Abstract
Building on the two rounds of exposure studies with human milk coordinated by the World Health Organization (WHO) in the mid-1980s and 1990s on polychlorinated biphenyls (PCB), polychlorinated dibenzo-p-dioxins (PCDD), and polychlorinated dibenzofurans (PCDF), five expanded studies on persistent organic pollutants (POPs) were performed between 2000 and 2019. After the adoption of the Stockholm Convention on POPs (the Convention) in 2001, WHO and the United Nations Environment Programme (UNEP) collaborated in joint studies starting in 2004. The collaboration aimed at provision of POPs data for human milk as a core matrix under the Global Monitoring Plan (GMP) to assess the effectiveness of the Convention as required under Article 16. Over time, the number of analytes in the studies expanded from the initial 12 POPs targeted by the Convention for elimination or reduction to the 30 POPs covered under the Stockholm Convention and two other POPs proposed for listing as of 2019. Many of these chemicals have numerous congeners, homologous groups, isomeric forms, and transformation products, which significantly extends the number of recommended analytes.
In the studies between 2000 and 2019, 82 countries from all five United Nations regions participated, of which 50 countries participated in more than one study. For the human milk samples of the 2016–2019 period, results are available for the full set of 32 POPs of interest for the Convention until 2019: (i) the 26 POPs listed by the start of the study in 2016; (ii) decabromodiphenyl ether [BDE-209] and short-chain chlorinated paraffins [SCCP] as listed in 2017; (3) dicofol and perfluorooctanoic acid [PFOA] as listed in 2019; (4) medium-chain chlorinated paraffins [MCCP] and perfluorohexane sulfonic acid [PFHxS] as proposed for listing. This is a unique characteristic among the core matrices under the GMP.
Four key messages can be derived:
-
(i).
These studies are an efficient and effective tool with global coverage as key contributor to the GMP. After collection of a large number of individual samples (usually 50) fulfilling protocol criteria, pooled samples are prepared using equal aliquots of individual samples (physical averaging) and are considered to be representative for a country, subregion or subpopulation at the time of the sampling. The analysis of pooled representative human milk samples by dedicated Reference Laboratories meeting rigorous quality criteria contributes to reliability and comparability and reduces uncertainty of the analytical results. Additionally, this concept is very cost-effective.
-
(ii).
These studies can be used for regional differentiation based on concentrations of individual POPs between and within the five UN Regional Groups (African Group, Asia-Pacific Group, Eastern European Group, Group of Latin American and Caribbean Countries; Western European and Others Group). For some POPs, a wide range of concentrations with up to three orders of magnitude between lower and upper concentrations was found, even for countries in the same UN region. Some countries had levels within the usual range for most POPs, but high concentrations for certain POPs. Findings of concentrations in the upper third of the frequency distribution may motivate targeted follow-up studies rather than if the observed level of a POP is found in the lower third of frequency distribution. However, the concentration of a POP has also to be seen in context of the sampling period and the history and pattern of use of the POPs in each country. Therefore, results are not intended for ranking of individual countries but rather to distinguish broader patterns.
-
(iii).
These studies can provide an assessment of time trends, as possible sources of variation were minimized by the survey concepts building on two factors (sampling design; analysis of the pooled samples by dedicated Reference Laboratories). The estimation of time trends based on comparison of median or mean concentrations in UN Regional Groups over the five surveys in five equal four-year periods between 2000 and 2019 provides a first orientation. However, the variation of the number of countries participating in a UN Regional Group in a certain period can influence the median or mean concentrations. Thus, it is more prudent to only use results of countries with repeated participation in these studies for drawing conclusions on temporal trends.
The reduction rates in countries should be seen in context with the concentration range: A differentiation of high levels and those in the range of the background contamination is meaningful. If high levels are found, sources might be detected which could be eliminated. This can lead to significant decrease rates over the following years. However, if low background levels are reported, no specific sources can be detected. Other factors for exposure, e.g. the contamination of feed and food by air via long-range transport and subsequent bioaccumulation, cannot be influenced locally.
However, only very few time points from most individual countries for most POPs of interest are available, which prevents the derivation of statistically significant temporal trends in these cases. Yet, the existing data can indicate decreasing or increasing tendencies in POP concentrations in these countries. Furthermore, pooling of data in regions allows to derive statistically significant time trends in the UN Regional Groups and globally. Global overall time trends using the data from countries with repeated participation were calculated by the Theil–Sen method. Regarding the median levels of the five UN Regional Groups, a decrease per 10 years by 58% was found for DDT, by 84% for beta-HCH, by 57% for HCB, by 32% for PBDE, by 48% for PFOS, by 70% for PCB, and by 48% for PCDD and PCDF (expressed as toxic equivalents). In contrast, the concentrations of chlorinated paraffins (CP) as “emerging POPs” showed increasing tendencies in some UN Regional Groups. On a global level, a statistically significant increase of total CP (total CP content including SCCP [listed in the Convention in 2017] and MCCP [proposed to be listed]) concentrations in human milk of 30% over 10 years was found.
-
(iv).
The studies can provide the basis for discussion of the relative importance (“ranking”) of the quantitative occurrence of POPs. This, however, requires a differentiation between two subgroups of lipophilic substances ([i] dioxin-like compounds, to be determined in the pg/g [=ng/kg] range, and [ii] non-dioxin-like chlorinated and brominated POPs, to be determined in the ng/g [=μg/kg] range; both groups reported on lipid base) and the more polar perfluorinated alkyl substances (PFAS); reported on product base [as pg/g fresh weight] or on volume base [ng/L]. For this purpose, results for the complete set of the 32 POPs of interest for the 2016–2019 period were considered.
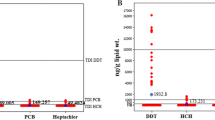
By far, the highest concentrations of lipophilic substances were found for DDT (expressed as “DDT complex”: sum of all detected analytes, calculated as DDT; maximum: 7100 ng/g lipid; median: 125 ng/g lipid) and for chlorinated paraffins (total CP content; maximum: 700 total CP/g lipid; median: 116 ng total CP/g lipid). PCB was next in the ranking and had on average an order of magnitude lower concentrations than the average of the total CP concentrations.
The high CP concentrations were caused predominantly by MCCP. If the pooled samples from mothers without any known major contamination source nearby showed a high level of CP, some individual samples (e.g. from local population close to emission sources, as a result of exposure to consumer products or from the domestic environment) might even have significantly higher levels. The lactational intake of SCCP and MCCP of the breastfed infant in the microgram scale resulting from the mothers’ dietary and environmental background exposure should therefore motivate targeted follow-up studies and further measures to reduce exposure (including in the case of MCCP, regulatory efforts, e.g. restriction in products).
Further, due to observed levels, targeted research should look at the balance among potential adverse effects against positive health aspects for the breastfed infants for three groups of POPs (dioxin-like compounds; non-dioxin-like chlorinated and brominated POPs; PFAS) regarding potentially needed updates of the WHO guidance.
As an overall conclusion, the seven rounds of WHO/UNEP human milk exposure studies are the largest global survey on human tissues with a harmonized protocol spanning over the longest time period and carried out in a uniform format. Thus, these rounds are an effective tool to obtain reliable and comparable data sets on this core matrix and a key contributor to the GMP. A comprehensive set of global data covering all POPs targeted by the Stockholm Convention, in all UN Regional Groups, and timelines covering a span of up to three decades allows to evaluate data from various perspectives. A widened three-dimensional view is necessary to discuss results and can be performed using the three pillars for assessments of the comprehensive data set, namely: analytes of interest; regional aspects; time trends. This can identify possible problems for future targeted studies and interventions at the country, regional, or global level. Long-term trends give an indication of the effectiveness of measures to eliminate or reduce specific POPs. The consideration of countries with repeated participation in these studies provides the best possible database for the evaluation of temporal trends. The continuation of these exposure studies is important for securing sufficient data for reliable time trend assessments in the future. Therefore, it is highly recommended to continue this monitoring effort, particularly for POPs that are of public health concern.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- WHO/UNEP-coordinated exposure studies
- Human milk
- Persistent organic pollutant (POP)
- UN Regional Groups
- Global
- Effective tool
- Key contributor to Global Monitoring Plan (GMP)
- Regional differentiation
- Time trend
- Relative importance of chemicals
- Stockholm Convention on POPs
1 Introduction
In Part I of this compendium on WHO/UNEP-coordinated exposure studies on human milk it was shown that human milk has served for many decades as a suitable matrix to assess the overall exposure of the general population to persistent organic pollutants (POPs) (Fürst 2023). In the mid-1980s and early 1990s, the World Health Organization (WHO) coordinated two exposure studies on concentrations of polychlorinated biphenyls (PCB), polychlorinated dibenzo-p-dioxins (PCDD), and polychlorinated dibenzofurans (PCDF) in human milk. After the adoption of the Stockholm Convention on Persistent Organic Pollutants (POPs) in 2001, WHO and the United Nations Environment Programme (UNEP) agreed to collaborate in joint studies starting in 2004 to support the implementation of the Convention by assessing its effectiveness as required under its Article 16. Between 2000 and 2019, WHO and UNEP performed five global studies on concentrations of POPs in human milk with the participation of 82 countries, 50 of them participating in more than one study (Malisch et al. 2023a). The countries were allocated to one of the five UN Regional Groups, namely, African Group, Asia and Pacific Group, Latin American and Caribbean Group, Eastern European Group, and Western European and Others Group (Table 1).
An overview of the Stockholm Convention, the Global Monitoring Plan (GMP) and its implementation by regional and global monitoring reports is given by (Šebková 2023). Human milk is a core matrix under the GMP. The objective of human biomonitoring within the GMP, which includes the WHO- and/or UNEP-coordinated human milk studies, is to identify temporal and, as appropriate, spatial trends in levels of POPs in humans to evaluate the effectiveness of the Convention.
