the Latin American and Caribbean Group.
In general, concentrations of HCB show a downwards trend: The median of all samples decreases from the 2000–2003 period (16 countries, 16.4 μg/kg lipid) to the 2016–2019 period (43 countries, 3.3 μg/kg lipid) by 80%. In the Eastern European Group, the median of the HCB concentrations drops from the 2000–2003 period (58 μg/kg lipid) to the 2012–2015 period and then plateaus at about 13 μg/kg lipid. In countries of the Western European and Others Group, a downward trend is observed from the 2000–2003 period (about 20 μg/kg lipid) to the 2008–2011 period and then levels out to around 10 μg/kg lipid. In other groups, the background contamination levels out at a concentration of up to 5 μg/kg lipid.
The following organochlorine pesticides and contaminants and their metabolites were mostly found in ranges of low background contamination; some were below the limit of quantification for the analytes of interest.
Aldrin is rapidly metabolized to dieldrin and therefore, aldrin was not detected in any sample (limit of quantification 0.5 μg/kg lipid). About 90% of the samples had dieldrin levels in a low background range below 5 μg/kg lipid. Four samples had dieldrin concentrations above 10 μg/kg with a maximum of 37.8 μg/kg lipid. A downward trend of the background levels for dieldrin from the 2000–2003 period to the 2016–2019 period is observed.
Recommended analytes for chlordane (chlordane complex) included initially cis-chlordane (or “alpha-chlordane”) and trans-chlordane (or “gamma-chlordane”) and the metabolite oxychlordane. Later, also cis- and trans-nonachlor were added (chlordane group), which can be found as impurities in chlordane. Based on the analysis for chlordane complex, determined as sum of cis- and trans-chlordane and oxychlordane, the results for 134 country samples show that 23% of the samples had levels below the limit of quantification (0.5 μg/kg lipid), 61% in a low background range below 5 μg chlordane complex/kg lipid, and 16% between 5 and 17 μg chlordane complex/kg lipid. A downward trend is observed from the 2000–2003 period to the 2016–2019 period. In 42 of 44 samples, the nonachlor concentrations were below 5 μg nonachlor /kg lipid, with two samples up to 12.4 μg nonachlor /kg lipid.
Endrin and its metabolite endrin ketone were not detected in any sample of the 2000–2019 period (limit of quantification 0.5 μg/kg lipid).
Heptachlor is rapidly metabolized in the body to heptachlor epoxide. In about half of the 134 country results, heptachlor epoxide was below the limit of quantification (0.5 μg/kg lipid). In most cases of quantifiable residues, cis-heptachlor epoxide was found with concentrations below 5 μg/kg lipid. Three samples were slightly above these low background levels with a maximum of 14.1 μg cis-heptachlor epoxide /kg lipid.
In about 80% of the samples, no mirex was detected (limit of quantification 0.5 μg/kg lipid); most of the remaining 20% of samples had concentrations in the range up to 3 μg/kg lipid. Only one sample had slightly higher levels (9.8 μg/kg lipid).
Monitoring for toxaphene (toxaphene complex) is based on the congeners Parlar 26 (P26), Parlar 50 (P50), and Parlar 62 (P62). In 67% of the samples, neither P26, P50 nor P62 was detected (limit of quantification 0.5 μg/kg lipid). In 32% of the samples, toxaphene was found in a range between 0.5 and 6 μg toxaphene complex/kg lipid, calculated as sum of P26, P50, and P62. Two samples of 2001 had slightly higher concentrations with a maximum of 16.2 μg toxaphene complex/kg lipid.
Chlordecone was not detected in any of 42 pooled samples submitted in the period 2016–2019 (limit of quantification 0.5 μg/kg lipid).
Analysis for endosulfan (endosulfan complex) comprises the determination of alpha-endosulfan, beta-endosulfan, and endosulfan sulfate. About 98% of all samples did not have quantifiable residues of these recommended analytes (limit of quantification 0.5 μg/kg lipid). Two samples had small traces of about 1 μg endosulfan complex/kg, while one sample had 6.3 μg endosulfan complex/kg.