From 2001 until 2019, the scope of POPs listed by the Stockholm Convention increased from 12 to 30 chemicals (28 chlorinated or brominated, 2 perfluorinated substances). Many of these chemicals have numerous congeners, homologous groups, isomeric forms, and transformation products, which significantly extends the number of recommended analytes. Furthermore, two POPs proposed for listing in 2019 were of interest (medium-chain chlorinated paraffins [MCCP] and perfluorohexane sulfonic acid [PFHxS]) and included on a preliminary basis. Thus, over time the surveys covered an increasing number of analytes of interest (UNEP, 2019). For the human milk samples of the 2016–2019 period, results for the complete set of 32 POPs of interest for the Convention until 2019 are available (including decabromodiphenylether [BDE-209] and short-chain chlorinated paraffins [SCCP] as listed in 2017, dicofol and perfluorooctanoic acid [PFOA] as listed in 2019, PFHxS as proposed for listing in 2019 and adopted in 2022, and MCCP as under review by the POPs Review Committee) (Table 2)—a unique characteristic among the core matrices.
The data are publicly available in the Data Warehouse of the Stockholm Convention Global Monitoring Plan (GMP DWH) (GMP DWH 2020).
This chapter presents summarizing conclusions of the WHO/UNEP-coordinated exposure studies and key messages.
2 Efficient and Effective Tool with Global Coverage as Key Contributor to the Global Monitoring Plan (GMP)
Generally, the concept of the WHO/UNEP-coordinated exposure studies of human milk has four basic elements (Malisch et al. 2023a):
-
(i).
Collection of a large number of individual samples from mothers guided by the criteria of the standardized WHO/UNEP protocols, under the supervision of a National Coordinator in each country
-
(ii).
Preparation of pooled (physically averaged) samples that are considered to represent the average levels of POPs in human milk for a country or subpopulation/region of that country at the time of sampling, using equal aliquots of individual samples
-
(iii).
Analysis of these pooled samples in designated Reference Laboratories to achieve a high degree of reliability and comparability of the analytical results
-
(iv).
Repeated participation of countries allowing conclusions on temporal trends
This concept has a number of advantages.
2.1 Possibility to Determine the Complete Set of Analytes of Interest
As no multi-method exists allowing to determine all POPs of interest to the Stockholm Convention by one single method, various analytical methods have to be applied. Due to the relatively high fat content of human milk and the large volume of the pooled (composite) sample, sufficient sample material was available to apply different methods for determination of all 30 POPs listed until 2019 and the two POPs proposed for listing (as of 2019). Consequently, results for this complete set of 32 POPs and related analytes of interest for the Convention are available for this core matrix for the 2016–2019 period.
2.2 Assurance of the Reliability and Comparability of Results over a Long Period (2000–2019) for the Wide Range of Analytes of Interest
The guidance document on the GMP for POPs addresses numerous factors that might affect the measured concentrations of POPs in environmental samples. Sampling design is considered to be an important factor. For most POPs, the precision of chemical analysis is generally believed to constitute only a minor part of the total variance in monitoring time-series of environmental data where sample variation is expected to be large, much larger compared to laboratory precision. This is especially true when the same accredited laboratory is used throughout the whole series (as in this case). In contrast, if different laboratories carry out the analysis from year to year, it could seriously decrease or challenge the possibility to evaluate time-series of POPs. This is also true if the same laboratory changes its methodology (UNEP 2007; UNEP 2015; UNEP 2019).
To minimize possible sources of variation for time trend analysis of POPs for the effectiveness evaluation of the Stockholm Convention, the concept of the WHO/UNEP-coordinated exposure studies builds on two factors:
-
1.
Minimization of possible sources of variation from the sampling design (see the above listed basic elements of the concept [i] for collection of a large number of individual samples fulfilling protocol criteria and [ii] preparation of pooled samples considered to be representative for a country or subpopulation at the time of sampling)
-
2.
Minimization of the variation from chemical analysis (see basic step [iii] mentioned above regarding the performance of the analyses of the pooled samples at the Reference Laboratories)
The determination of small changes in levels of POPs is necessary and requires that variability and uncertainty in the sampling process be minimized as far as possible, while maintaining an adequate number of qualified donors. Therefore, the revised WHO protocol guidelines for the 2004–2007 study and subsequent rounds called for the recruitment of 50 individual donors per pooled sample in countries with up to 50 million population. It is recognized that some flexibility is necessary for countries with smaller populations and/or low birth rates. In some cases, reducing the number of donors was unavoidable. On the other hand, huge countries with populations well over 50 million are encouraged to prepare a second pooled sample (or more) if feasible.
The Reference Laboratory for chlorinated and brominated POPs in the period 2000–2019 was the CVUA Freiburg, Germany, and for perfluoroalkyl substances in the period 2009–2019 was at Örebro University, Sweden. Accuracy depends on systematic errors and random components. “Trueness” (closeness of the agreement between the expectation of a test result or a measurement result and a true value) and “Precision” (closeness of the agreement between independent test/measurement results obtained under stipulated conditions) are used to describe accuracy and are therefore important criteria for assessment of reliability of analytical methods. Three articles in Part II of this compendium demonstrate the application of a comprehensive long-term analytical quality control to achieve a high degree of reliability for chlorinated and brominated POPs and to estimate trueness and long-term precision (including as key elements, e.g. procedural blanks, use of fortified material, numerous quality control samples as an in-house reference material, duplicate analyses, and successful participation in proficiency tests) (Malisch and Schächtele 2023; Hardebusch et al. 2023; Schächtele et al. 2023a). The analytical methods for perfluoroalkyl substances (PFAS) were published separately (Sadia et al. 2020; Fiedler and Sadia 2021; Fiedler et al. 2022).
2.3 Cost-Effectiveness
In all WHO- and UNEP-coordinated exposure studies of human milk, only pooled samples were used for analysis. By analysis of one or a few representative pooled human milk samples for a country, an estimate of the average human body burden can be obtained, which is the result of long-term exposure in the respective different countries. The analysis of one or a few pooled human milk samples considered to be representative is far less expensive than the analysis of a high number of individual samples, particularly for PCDD and PCDF or chlorinated paraffins. Thus, this approach is very cost-effective.
2.4 Capacity Building and Global Human Milk Bank
Since 2005, the WHO guidelines for organization, sampling, and analysis specified the collection of 50 individual samples of 50 ml each. For the analysis of analytically simple POPs, such as organochlorine pesticide POPs and marker PCB, a 25 ml aliquot of each individual sample was taken and sent to a qualified laboratory chosen by the National Coordinator, usually in the country. For the pooled sample, the remaining 25 ml from each of the 50 individual samples were used to make one pooled sample and shipped frozen to CVUA Freiburg as the Reference Laboratory for chlorinated and brominated POPs for the 2000–2019 surveys. Of this pooled sample, an aliquot was sent to the PFAS Reference Laboratory at Örebro University.
The option to have individual samples analysed for the initial organochlorine pesticide POPs and marker PCB in a competent national laboratory and the analysis of pooled samples by the Reference Laboratory, is a contribution to capacity building, particularly in developing countries. The comparison of the mean of the individual samples for these analytes with the result of the Reference Laboratory serves as an external check, as the average of the results of the individual samples should be the same as the result of the pooled sample, which is prepared from equal aliquots of the individual samples. Analysis of individual samples for the analytically simple POPs also provides information on the range of levels and geographic distribution, especially when a high value is encountered.
After analysis, any remaining pooled sample was stored at CVUA Freiburg in the Global Human Milk Bank at −20 °C. This bank is used when new POPs are added to the Stockholm Convention to allow for a retrospective analysis.
2.5 Conclusion
Table 3 provides an overview on the number of countries participating in all seven rounds of WHO- and/or UNEP-coordinated exposure studies performed between 1987 and 2019, including the 82 different countries participating between 2000 and 2019, and the respective coverage of POPs, including the determination of all 30 listed POPs and two POPs recommended for listing analysed in the 2016–2019 round. All these studies were performed based on collection of individual samples from mothers according to sampling protocol criteria, the preparation of pooled samples considered to be representative for a country or subpopulation, and analysis in Reference Laboratories.
As a conclusion, the WHO/UNEP surveys are the largest global surveys on human tissues with a harmonized protocol spanning the longest time period and carried out in a uniform format. Thus, these studies are an efficient and effective tool to obtain reliable comprehensive data sets for this GMP core matrix and a key contributor to the Stockholm Convention.
3 Regional Group Differentiation
Results presented in this compendium are shown for United Nations Regional Groups. The concentrations of individual POPs were compared between and within regions, however, they are not intended for ranking of individual countries. For some POPs, a wide range of concentrations with up to three orders of magnitude between lower and upper concentrations was found, even for countries in the same UN Regional Group. Some countries had levels within the usual range for most POPs, but high concentrations for a certain POP. Findings of concentrations in the upper third of the frequency distribution may motivate targeted follow-up studies rather than if the observed level of a POP is found in the lower third of frequency distribution. However, the levels of concentrations have also to be seen in context with the sampling period and POPs use patterns/history in each country.
Comprehensive articles in Part III and Part IV of this review book present the complex discussion for the 32 POPs of interest in detail. Further, two selected examples (PCB and DDT) below illustrate some important regional aspects. For the convenience of readers, the supplementary information for this article (appendix, section “Regional Differentiation”) contains short summaries of regional aspects also for other selected POPs: (i) Toxic equivalents [TEQ] of PCDD/PCDF and dioxin-like PCB [WHO2005-TEQ]); (ii) beta-HCH; (iii) HCB; (iv) dieldrin; (v) PBDE; (vi) PFOS; and (vii) chlorinated paraffins. For all other POPs and the detailed discussion refer to the articles mentioned above.
3.1 PCB
The range of concentration levels detected for PCB (expressed as the sum of 6 indicator PCB [ΣPCB6]) in 232 pooled human milk samples from 82 countries collected between 2000 and 2019 varied approximately between 1 and 1000 ng/g lipid. The highest concentrations were found in the Eastern European Group (median of 121 ng/g lipid and maximum of 1009 ng/g lipid), followed by the group of Western European and Other States (median 75 ng/g lipid, maximum 467 ng/g lipid). In all other groups, considerably lower PCB levels were found (median between 15 and 22 ng/g, maximum lower than 100 ng/g lipid) (Fig. 1). Note that Australia, Israel, New Zealand, and the USA are included as “Others” in the Western European and Others Group; in these countries, lower PCB concentrations were found comparable to non-European countries. Overall, observed PCB concentrations were considerably higher in Europe than in the other geographic regions.
Range of concentrations of the sum of 6 Indicator PCB (ΣPCB6) among UN Regional Groups (ng/g lipid, upper bound; N = 232 pooled samples from 82 countries) [Box plot; minimum and maximum: as circles; fifth and 95th percentile: as whiskers; lower (25–50%) and upper (50–75%) quartiles, separated by the line for the median: as box; mean: as asterisk]
Figure 2 illustrates the regional differentiation (indicating the five UN Regional Groups by different colours) with temporal tendencies over the five rounds. Between 2000 and 2003, the majority of participants were European countries. Later, WHO and UNEP encouraged countries of other groups to participate through special programmes. As shown above, European countries had by far the highest ΣPCB6 concentrations. This is supported by the results of the 2000–2003 round, when only Eastern and Western countries were found in the upper third and middle part of the frequency distribution. The 2004–2007 round also showed considerably higher non-dioxin-like PCB concentrations in European countries. In these rounds, the highest ΣPCB6 concentrations were found in samples from the Czech and the Slovak Republics. In one of the three pooled samples submitted by the Czech Republic in 2001, the highest concentration of 1009 ng/g was found. The median of the three submitted samples was 502 ng/g. However, the PCB concentrations in samples of these two countries decreased considerably to 109 ng/g in the Czech Republic and 78 ng/g in the Slovak Republic in the samples from 2019. For a detailed discussion, see Part III of this compendium (Malisch et al. 2023b).
Results of the 2000–2019 surveys for the sum of 6 Indicator PCB (ΣPCB6) in 232 pooled human milk samples from 82 countries of the five UN Regional Groups (with split of the Asia-Pacific Group into two subgroups Asia and Pacific Islands) indicated by different colours and classified into five periods of sample submission (ng ΣPCB6/g lipid). Whiskers indicate the range of concentrations found in the pooled samples around the median concentration, if two or more samples were submitted by a country in a certain period
3.2 DDT
The highest median levels of DDT (expressed as “DDT complex”: sum of all detected analytes, calculated as DDT [Malisch et al. 2023c]) were found in the African Group (517 μg DDT complex/kg lipid), followed by the Eastern European Group (449 μg DDT complex/kg lipid), the lowest median levels in the Western European and Others Group (147 μg DDT complex/kg lipid) (Fig. 3). However, large differences were seen between countries even in the same region. In the African Group, the range of DDT complex concentrations stretched over three orders of magnitude with a minimum of 17 μg/kg lipid found in 2019 in one country and a maximum of 23,500 μg/kg lipid found in 2012 in another country. The DDT complex concentrations in the Asia-Pacific Group covered a range between 189 and 8500 μg/kg lipid.
Range of concentrations of DDT (expressed as μg DDT complex/kg lipid) in human milk among UN Regional Groups (N = 134 country results [using the median, if results for two or more pooled samples are available in a certain period] from 82 countries, comprising the five periods from 2000–2019) [Box plot; minimum and maximum: as circles; fifth and 95th percentile: as whiskers; lower (25–50%) and upper (50–75%) quartiles, separated by the line for the median: as box; mean: as asterisk]
In all UN Regional Groups, the median of the DDT complex concentrations was higher in the 2000–2003 period than in the 2016–2019 period. However, in the three rounds in-between, a considerable variation within the regions was observed; caused by the variation of countries participating in a certain UN Regional Group in a certain period. In this way, the elevated concentrations in the 2008–2011 period were found in Tajikistan (8500 μg DDT complex/kg lipid) and on the Solomon Islands (4760 μg DDT complex/kg lipid), and in the 2012–2015 period in Ethiopia (23,500 μg DDT complex/kg lipid). However, the 2019 samples from the Solomon Islands and Ethiopia showed considerable downward trends with 1390 and 7100 μg DDT complex/kg lipid, respectively (Fig. 4). For a detailed discussion, see Part III of this compendium (Malisch et al. 2023c).
4 Assessment of Time Trends
The estimation of time trends based on data from different countries reported in the literature is difficult, as different sampling concepts were applied and the comparability of the analytical results is not always clear (Fürst 2023). However, for the long-term, the aim of the WHO/UNEP-coordinated exposure studies is to support the Convention’s implementation by providing data to the effectiveness evaluation as required under Article 16. Therefore, as explained above in subsection 2.2, possible sources of variation for time trend analysis were minimized by the concept building on the two factors (sampling design; performance of the analyses of the pooled samples at the Reference Laboratories).
For the evaluation of time trends, participation in different sampling periods is necessary. Countries were encouraged to participate repeatedly. The first sampling period is conducted to determine baseline concentrations for POPs in randomly selected individual samples of human milk and pooled samples made from them. A second sampling period should be conducted with a similarly selected cohort, e.g. four or five years later (or other time period deemed appropriate). Future samplings should be undertaken at regular intervals and the monitoring of human milk for POPs should be considered a long-term activity.
To provide reliable and comparable monitoring information for the Parties to the Stockholm Convention, the GMP guidance document proposed a quantitative objective for temporal trend studies: These studies should be able to detect a 50% decrease in the levels of POPs within a 10-year period (UNEP 2015; UNEP 2019). However, in distinction from this goal for abilities of temporal trend studies to detect changes over time, there is no stipulation of a quantitative goal for the rate of reduction/decrease in POPs levels by the Convention. The Convention’s objectives are either to eliminate or to restrict production, use, and releases, depending on the annex where a chemical is listed, but the rate of decline is nowhere specified.
A period of at least 10–15 years is estimated to be necessary to detect significant temporal changes of moderate size for most POPs. For example, a change of 7% per year would be necessary for a 50% decrease over 10 years. Furthermore, at least 4 to 5 years of monitoring would be necessary to give reliable estimates taking into account random within- and between-year variation and other components of variance (UNEP 2019). In addition, the rate of decrease will vary among POPs. Moreover, the reduction rates depend on the concentration range (levels above or in the range of background contamination), see subsection 4.1 “DDT” in the following.
The estimation of time trends based on comparison of median or mean concentrations in UN Regional Groups in the five studies by classification into equal four-year periods between 2000 and 2019 provides a first orientation. However, the variation of countries participating in a certain UN Regional Group in a certain period can influence the median or mean concentrations. In particular, if the number of countries differs considerably between periods or if quite high concentrations are found in a sample submitted by a country with single participation in a certain period, the resulting median or mean can be distorted. Thus, it is more precise to only use results from countries with repeated participation in these studies: This allows drawing conclusions on temporal trends, which are not potentially influenced by these possible factors.
In the following, short summaries of three examples of the “initial POPs” (DDT; PCB; Toxic equivalents of PCDD/PCDF and dioxin-like PCB [WHO2005-TEQ]) and of one emerging POP (chlorinated paraffins [CP]) illustrate these important aspects for conclusions on time trends. For the convenience of readers, more examples are given in the supplementary information (appendix, section “Assessments of Time Trends”): (1) beta-HCH; (2) HCB; (3) PBDE; (4) PFOS. For other POPs and the full view, comprehensive articles appear in Part III and Part IV of this compendium.
4.1 DDT
As general estimation of time trends, the median of the DDT complex concentrations of all country results (N = 134) shows a decrease of 72% from the 2000–2003 period (median for 16 countries: 445 μg/kg lipid) to the 2016–2019 period (median for 43 countries: 125 μg/kg lipid). The downward trend between these two periods was found in all regions, but with considerable differences among regions and a great variation among the three rounds in-between, as illustrated by Fig. 5. In all groups, the median of the DDT complex concentrations was higher in the 2000–2003 period than in the 2016–2019 period. However, in the three rounds in-between, a considerable variation within UN Regional Groups was observed; obviously with a substantial maximum in Latin American and Caribbean countries in the 2004–2007 period and elevated levels also in Africa at that time period. If calculated as the mean of all country results, the DDT complex concentration was higher in the 2000–2003 period than in the 2016–2019 period in all regions except Africa. Again, in the three rounds in-between, a considerable variation was observed, with a substantial maximum in Africa in the 2012–2015 period. This shows that the mean is misleading, if substantial maxima occur (Fig. 6).