Pentachlorophenol (PCP) does not bioaccumulate in humans; however, the metabolite pentachloroanisole (PCA) can be found after use of PCP. While none of 43 pooled samples of the 2016–2019 period had residues of PCP, only 2 of these samples had residues of PCA (limit of quantification 0.5 μg/kg lipid). One sample had traces of PCA (1.1 μg/kg lipid) and another sample had 33.3 μg/kg lipid.
Dicofol was not detected in 40 out of 41 samples, which were submitted between 2017 and 2019 (limit of quantification 0.5 μg/kg lipid). In one sample from the African group, a level of 3 μg/kg lipid was found.
In 90 of 99 pooled samples of the periods covering 2008–2019, no pentachlorobenzene (PeCB) was found (limit of quantification 0.5 μg/kg lipid); in nine samples, traces between 0.5 and 1.2 μg/kg lipid were found.
No residues of hexachlorobutadiene (HCBD) were detected (limit of quantification 0.5 μg/kg lipid) in any of the 43 pooled samples, submitted in the period 2016–2019.
In conclusion, these results demonstrate that for many of the POPs covered by this article, the levels of POPs in human milk have generally fallen over the last 20 years with higher levels seen only in sporadic cases. For other POPs, the results were low or below the limit of quantification. Most significantly, human milk samples are a key matrix for the effectiveness evaluation of the Stockholm Convention; their analysis as pooled samples considered to represent the average levels of POPs for a country at the time of sampling has been shown to be a very cost-effective approach for monitoring levels POPs on a global level, allowing to draw conclusions (i) on the relative importance of particular POPs in regions, (ii) on the priority of the individual POPs among the whole list of 30 compounds as covered under the Stockholm Convention as of 2019, and (iii) on temporal trends.
References
DG-SANTE (2021) Analytical quality control and method validation procedures for pesticide residues analysis in food and feed, Document SANTE/12682/2019, implemented by 01.01.2020, https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727&LabID=100&Lang
Fång J, Nyberg E, Winnberg U, Bignert A, Bergman A (2015) Spatial and temporal trends of the Stockholm Convention POPs in mothers’ milk—a global review. Environ Sci Pollut Res 22:8989–9041
Fiedler H, Abad E, van Bavel B, de Boer J, Bogdal C, Malisch R (2013) The need for capacity building and first results for the Stockholm Convention Global Monitoring Plan. Trends Anal Chem 46:72–84
Fujii Y, Haraguchi K, Harada KH, Hitomi T, Inoue K, Itoh Y, Watanabe T, Takenaka K, Uehara S, Yang H-R, Kim M-Y, Moon C-S, Kim H-S, Wang P, Liu A, Nguyen NH, Koizumi., A. (2011) Detection of dicofol and related pesticides in human breast milk from China, Korea and Japan. Chemosphere 82(2011):25–31
Fürst P (2023) Human milk surveys on persistent organic pollutants from a historical perspective. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Gebremichael S, Birhanu T, Tessema DA (2013) Analysis of organochlorine pesticide residues in human and cow’s milk in the towns of Asendabo, Serbo and Jimma in South-Western Ethiopia. Chemosphere 90:1652–1657
Global Monitoring Plan Data Warehouse (2020) Global Monitoring Plan Data Warehouse of the Stockholm Convention on Persistent Organic Pollutants: visualisation platform and on-line tool for the analysis of global levels of POPs in air, water, breast milk and blood, online at http://www.pops-gmp.org (Hůlek R, Borůvková J, Kalina J, Bednářová Z, Šebková K, Hruban T, Novotný V, Ismael M, Klánová J, Masaryk University)
Hardebusch B, Dambacher B, Polley J, Lippold R, Schächtele A (2023) Analysis and quality control of WHO- and UNEP-coordinated exposure studies 2000-2019 – organochlorine pesticides and industrial chemicals. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part II)
Hedley AJ, Hui LL, Kypke K, Malisch R, van Leeuwen FXR, Moy G, Wong TW, Nelson EAS (2010) Residues of persistent organic pollutants (POPs) in human milk in Hong Kong. Chemosphere 79:259–265