Time trends for median concentrations of DDT (expressed as μg DDT complex/kg lipid) in human milk in the five UN Regional Groups (with split of the Asia-Pacific Group [APG] into two subgroups Asia and Pacific Islands; GRULAC = Group of Latin American and Caribbean Countries; EEG = Eastern European Group; WEOG = Western European and Others Group) in five periods between 2000 and 2019 based on all country results (using the median, if results for two or more pooled samples are available in a certain period) from 82 countries
Time trends for mean concentrations of DDT (expressed as μg DDT complex/kg lipid) in human milk in the five UN Regional Groups (with split of the Asia-Pacific Group [APG] into two subgroups Asia and Pacific Islands; GRULAC = Group of Latin American and Caribbean Countries; EEG = Eastern European Group; WEOG = Western European and Others Group) in five periods between 2000 and 2019 based on all (N = 134) country results (using the median, if results for two or more pooled samples are available in a certain period) from 82 countries
As a result, the median, mean, and ranges of DDT complex concentrations found in the UN Regional Groups in five periods over these 20 years cannot be used directly to derive continuous time trends. The main problem is the variation of countries and the low number of countries participating from a certain UN Regional Group in a certain period, in particular, if a country with a very high concentration participated in a single period: This could have a considerable effect on median or mean concentrations for this UN Regional Group in that period. The substantial maximum in Latin American and Caribbean countries in the 2004–2007 period was caused by high DDT concentrations found in Haiti in 2005 (2830 μg/kg lipid) as only country of this UN Regional Group in this period; however, in Haiti, these concentrations decreased considerably in the following years (in 2011: 574 μg/kg lipid, in 2015: 263 μg/kg lipid). The maximum of the mean values in African countries in the 2012–2015 period was caused by the high DDT concentration in human milk from Ethiopia in 2012 (23,500 μg/kg lipid; participating as one of three African countries in this period). However, also in Ethiopia, the DDT concentrations decreased considerably until 2019 (7100 μg/kg lipid). For more information and discussion of the findings of DDT in UN Regional Groups, see (Malisch et al. 2023c).
The assessment of time trends based only on results of countries with repeated participation allows more certainty in drawing the conclusions, and therefore is advisable for the evaluation of changes over time for the effectiveness evaluation for the purpose of Article 16. Figure 7 illustrates the temporal tendencies observed in 44 countries of the five UN Regional Groups that participated in the survey two or more times. In nearly all countries, decreasing tendencies are observed, with a median of 59% decrease over the years between the first and the last participation.
The reduction rates in countries should be seen in context and discussed within the total concentration range: A differentiation among levels above or within the range of the background contamination seems to be necessary. If high levels are found, sources might be detected which could be eliminated, as e.g. described for Ethiopia (Gebremichael et al. 2013). However, at low/background levels, other factors, e.g. secondary contamination of feeds and food via long-range atmospheric transport and subsequent bioaccumulation, cannot be influenced locally.
It should also be noted that a limited number of data points of concentrations over the period of 2000–2019 are available from the individual countries which prevents the determination of statistically significant individual temporal trends in most cases. Nevertheless, existing data do indicate decreasing or increasing tendencies in POP concentrations. Furthermore, pooling of/combining data collected in regions allows deriving statistically significant time trends in the UN Regional Groups and overall globally.
For the 44 countries with repeated participation, the overall decrease of POP concentrations per 10 years was calculated by the non-parametric linear Theil–Sen trend estimator (Sen 1968; Theil 1992). Secondly, a method for deriving the regional group trend as a median of trends in countries within the group was used (“median method”) (for methods of statistical data treatment, see Malisch et al. 2023d). A statistically significant overall decrease within a 10-year period between 50% and 80% was achieved for DDT levels in the African Group, the Asian-Pacific Group and the Group of Latin American and Caribbean Countries, and at a global level. Lower decrease rates were observed in the Eastern European Group and the Western European and Others Group, which had banned DDT much earlier. As the median of the five UN Regional Groups, a decrease per 10 years by 58% was found for DDT (Table 4). For more details, see (Malisch et al. 2023e).
4.2 PCB
As shown above (subsection 3.1), in comparison to other geographic regions, European countries had by far the highest ΣPCB6 concentrations (Indicator PCB) in the 2000–2019 period. This period can be expanded by the inclusion of results for non-dioxin-like PCB of the first (1987–1988) and second (1992–1993) rounds of WHO-coordinated exposure studies (WHO 1989, 1996), to cover in total about three decades. For the assessment of temporal trends based only on countries with repeated participation, data from 25 European countries are available, of which 15 countries participated already in the 1987–1988 and/or the 1992–1993 round (Fig. 8).
Overview of the development of Indicator PCB concentrations (ng ΣPCB6/g lipid; country results as median of multiple sample results, if two or more pooled samples were submitted in a certain period) over time between 1987 and 2019 for 25 countries of the Eastern European and Western European and Others Regional Groups that participated two or more times
At the end of the 1980s and early 1990s, ΣPCB6 concentrations in European countries were in a range up to 800 ng/g lipid and decreased until 2019 to concentrations mostly below 100 ng/g lipid. After detection of PCB in environmental samples in 1966 and measures taken since the 1970s, significant reductions were already achieved in the 1990s. Therefore, a decrease of ΣPCB6 concentrations in human milk by up to 95% was achieved since the end of the 1980s and resulting in low background levels observed in 2019.
Also, in most countries from the other UN Regional Groups, a decrease between different periods was observed, but these decreases began at considerably lower concentrations (below about 100 ng/g lipid). Similarly to 15 European countries, two Asian countries (Vietnam, Thailand) were monitored over three decades (with first participation already in the 1987–1988 POPs exposure study), whereas the studies in some African, Latin American and Caribbean countries comprise at best two decades (with first participation in the 2000–2003 or 2004–2007 study) but rather a decade—starting from the 2008–2011 survey round (Fig. 9).
Overview of the development of Indicator PCB concentrations (ng ΣPCB6/g lipid; country results as median of multiple sample results, if two or more pooled samples were submitted in a certain period) over time between 1987 and 2019 for 32 countries of the African Group, the Asia-Pacific Group and the Group of Latin American and Caribbean Countries that participated two or more times
In the majority of UN Regional Groups and at a global level, a decrease between 50% and 70% within a 10-year period was achieved for the levels of ΣPCB6. The Latin American and Caribbean countries had lower ΣPCB6 concentrations in comparable periods, obviously resulting in lower decrease rates. This is an indication that the decrease might be faster in regions with higher concentration, compared to a slower decrease in less polluted regions. All trends were statistically significant (p-value <0.001) (Table 5).
For details of the first general estimation of temporal trends from all 82 countries participating between 2000 and 2019, see (Malisch et al. 2023b); for assessment of time trends only of 57 countries with repeated participation in WHO/UNEP-coordinated exposure studies performed between 1987 and 2019, see (Malisch et al. 2023d).
4.3 Toxic Equivalents of PCDD and PCDF (WHO-PCDD/PCDF-TEQ [2005])
As for non-dioxin-like PCB, also for PCDD and PCDF data are available from the first two rounds of human exposure studies coordinated by WHO in the mid-1980s and 1990s to determine the concentrations of PCB, PCDD, and PCDF in human milk. As dioxin-like PCB were of interest starting from the second WHO round (1992–1993), time trends for total TEQ (WHO2005-TEQ) can be derived only beginning with that period. Therefore, the time trends for WHO-PCDD/PCDF-TEQ (calculated using the toxic equivalency factors [TEF]) proposed in 2005 [WHO2005-TEF] [van den Berg et al. 2006]; for calculation and use of the terms for sum parameters for toxic equivalents including “WHO-PCDD/PCDF-TEQ [2005]”, see Malisch et al. 2023b) are summarized, which can be derived beginning with the 1987–1988 period covering again about three decades. For the assessment of temporal trends of PCDD and PCDF based only on countries with repeated participation, data from 19 countries (15 European and New Zealand, Thailand, USA, and Vietnam) are available, which participated already in the 1987–1988 and/or the 1992–1993 round (Fig. 10). In the Netherlands, Belgium, and Germany with highest WHO-PCDD/PCDF-TEQ concentrations in the 1987–1998 period, a decrease of about 90% was achieved until the 2012–2015 or 2016–2019 period.
Overview of the development of WHO-PCDD/PCDF-TEQ (2005) concentrations (pg/g lipid; country results as median of multiple sample results, if two or more pooled samples were submitted in a certain period) over time for countries with repeated participation in WHO/UNEP-coordinated exposure studies between 1987 and 2019 and first participation in the first or second round
In the UN Regional Group with Western European and other countries with relatively high WHO-PCDD/PCDF-TEQ concentrations in human milk in the 1987–1988 and 1992–1993 rounds, a decrease of 51% over 10 years was achieved. Most Eastern European countries participated for the first time in the 1992–1993 period with on average lower WHO-PCDD/PCDF-TEQ concentrations in human milk than in Western European countries; for this group the decrease within a 10-year-period was 35%. Large differences between the decrease calculated by the Theil–Sen method or the median method indicate a considerable variation between countries in that region. Lower decrease rates were observed in some countries, but this has to be seen in context with the quite low initial levels in these countries. Therefore, these regional trends have also to be seen in context with the variation among participating countries. As median of the five UN Regional Groups, a decrease per 10 years by 48% was found for WHO-PCDD/PCDF-TEQ (Table 6). For more details and for the assessment of temporal trends of total TEQ (WHO2005-TEQ), see (Malisch et al. 2023d).
4.4 Chlorinated Paraffins
Average CP levels have increased between two time spans (2009–2014 vs 2015–2019) in four out of the five UN Regional Groups: in the African Group (AFR), the Asia-Pacific Group (ASPAC), the Eastern European Group (EEG), and the Group of Latin America and Caribbean Countries (GRULAC) (Fig. 11). On the other hand, average CP levels in the Western European and Others Group (WEOG) seem to have decreased in the latter time period, with a comparatively close grouping of the results. As only two data points per country were available, it is not possible to derive statistically significant time trends for individual countries but statistically not significant temporal tendencies (Krätschmer et al. 2023).
Range of sum of CP determined in pooled human milk samples from the 2009–2019 period of WHO/UNEP-coordinated human milk studies. 27 of the samples collected before 2015 were only analysed for their total CP content, for all other samples, SCCP and MCCP levels were reported. AFR: Africa, ASPAC: Asia-Pacific Group, EEG: Eastern European Group, GRULAC: Group of Latin American and Caribbean Countries, WEOG: Western European and Others Group (including Australia)
Temporal trends over a ten-year period of the CP levels in the five UN regional groups were assessed by the Theil–Sen method. No significant changes in CP levels were observed in the African and GRULAC groups as a whole, while a decrease by 63% was calculated in the WEOG group over 10 years (p = 0.001). In contrast, in the Asia-Pacific and Eastern European regional groups an increase of CP over a decade by up to 200% was calculated (p < 0.010). The ten-year-trend globally, based on all 84 results of 57 countries covering the period between 2009 and 2019, indicated an increase of total CP in human milk by 30% (p < 0.001) (Table 7) (Krätschmer et al. 2023). This indication of increasing CP levels in the background contamination of human milk is cause for concern.
5 Relative Importance of POPs
Another important aspect is the share of the individual 30 POPs and 2 additional chemicals proposed for listing in “total POPs concentrations”. Results for the complete set of these “30 plus 2” POPs of interest are available for the core matrix “human milk” for the 2016–2019 period—a unique characteristic among the core matrices under the GMP. For discussion of the relative importance (“ranking”), the following differentiation is necessary:
-
The lipophilic chlorinated and brominated POPs are reported on lipid base. Here, dioxin-like compounds (PCDD, PCDF, and dioxin-like PCB contributing to toxic equivalents [TEQ]); furthermore PCN, which according to peer reviewed publications have also dioxin-like toxic toxicity, have to be determined in the pg/g range, whereas the other chlorinated and brominated POPs are usually determined in the ng/g (=μg/kg) range.
-
The more polar perfluoroalkyl substances (PFAS) data are usually reported on product base (as pg/g fresh weight) or on volume base (ng/L).
5.1 Dioxin-Like Compounds
In the 44 samples submitted in the 2016–2019 period, total TEQ (WHO2005-TEQ) resulting from PCDD, PCDF, and dioxin-like PCB were in the range 1.29–11.6 pg/g lipid (median: 3.90 pg/g lipid). On average, 73% resulted from PCDD and PCDF (range 44–92%; median: 2.63 pg WHO-PCDD/PCDF-TEQ/g lipid, range 1.02–9.97 pg/g lipid) and 27% from dioxin-like PCB (range 8–56%; median: 1.00 pg WHO-PCB-TEQ/g lipid, range 0.27–3.70 pg/g lipid) (Malisch et al. 2023b).
With regard to dioxin-like compounds (DLCs), at present there is no health-based guidance value (HBGV) available for the breastfed infant. Although formally these HBGVs have been set to protect human health for a lifetime exposure period, much of the underlying experimental data focus on the perinatal and/or childhood period. Therefore, it is justifiable to use these HBGVs for early life and shorter than lifetime exposures, e.g. breastfeeding. With this approach the present HBGVs for DLCs were generally exceeded one order of magnitude or more in industrialized countries over the period 2000 to 2019. If HBGVs of 1 or 0.1 pg TEQ/kg/day are used to calculate toxicological acceptable levels of DLCs in human milk, it can be estimated that such levels will not be reached before, respectively, 2030 or 2050. When the subtle adverse health effects of DLCs in the breastfed infant reported in the 1990s were compared with benefits of breastfeeding for the infant and mother, it is concluded that benefits grossly outweigh the potential adverse health. Therefore, it is concluded that the WHO has rightfully encouraged breastfeeding for the last decades (van den Berg et al. 2023).
The concentrations of polychlorinated naphthalenes (PCN) were determined in 40 pooled human milk samples from 39 countries (Tschiggfrei et al. 2023). The median concentration of the sum of 26 PCN was 55 pg/g lipid (range 27 to 170 pg/g). Among other adverse biological effects, PCN also show dioxin-like toxicity and this was estimated by calculating the toxic equivalents in these samples using two sets of relative effect potency (REP) values: a set that has been used in earlier human exposure studies, and REPs suggested by Falandysz et al. (2014). The median PCN-TEQ concentration in human milk was 0.07 pg PCN-TEQ/g lipid (range 0.03 pg/g to 0.23 pg/g), when calculated using the human biomonitoring study REPs, and 0.03 pg PCN-TEQ/g lipid (range 0.01 pg/g to 0.10 pg/g), when calculated with other suggested REPs. The vast majority, about 90%, of this TEQ can be attributed to the PCN 66/67 congener pair. The assessment of PCN 66 and PCN 67 in order to obtain confirmed TEFs would be most important for calculations of the dioxin-like toxicity of PCN congeners, followed by PCN 69. Minor contributions to PCN-TEQ concentrations in human milk come from PCN 52/60, PCN 64/68, PCN 70, and PCN 73.
On average, the contribution of PCN-TEQ to the cumulative TEQ (including the overall sum of toxic equivalents of PCDD, PCDF, and dioxin-like PCB [WHO2005-TEQ]) was between 1% and 2%, with a wider range up to 5% for the 39 countries of this study. This is about an order of magnitude lower than the contribution of dioxin-like PCB to the cumulative TEQ (median 26%). In line with the observed higher total PCN concentrations, European countries also showed considerably higher levels of PCN-TEQ than the other regions. PCN-TEQ calculated with REPs used in human biomonitoring studies add on average about 2% to the cumulative TEQ of dioxin-like contaminants in Africa, Asia-Pacific and Latin American and Caribbean countries and about 4% in European countries. The corresponding contribution of PCN-TEQ calculated with REPs suggested by Falandysz et al. (2014) would be 1% in non-European countries and 2% in European countries.
In addition to the 30 chemicals listed by the Convention and 2 chemicals proposed for listing as of 2019, in 38 pooled samples from 28 countries collected between 2001 and 2009 concentrations of polybrominated dibenzo-p-dioxins and -furans (PBDD/PBDF) were determined to assess their contribution to the total TEQ (WHO2005-TEQ) resulting from PCDD, PCDF, and dioxin-like PCB. PBDD and PBDF provided on average about 10% to the overall TEQ calculated as sum of WHO-PCDD/PCDF-TEQ and WHO-PBDD/PBDF-TEQ, when assuming the same TEFs for brominated congeners as assigned to their chlorinated analogues. No correlations between PCDD/PCDF and PBDD/PBDF, or PBDD/PBDF and PBDEs (calculated as ∑ PBDE6) were found (Kotz et al. 2005; Schächtele et al. 2023b).
5.2 Non-dioxin-Like Chlorinated and Brominated POPs
The range of the non-dioxin-like chlorinated and brominated POPs found in the 2016–2019 survey is illustrated by Figs. 12 and 13. By far highest concentrations were found for DDT and for chlorinated paraffins (total CP including SCCP and MCCP). The maximum found for DDT (7100 ng DDT complex/g lipid) was a factor of 10 higher than the maximum of total CP (700 ng/g lipid for the sum of SCCP and MCCP). However, the median of CP concentrations (116 ng total CP/g lipid) was comparable to the median of DDT concentrations (125 ng DDT complex/g lipid). The high CP concentrations were caused predominantly by MCCP (median 66 ng/g lipid; maximum 536 ng/g lipid), with SCCP concentrations of 51 ng/g lipid as median and 188 ng/g lipid as maximum. PCB as next following group in the ranking had on average an order of magnitude lower concentrations than the total CP concentrations (median 12.7 ng ∑ PCB6/g lipid, maximum 109 ng/g lipid).
Range of concentrations of lipophilic chlorinated and brominated POPs in human milk (ng/g lipid) from 43 countries in the period 2016–2019 (median with error bars indicating the minimum and maximum) (This figure is scaled to 100 ng/g lipid as maximum value allowing a visual comparison also at lower concentration ranges)
Median concentrations between 1.0 ng/g lipid and 3.3 ng/g lipid were found for nonachlor, ∑ PBDE6 (and ∑ PBDE7 including BDE-209), dieldrin, beta-HCH, and HCB; maximum levels between 10 ng/g lipid and 110 ng/g lipid for pentachloroanisole, nonachlor, beta-HCH, HCB, and ∑ PBDE6. The concentrations of other chlorinated and brominated POPs were frequently below LOQ (0.5 ng/g lipid) or, if quantifiable, below 10 ng/g lipid. The quite low concentrations for ∑ PCN26 (median 55 pg/g lipid; range 27 to 170 pg/g) have to be assessed with regard to their dioxin-like toxicity (see above).
Regarding PBDE, deca-BDE (BDE-209) contributed on average about 13% to ∑PBDE7. The contribution of the octa-brominated diphenyl ether (octa-BDE) BDE-197 and nona-BDEs-206, - 207 and -208 to the sum of 25 PBDE was in the range of the six recommended analytes and BDE-209. Therefore, their addition to the list of recommended analytes should be considered (Schächtele et al. 2023b).
The range below 5 ng/g lipid can be seen as background concentrations of non-dioxin-like chlorinated and brominated POPs. Background concentrations are defined as that portion of the measured human milk levels that is found in the absence of specific sources and therefore is not attributable to a known exposure, e.g. to use of the chemical of interest or to emissions within the study area. In contrast to findings of high concentrations, e.g. after use of chemicals, and/or after a sufficient long withdrawal period (measures restricting their manufacture and use) for many POPs, the levels are described as “low background levels”. However, the term “background level” does not imply per se any level of safety. With respect to potential adverse effects, risk assessments need to consider many factors, including the toxicity of the chemical of interest and the determined concentration range (van den Berg et al. 2016). Further, due to observed levels, targeted research should look at the balance among potential adverse effects against positive health aspects for the breastfed infants for three groups of POPs (dioxin-like compounds; non-dioxin-like chlorinated and brominated POPs; PFAS) regarding potentially needed updates of the WHO guidance.