Hui LL, Hedley AJ, Kypke K, Cowling BJ, Nelson EAS, Wong TW, van Leeuwen FXR, Malisch R (2008) DDT levels in human milk in Hong Kong, 2001–2002. Chemosphere 73:50–55
Malisch R, Kypke K, van Leeuwen FXR, Moy G, Park S (2008) Evaluation of WHO-coordinated exposure studies on levels of persistent organic pollutants (POPs) in human milk with regard to the Global Monitoring Plan. Organohalogen Comp 70:228–232
Malisch R, Kypke K, Kotz A, Wahl K, Bitomsky N, Moy G, Park S, Tritscher A, Lee SY, Magulova K, Fiedler H (2010) WHO/UNEP-coordinated exposure study (2008-2009) on levels of persistent organic pollutants (POPs) in human milk with regard to the Global Monitoring Plan. Organohalogen Comp 72:1766–1769
Malisch R, Malisch K, van Leeuwen FXR, Moy G, Tritscher A, Witt A, Alvarez J (2023a) Overview of WHO- and UNEP-coordinated human milk studies and their link to the Stockholm Convention on Persistent Organic Pollutants. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Malisch R, Hardebusch B, Lippold R, van Leeuwen FXR, Moy G, Tritscher A, Šebková K, Klánová J, Kalina J (2023b) Time trends in human milk derived from WHO- and UNEP-coordinated exposure studies, Chapter 2: DDT, beta-HCH and HCB. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part IV)
OECD (2011) OECD Environment, Health and Safety Publications, Series on Pesticides No. 66 and Series on Testing and Assessment No. 164, Guidance Document on Crop Field Trials, ENV/JM/MONO(2011)50
Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC
Šebková K (2023) The Stockholm Convention, Global Monitoring Plan and its implementation in regional and global monitoring reports. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Smith D (1999) Worldwide trends in DDT levels in human breast milk. Int J Epidemiol 28(2):179–188. https://doi.org/10.1093/ije/28.2.179. PMID: 10342677
Umweltbundesamt (2021) DDT und Metabolite, Umweltprobenbank.de. https://www.umweltprobenbank.de/de/documents/profiles/analytes/10059
UNEP (2001) Stockholm Convention on Persistent Organic Pollutants (POPs), Text and Annexes. http://www.pops.int
UNEP (2006a) Report of the Persistent Organic Pollutants Review Committee on the work of its second meeting, Addendum “Risk profile on lindane”. UNEP/POPS/POPRC.2/17/Add.4
UNEP (2006b) Report of the Persistent Organic Pollutants Review Committee on the work of its third meeting, Addendum “Risk profile on beta hexachlorocyclohexane”. UNEP/POPS/POPRC.3/20/Add.9
UNEP (2007) Guidance on the global monitoring plan for persistent organic pollutants, preliminary version, February 2007, Amended in May 2007
UNEP (2009) Report of the conference of the parties of the Stockholm Convention on Persistent Organic Pollutants on the work of its fourth meeting. UNEP/POPS/COP.4/38
UNEP (2013) Conference of the parties to the Stockholm Convention on Persistent Organic Pollutants, Sixth meeting, Geneva, 28 April–10 May 2013: Results of the global survey on concentrations in human milk of persistent organic pollutants by the United Nations Environment Programme and the World Health Organization. UNEP/POPS/COP.6/INF/33
UNEP (2014) Global monitoring plan for persistent organic pollutants, second regional monitoring report, Central and Eastern European (CEE) and Central Asian Region
UNEP (2017) Second global monitoring report. UNEP/POPS/COP.8/INF/38
UNEP (2019) Guidance on the global monitoring plan for persistent organic pollutants. UNEP/POPS/COP.9/INF/36. http://www.pops.int/TheConvention/ConferenceoftheParties/Meetings/COP9/tabid/7521/Default.aspx
UNEP (2020) All POPs listed in the Stockholm Convention, http://www.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx (Stockholm Convention > The Convention > The POPs > All POPs)
UNEP (2022a) Monitoring reports. Link to first monitoring reports, second monitoring reports and third monitoring reports of the regional organization groups and the global coordinating group. http://www.pops.int/implementation/globalmonitoringplan/monitoringreports/tabid/525/default.aspx (Stockholm Convention > Implementation > Global Monitoring Plan > Monitoring Reports)