The relative share of SCCP and MCCP concentrations in addition to remaining 28 chlorinated and brominated analytes as listed in 2019 is illustrated by Fig. 14 (Krätschmer et al. 2023). The Stockholm Convention on POPs was sorted into two groups:
-
Pesticide group: aldrin, chlordane, chlordecone, DDT, dicofol; dieldrin, endosulfan, endrin, heptachlor, alpha-hexachlorocyclohexane (HCH), beta-HCH; gamma-HCH; mirex, pentachlorobenzene, pentachlorophenol (including pentachloroanisole) and toxaphene
-
(Other) Industrial chemicals and by-products: hexabromobiphenyl (HBB), hexabromocyclododecane (HBCDD), HCB, hexachlorobutadiene; PBDE (tetra- and pentabromodiphenyl ether; hexa- and heptabromodiphenyl ether; decabromobiphenyl ether), PCB, PCDD, PCDF, and PCN
Median sum of all POPs analysed in pooled human milk samples from 2015–2019, sorted by UN regions and broken down into Stockholm Convention POPs groups (bar charts) and further into components of the “industrial chemicals” POPs group (pie charts). AFR: Africa, ASPAC: Asia-Pacific Group, EEG: Eastern European Group, GRULAC: Group of Latin American and Caribbean Countries, WEOG: Western European and Others Group
SCCP and MCCP (proposed to be listed to the Convention) dominated over the POPs grouped as industrial chemicals and by-products in the African, the Asia-Pacific and the Latin American and Caribbean Regional Groups. For European countries, the contrary was observed—proportion of “other industrial POPs and by-products” (mainly PCB) in the sum of concentrations of all POPs was much higher than that of SCCP and MCCP, respectively.
Individual samples (from individual donors) can provide additional information on the distribution of exposures and on factors possibly contributing to such exposure. Compared to pooled samples, they can span a broad range of concentrations. If significantly elevated levels are found in pooled samples, a follow-up survey/monitoring is usually recommended; if levels are quite low, no particular additional effort would generally seem to be necessary. Given the design of the milk studies, i.e. analysis of pooled samples, the dominance of SCCP and MCCP in comparison to levels of most other POPs in UN Regional Groups gives cause for concern. If the pooled samples from mothers without any known major POPs contamination sources already show this consistent pattern with a dominance of CP, individual samples (e.g., from local population close to POPs emission sources, as a result of exposure to consumer products or from the domestic environment) might be even markedly higher. The lactational intake of SCCP and MCCP of the breastfed infant in the microgram scale resulting from the mothers’ dietary and environmental background exposure should therefore motivate targeted follow-up studies and further regulatory efforts (or, in the case of MCCP, any, e.g. restriction in products) (Krätschmer et al. 2023).
5.3 Perfluoroalkyl Substances (PFAS)
As mentioned above, PFAS concentrations are reported on product basis and therefore cannot be compared with concentrations of lipophilic POPs determined on lipid basis. 44 samples from 42 countries collected in the period 2016–2019 were analysed for PFAS. Within this group, the PFOS concentrations were in a wide range of <6.2–212 pg/g fresh weight (fw) (median: 13.2 pg/g fw) and the PFOA concentrations in a quite narrow range of 6.2–37.4 pg/g fw (median: 15.8 pg/g fw). PFHxS concentrations in 40 samples were below LOQ (<5.5 pg/g fw); four samples had concentrations between 7.3 pg/g fw and 111 pg/g fw (Fiedler and Sadia 2021).
6 Overall Conclusions
The concept of the WHO UNEP-coordinated exposure studies with standardized protocols for preparation of pooled samples considered to be representative for a country or subpopulation in a country and analysis in designated Reference Laboratories provides reliable and comparable data for POPs for human milk. Such studies have been performed over three decades (starting in 1987 with the focus on PCDD/PCDF and PCB and expanding to cover all 30 POPs listed by the Stockholm Convention in 2019 and in addition two other candidate POPs proposed for listing).
For discussion of country-specific results obtained for a certain analyte at a certain time, the three-dimensional picture can be derived by the assessments of the three pillars of this core matrix (Fig. 15): (1) the relative importance (“ranking”) of the analytes of interest (30 listed and 2 candidate POPs proposed for listing as of 2019); (2) regional aspects (at the level of countries, UN Regional Groups or globally), and (3) time trends and their assessments for the effectiveness evaluation of the Stockholm Convention as requested by its Article 16.
The consideration of countries with repeated participation provides the best possible data set for the evaluation of temporal trends and for an assessment of the effectiveness of adopted measures. It is important to continue this existing monitoring arrangement in the same setting to secure sufficient data for reliable time trend assessments in the future. Due to the long half-life of POPs, only long-term trends can give an indication of the effectiveness of measures, and can identify possible problems to allow for targeted intervention.
References
Falandysz J, Fernandes A, Gregoraszczuk E, Rose M (2014) The toxicological effects of halogenated naphthalenes: a review of aryl-hydrocarbon receptor-mediated (dioxin-like) relative potency factors. Journal of Environmental Science and Health, Part C: Environmental carcinogenesis and ecotoxicology reviews 32(3):239–272. https://doi.org/10.1080/10590501.2014.938945
Fiedler H, Sadia M (2021) Regional occurrence of perfluoroalkane substances in human milk for the global monitoring plan under the Stockholm convention on persistent organic pollutants during 2016-2019. Chemosphere 277:130287
Fiedler H, Sadia M, Krauss T, Baabish A, Yeung LWY (2022) Perfluoroalkane acids in human milk under the global monitoring plan of the Stockholm Convention on Persistent Organic Pollutants (2008–2019). Front Environ Sci Eng 16(10):132. https://doi.org/10.1007/s11783-022-1541-8
Fürst P (2023) Human milk surveys on persistent organic pollutants from a historical perspective. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Gebremichael S, Birhanu T, Tessema DA (2013) Analysis of organochlorine pesticide residues in human and cow’s milk in the towns of Asendabo, Serbo and Jimma in South-Western Ethiopia. Chemosphere 90:1652–1657
GMP DWH (2020) Hůlek, R., Borůvková, J., Kalina, J., Bednářová, Z., Šebková, K., Hruban, T., Novotný, V., Ismael, M., Klánová J. Global monitoring plan data warehouse of the Stockholm convention on persistent organic pollutants: visualisation platform and on-line tool for the analysis of global levels of POPs in air, water, breast milk and blood [online]. Masaryk University, 2020. Available from: https://www.pops-gmp.org
Hardebusch B, Dambacher B, Polley J, Lippold R, Schächtele A (2023) Analysis and quality control of WHO- and UNEP-coordinated human milk studies 2000-2019: organochlorine pesticides and industrial chemicals. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part II)
Kotz A, Malisch R, Kypke K, Oehme M (2005) PBDE, PBDD/F and mixed chlorinated-brominated PXDD/F in pooled human milk samples from different countries. Organohalogen Compd 67:1540–1544
Krätschmer K, Malisch R, Vetter W (2023) WHO- and UNEP-coordinated exposure studies 2000-2019: findings of chlorinated paraffins. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
Malisch R, Schächtele A (2023) Analysis and quality control of WHO- and UNEP-coordinated human milk studies 2000-2019: polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part II)
Malisch R, Malisch K, van Leeuwen FXR, Moy G, Tritscher A, Witt A, Alvarez J (2023a) Overview of WHO- and UNEP-coordinated human milk studies and their link to the Stockholm convention on persistent organic pollutants. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Malisch R, Schächtele A, van Leeuwen FXR, Moy G, Tritscher A (2023b) WHO- and UNEP-coordinated exposure studies 2000-2019: findings of polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
Malisch R, Kypke K, Dambacher B, Hardebusch B, Lippold R, van Leeuwen FXR, Moy G, Tritscher A (2023c) WHO- and UNEP-coordinated exposure studies 2000-2019: findings of selected organochlorine pesticides and industrial chemicals. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
Malisch R, Schächtele A, van Leeuwen FXR, Moy G, Tritscher A, Šebková K, Klánová J, Kalina J (2023d) Time trends in human milk derived from WHO- and UNEP-coordinated exposure studies, chapter 1: polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part IV)
Malisch R, Hardebusch B, Lippold R, van Leeuwen FXR, Moy G, Tritscher A, Šebková K, Klánová J, Kalina J (2023e) Time trends in human milk derived from WHO- and UNEP-coordinated exposure studies, chapter 2: DDT, beta-HCH and HCB. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part IV)
Malisch R, Fürst P, Šebková K, Klánová J, Sapunova D, Kalina J (2023f) Time trends in human milk derived from WHO- and UNEP-coordinated exposure studies, chapter 3: perfluorinated alkyl substances (PFAS). In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part IV)
Sadia M, Yeung LWY, Fiedler H (2020) Trace level analyses of selected perfluoroalkyl acids in food: method development and data generation. Environ Pollut 263:113721
Schächtele A, Hardebusch B, Krätschmer K, Tschiggfrei K, Zwickel T, Malisch R (2023a) Analysis and quality control of WHO- and UNEP-coordinated human milk studies 2000-2019: polybrominated diphenylethers, hexabromocyclododecanes, chlorinated paraffins and polychlorinated naphthalenes. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part II)
Schächtele A, Malisch R, Hardebusch B, van Leeuwen R, Moy G, Tritscher A, van Duursen M, van den Berg M, Šebková K, Klánová J, Kalina J (2023b) WHO- and UNEP-coordinated human milk studies 2000–2019: findings of polybrominated substances. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
Šebková K (2023) The Stockholm convention, global monitoring plan and its implementation in regional and global monitoring reports. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Sen PK (1968) Estimates of the regression coefficient based on Kendall’s tau. J Am Stat Assoc 63:1379–1389. https://doi.org/10.1080/01621459.1968.10480934
Theil H (1992) A rank-invariant method of linear and polynomial regression analysis. In: Raj B, Koerts J (eds) Henri Theil’s contributions to economics and econometrics. Advanced studies in theoretical and applied econometrics, vol 23. Springer, Dordrecht, pp 345–381. https://doi.org/10.1007/978-94-011-2546-8_20
Tschiggfrei K, Schächtele A, Fernandes AR, Falandysz J, van Duursen M, van den Berg M, Malisch R (2023) WHO- and UNEP-coordinated human milk studies 2000–2019: findings of polychlorinated naphthalenes. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
UNEP (2007). Guidance on the global monitoring plan for persistent organic pollutants, preliminary version, February 2007
UNEP (2015) Guidance on the global monitoring plan for persistent organic pollutants, January 2013. UNEP/POPS/COP.7/39 (Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Geneva, 4–15 May 2015)
UNEP (2019) Guidance on the global monitoring plan for persistent organic pollutants, updated draft January 2019. UNEP/POPS/COP.9/INF/36 (Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Geneva, 29 April–10 May 2019)
van den Berg M, Birnbaum L, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson E (2006) The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors (TEFs) for dioxins and dioxin-like compounds. Toxicol Sci 93(2):223–241
van den Berg M, Kypke K, Kotz A, Tritscher A, Lee SY, Magulova K, Fiedler H, Malisch R (2016) WHO/UNEP global surveys of PCDDs, PCDFs, PCBs and DDTs in human milk and benefit–risk evaluation of breastfeeding. Arch Toxicol 91:83. https://doi.org/10.1007/s00204-016-1802-z
van den Berg M, van Duursen MBM, Tritscher A, Malisch R, Peterson RE (2023) Risk-benefit analysis for the breastfed infant based on the WHO- and UNEP human milk surveys for dioxin-like compounds. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part IV)
WHO (1989) Levels of PCBs, PCDDs, and PCDFs in breast milk – results of WHO-coordinated interlaboratory quality control studies and analytical field studies. WHO Regional Office for Europe. Environ Health 34:1–92. isbn 87-7437-254-8
WHO (1996) Levels of PCBs, PCDDs and PCDFs in human milk – second round of WHO-coordinated exposure study. WHO European Centre for Environment and Health, Environmental Health in Europe No 3, 1–121
Acknowledgements
This publication was developed in the framework of the projects titled “Implementation of the POPs Monitoring Plan in the Asian Region” and “Continuing regional Support for the POPs Global Monitoring Plan under the Stockholm Convention in the Africa, Asia-Pacific and Latin-American and Caribbean Region”, funded by the Global Environment Facility and in close collaboration with and support of CVUA Freiburg.