UNEP (2022b) DDT—Overview http://www.pops.int/Implementation/PesticidePOPs/DDT/Overview/tabid/378/Default.aspx (Stockholm Convention > Implementation > Pesticide POPs > DDT > Overview)
van den Berg M, Kypke K, Kotz A, Tritscher A, Lee SY, Magulova K, Fiedler H, Malisch R (2016) WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit–risk evaluation of breastfeeding. Arch Toxicol. https://doi.org/10.1007/s00204-016-1802-z
WHO (1989) Levels of PCBs, PCDDs, and PCDFs in breast milk. Environmental Health Series 34, 1–92 (WHO Regional Office for Europe)
WHO (1996) Levels of PCBs, PCDDs and PCDFs in human milk. Environmental Health in Europe No. 3, 1–121 (WHO European Centre for Environment and Health)
Acknowledgments
This publication was developed in the framework of the projects titled “Implementation of the POPs Monitoring Plan in the Asian Region” and “Continuing regional Support for the POPs Global Monitoring Plan under the Stockholm Convention in the Africa, Pacific and Latin-American and Caribbean Region,” funded by the Global Environment Facility and in close collaboration with and support of CVUA Freiburg.
The worldwide implementation of the Global Monitoring Plan for POPs, including that of the UNEP/WHO global human milk studies, is made possible thanks to the generous contributions to the Stockholm Convention Voluntary Trust Fund by the Governments of Japan, Norway, and Sweden and through the European Union’s Global Public Goods and Challenges Programme (GPGC). Further, the substantial contributions made by the Global Environment Facility to support POPs monitoring activities in regions implemented by UNEP, in close collaboration with WHO, particularly for the global human milk surveys, are greatly appreciated.
The authors express their gratitude to the National Coordinators of the WHO- and UNEP-coordinated exposure studies for their excellent work to collect the human milk samples and to prepare and send the pooled samples to the WHO/UNEP Reference Laboratories, which included great efforts to plan and implement the national studies with the assistance of the health, environment, laboratory, and administrative staff. The continuous exchange of information between the National Coordinators and WHO, UNEP, and the Reference Laboratories was an important aspect for the successful organization of these studies on a global level.
Hae Jung Yoon, Seongsoo Park, and Philippe Verger (WHO Department of Food Safety and Zoonoses) are acknowledged for their coordinating support during their time at WHO, and Lawrence Grant (WHO) for the statistical analysis of the sampling protocols.
We thank Katarina Magulova and Ana Witt (Secretariat of the Basel, Rotterdam, and Stockholm Conventions) and Jacqueline Alvarez, Haosong Jiao, and Gamini Manuweera (United Nations Environment Programme, Economy Division, Chemicals and Health Branch) for their support and contributions to these surveys, furthermore Heidelore Fiedler for the conception and implementation of the GMP projects during her time at the UNEP Economy Division, Chemicals and Health Branch.
We also thank the team at CVUA Freiburg for the performance of the analyses of the POPs presented in this publication.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors alone are responsible for the views expressed in this publication, which do not necessarily represent the decisions, policy, or views of the World Health Organization and the United Nations Environment Programme.
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Malisch, R. et al. (2023). WHO- and UNEP-Coordinated Exposure Studies 2000–2019: Findings of Organochlorine Pesticides and Industrial Chemicals. In: Malisch, R., Fürst, P., Šebková, K. (eds) Persistent Organic Pollutants in Human Milk. Springer, Cham. https://doi.org/10.1007/978-3-031-34087-1_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-34087-1_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34085-7
Online ISBN: 978-3-031-34087-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)