The worldwide implementation of the Global Monitoring Plan for POPs, including that of the WHO/UNEP global human milk studies, is made possible thanks to the generous contributions to the Stockholm Convention Voluntary Trust Fund by the Governments of Japan, Norway and Sweden and through the European Union’s Global Public Goods and Challenges Programme (GPGC). Further, the substantial contributions made by the Global Environment Facility to support POPs monitoring activities in regions implemented by UNEP, in close collaboration with WHO, particularly for the global human milk studies, are greatly appreciated.
The authors express their gratitude to the National Coordinators of the WHO/UNEP-coordinated exposure surveys for their excellent work to collect the human milk samples and to prepare and send the pooled samples to the Reference Laboratories, which included great efforts to plan and implement the national studies with the assistance of the health, environment, laboratory, and administrative staff.
The authors thank Katarina Magulova and Ana Witt (Secretariat of the Basel, Rotterdam and Stockholm Conventions) and Haosong Jiao (UNEP Economy Division, Chemicals and Health Branch) for their support and contributions to these studies, and Heidelore Fiedler for the conception and implementation of the Global Monitoring Plan projects at her time at United Nations Environment Programme, Economy Division, Chemicals and Health Branch.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors alone are responsible for the views expressed in this publication, which do not necessarily represent the decisions, policy, or views of the World Health Organization and the United Nations Environment Programme.
Appendix
Appendix
1.1 Regional Differentiation
1.1.1 Toxic equivalents of PCDD/PCDF and Dioxin-like PCB (WHO2005-TEQ)
The range of concentrations of toxic equivalents (TEQ) of PCDD/PCDF and dioxin-like PCB (expressed as WHO2005-TEQ; long term “WHO-PCDD/PCDF-PCB-TEQ [2005]”, shortly “Total TEQ”) in 232 pooled samples from 82 countries collected between 2000 and 2019 varies between 1.29 and 49 pg WHO2005-TEQ/g, with a median of 7.24 pg/g. The highest median WHO2005-TEQ concentrations were found in countries of the Eastern European Group and the Western European and Others Group with 12.0 pg/g and 10.3 pg/g, respectively. The widest variation was in Africa (range 1.29 to 49 pg/g). With median concentrations between 4 and 5 pg/g and maximum levels between 10 and 12 pg/g, the Pacific region in the Asia-Pacific Group and countries from the Latin American and Caribbean Group were at the lower end of the distribution (Fig. 16).
Range of concentrations of total TEQ (WHO-PCDD/PCDF-PCB-TEQ [2005]) among UN regions (with split of the Asia-Pacific Group into the subgroups Asia and Pacific Islands; pg/g lipid, N = 232 pooled samples from 82 countries) [Box plot; minimum and maximum: as circles; 5th and 95th percentile: as whiskers; lower (25–50%) and upper (50–75%) quartiles, separated by the line for the median: as box; mean: as asterisk]
Time trends and changes in the fraction of regional groups over these periods have to be taken into consideration for this overall picture for a period of 20 years. Whereas in the 2000–2003 period, the majority of participants came from countries of the Eastern European Group and Western European and Others Group, in the 2016–2019 round, the majority came from the African Group, followed by the Group of Latin American and Caribbean Countries and then the Asia-Pacific Group. Figure 17 illustrates the regional differentiation (indicating the five UN Regional Groups by different colours) with temporal tendencies over the five rounds. In the 2000–2003 round, 21 of the 26 countries participating were from the Eastern European or Western European and Others Groups. In comparison to other regions, countries from these groups had quite high total TEQ concentrations (up to 27 pg WHO2005-TEQ/g lipid in the pooled samples). In this period, Egypt had total TEQ concentrations comparable to European countries decreasing considerably until 2019. In the 2016–2019 round, four countries from the African Group and the Marshall Islands are found at the upper end of the frequency distribution curve with concentrations between 6.7 and 11.6 pg/g. On the other side of the frequency distribution curve was Ethiopia with the lowest levels of total TEQ of all countries in the 2000–2019 studies (1.54 pg WHO2005-TEQ/g in 2012 and 1.29 pg/g in 2019). For a detailed discussion, see Part III of this compendium (Malisch et al., 2023b).
Results of the 2000–2019 surveys for total TEQ in 232 pooled human milk samples from 82 countries of the five UN Regional Groups (with split of the Asia-Pacific Group into the subgroups Asia and Pacific Islands) indicated by different colours and classified into five periods of sample submission (pg WHO-PCDD/PCDF-PCB-TEQ [2005])/g lipid). Whiskers indicate the range of concentrations found in samples around the median concentration, if two or more samples were submitted by a country in a certain period
1.1.2 Beta-HCH
As result of the metabolization of hexachlorocyclohexanes (HCH) in humans, the concentrations of alpha-HCH and gamma-HCH were in most human milk samples below the limit of quantification (<0.5 μg/kg lipid), whereas mainly beta-HCH accumulates in humans. Great differences of beta-HCH concentrations between UN Regional Groups and between countries in the same region were found, covering a range of three orders of magnitude with a minimum of <0.5 μg beta-HCH/kg lipid found in few countries and a maximum of 1020 μg beta-HCH/kg lipid found in 2002 in the Asia-Pacific Group. On average (as median and mean), the highest beta-HCH concentrations were found in the Eastern European Group (median 25.1 μg/kg lipid, mean 95.2 μg/kg lipid) with a range up to 476 μg/kg lipid (Fig. 18).
Range of concentrations of beta-HCH in human milk among UN regions (μg/kg lipid; N = 134 country results [with median, if results for two or more pooled samples are available in a certain period] from 82 countries, comprising the five periods from 2000 to 2019) [Box plot; minimum and maximum: as circles; 5th and 95th percentile: as whiskers; lower (25–50%) and upper (50–75%) quartiles, separated by the line for the median: as box; mean: as asterisk]
The findings of high beta-HCH concentrations in different periods were influenced by the variation of countries participating in a certain UN region in a certain period. The highest concentration was found in 2002 in in Hong Kong SAR of China (1020 μg beta-HCH/kg lipid as the median of 10 pooled samples). Hong Kong participated twice, with a considerable decrease to the 2009 level of 290 μg beta-HCH/kg lipid (median of 4 samples from different population groups). A decrease was also found in countries of the Eastern European Group with repeated participation, e.g. the maximum level of 476 μg beta-HCH/kg lipid found in 2009 in Moldova decreased considerably to 150 μg beta-HCH/kg lipid in 2015 (Fig. 19). For a detailed discussion, see Part III of this compendium (Malisch et al., 2023c).
1.1.3 HCB
The maximum levels found for hexachlorobenzene (HCB) and therefore the ranges were much lower than for DDT and beta-HCH, with a minimum of about 1–2 μg/kg lipid found in some countries and a maximum of 154 μg/kg lipid found in 2009 in an Eastern European country. The median of 134 country results was 5.1 μg/kg lipid. All countries from Africa were at all times in this low background range below 5 μg/kg lipid, and many countries from the Pacific Islands and Latin America and the Caribbean were as well (Fig. 20).
Range of concentrations of HCB in human milk among UN regions (μg/kg lipid; N = 134 country results [with median, if results for two or more pooled samples are available in a certain period] from 82 countries, comprising the five periods from 2000 to 2019) [Box plot; minimum and maximum: as circles; 5th and 95th percentile: as whiskers; lower (25–50%) and upper (50–75%) quartiles, separated by the line for the median: as box; mean: as asterisk]
In the Eastern European Group, a wide range of concentrations was observed between the background contamination as found in Croatia, 2014 (7.6 μg/kg lipid) and Bulgaria, 2014 (7.7 μg/kg lipid), in comparison to 154 μg/kg found in Moldova, 2009. In Moldova, these concentrations decreased considerably to 46.7 μg HCB/kg lipid in 2015. The Czech Republic participated four times between 2000 and 2019; here a continuous downtrend is found from 76 μg/kg lipid in 2001 to 12.5 μg/kg lipid in 2019. Also in Western European countries, a gradual downward trend was observed from participation of countries in the 2000–2003 period until the 2016–2019 period (Fig. 21). For a detailed discussion, see Part III of this compendium (Malisch et al., 2023c).
1.1.4 Dieldrin
In the analyte group “Organochlorine pesticides and industrial contaminants”, maximum concentrations above 20 μg/kg lipid were found for DDT, beta-HCH, HCB, and dieldrin. However, the dieldrin concentrations were considerably lower than those for the other three POPs. A maximum of 37.8 μg/kg lipid was found in a sample of the Asia-Pacific Region (sample from Tajikistan, 2009). The median concentration was between 1 and 2 μg/kg lipid in all UN Regional Groups except the Western European and Others Group (median 3.57 μg/kg lipid) (Fig. 22). Based on 134 country results, nearly 90% of the samples had dieldrin levels in a low background range below 5 μg/kg lipid (Fig. 23). For a detailed discussion, see Part III of this compendium (Malisch et al., 2023c).
Range of concentrations of dieldrin in human milk among UN regions (μg/kg lipid; N = 134 country results [with median, if results for two or more pooled samples are available in a certain period] from 82 countries, comprising the five periods from 2000 to 2019) [Box plot; minimum and maximum: as circles; 5th and 95th percentile: as whiskers; lower (25–50%) and upper (50–75%) quartiles, separated by the line for the median: as box; mean: as asterisk]
1.1.5 PBDE
Large differences in levels of 124 country results (using the median, if results for two or more pooled samples are available in a certain period) from 80 countries collected between 2000 and 2019 were found for the sum of six polybrominated diphenyl ethers (PBDE) (BDE-47, BDE-99, BDE-100, BDE-153, BDE-154, BDE-183) (Figs. 24 and 25). 80% of these samples were in a range below 5 ng ∑ PBDE6/g lipid, including all samples from Africa and the Eastern European Group. The highest concentration of 223 ng ∑ PBDE6/g lipid was found in the 2000–2003 period in the Western European and Others Group (comprising Australia, Israel, New Zealand, and USA as “Others”) in the USA, followed by 107 ng ∑ PBDE6/g lipid in the Asia-Pacific Group in the 2016–2019 period (Marshall Islands). A closer look into highest country result found in the USA in 2003 reveals that the 223 ng ∑ PBDE6/g lipid was calculated as median of two pooled samples from North Carolina [92.6 ng/g lipid] and California [352 ng/g lipid]. This was about three orders of magnitude above the lowest concentration found in 2009 in Syria (0.28 ng ∑ PBDE6/g lipid). For a detailed discussion, see Part III of this compendium (Schächtele et al., 2023b).
Range of concentrations of ∑ PBDE6 in human milk among UN regions (ng/g lipid; N = 124 country results [with median, if results for two or more pooled samples are available in a certain period] from 80 countries, comprising the five periods from 2000 to 2019) [Box plot; minimum and maximum: as circles; 5th and 95th percentile: as whiskers; lower (25–50%) and upper (50–75%) quartiles, separated by the line for the median: as box; mean: as asterisk]
1.1.6 PFOS
The range of concentrations of perfluorooctane sulfonic acid (PFOS) in 86 pooled samples from 59 countries collected between 2008 and 2019 varies between <6.4 ng/l and 218 ng/L total PFOS (∑PFOS), calculated as sum of linear PFOS (L-PFOS) and branched isomers (br-PFOS), with median concentrations between 14 ng/L in the African region and 34 ng/L in the Eastern European region (Fig. 26). Most samples originated from the African region (N = 27), the fewest from the Western European and Others Group (N = 8; with no sample from the 2008–2011 period). The maximum concentration of 218 ng ∑PFOS/L was found in a sample from Kiribati collected in the 2016–2019 period. The range of concentrations found in 27 samples of the 2008–2011 period, in 15 samples of the 2012–2015 period, and 44 samples of the 2016–2019 period is illustrated in Fig. 27 with identification of the UN Regional Groups in different colours. For a detailed discussion including the presentation of results for other perfluorinated alkane substances (PFAS), in particular perfluorooctanoic acid (PFOA; listed in 2019) and perfluorohexane sulfonic acid (PFHxS; proposed for listing as of 2019), see Fiedler and Sadia (2021) and Fiedler et al. (2022).
Range of concentrations of ∑PFOS in human milk among UN regions (ng/L; N = 86 pooled samples from 59 countries, comprising the period from 2008 to 2019) [Box plot; minimum and maximum: as circles; 5th and 95th percentile: as whiskers; lower (25–50%) and upper (50–75%) quartiles, separated by the line for the median: as box; mean: as asterisk]
1.1.7 Chlorinated Paraffins
Chlorinated paraffins (CP) are very complex mixtures of several million theoretically possible compounds. Contrary to medium-chain CP (MCCP, C14-C17), short-chain chlorinated paraffins (SCCP, C10-C13) have been listed in 2017 in Annex A (Elimination) of the Stockholm Convention on Persistent Organic Pollutants. The concentrations of CP were determined in 84 pooled human milk samples collected between 2009 and 2019 in 57 countries (Krätschmer et al., 2023). Until 2015, only total CP content was determined. Thus, for 27 samples of the 2009 to 2011 period and some samples of the 2012 to 2013 period, only the sum of CP is available. Later, a new quantification method was developed able to differentiate between SCCP and MCCP and to give information on homologue group patterns. Some samples of the period 2012 to 2013 and all samples arriving after 2014 were analysed with this new quantification method, altogether 57 samples. Therefore, the sum of all detected CP is used as parameter to present all analysed samples here. CP were present in all 84 samples, ranging 8.7–700 ng/g lipid. On average, African and Asia/Pacific pooled samples had the highest combined SCCP and MCCP content of the five UN regional groups: With a median of 160 ng/g lipid each, they surpassed median levels of the other three groups by a factor of 2–3 (Fig. 28).
Range of concentrations of sum of CP in human milk among UN regions (ng/g lipid; N = 84 samples from 57 countries, comprising the 2009–2019 period) [Box plot; minimum and maximum: as circles; 5th and 95th percentile: as whiskers; lower (25–50%) and upper (50–75%) quartiles, separated by the line for the median: as box; mean: as asterisk]
As up to 2022 only SCCP were listed, the relation between SCCP and MCCP is important for interpretation of the sum parameter. In most pooled country samples of all UN regional groups, MCCP concentrations at least equalled SCCP concentrations, contributing 24–85% to the total CP levels reported here (Fig. 29). In 36 of the 53 countries where distinct data is available, MCCP even surpassed SCCP. Using the SCCP/MCCP ratio as an indication of this relation, it is interesting to see that African and Eastern European country samples had a tendency towards a higher dominance of MCCP, whereas in the other UN regional groups, median SCCP levels were closer to equal distribution compared to MCCP (African group 58% MCCP, Asian-Pacific Group 55%, Group of Latin America and Caribbean Countries 53%, Eastern European Group 67%, Western European and Others Group 54%).
Range of SCCP/MCCP ratios, sorted by UN Regional Groups. The dotted horizontal line indicates ratio = 1, i.e. equal presence of SCCP and MCCP. AFR African Group, ASPAC Asian-Pacific Group, EEG Eastern European Group, GRULAC Group of Latin America and Caribbean Countries, WEOG Western European and Others Group
Figure 30 illustrates the differences in levels of 84 pooled samples from 57 countries collected between 2009 and 2019 for the sum of CP. Most notably, the samples from Mongolia (2018, 700 ng CP/g lipid) and Niger (2015, 680 ng CP/g lipid) were higher than other samples from their regional groups. For a detailed discussion, see Part III of this compendium (Krätschmer et al., 2023).
1.2 Assessments of Time Trends
1.2.1 Beta-HCH
Conclusions on time trends for beta-HCH in the different UN regions cannot easily be drawn. As shown already for DDT in subsection 4.1, also the time trends of the median beta-HCH concentrations in the UN regions are not consistent. For the median of the samples in all UN regions, a decrease of 91% was found from the 2000–2003 period (25.3 μg/kg lipid) to the 2016–2019 period (2.4 μg/kg lipid). A considerable decrease is also seen in the individual UN regions if the median of the beta-HCH concentrations of the 2000–2003 period is compared to the 2016–2019 period. However, in the three rounds in-between, considerable variations were observed, notably in Asian and in the Eastern European countries starting with an initial downward trend from 2000–2003 to 2004–2007. This, however, is followed by an increase in the period 2008–2011 before the concentrations decrease again to 2016–2019. Other than for DDT, the temporal trends using the mean concentrations look quite comparable to the trends derived from the median with no significant maxima in other periods (Malisch et al., 2023c).
The evaluation only of countries with repeated participation shows decreasing tendencies in nearly all countries. Figure 31 illustrates the temporal tendencies in 44 countries of the five UN regional groups that participated two or more times. In nearly all countries, decreasing tendencies are observed, with a median of 65% decrease over the years between first and last participation. As for DDT, a differentiation of levels above or in the range of background contamination seems to be advised. If high levels are found, sources might be detected which could be eliminated, whereas at low background levels, other factors cannot be influenced. Therefore, in some countries a variation at low background levels (<5 μg/kg) over time is observed when concentrations tend to level out.
An overall decrease within a 10-year period between 50% and 98% was achieved for beta-HCH levels in all the UN regions and at a global level. The overall decrease per 10 years calculated by the Theil–Sen method for exponential trends and the additionally applied median method to derive time trends were quite comparable in nearly all UN regions and at the global level (Table 8). On a global level and in all UN regions except Latin America and the Caribbean, all trends were significant (p-value <0.001 in three regions and globally, 0.037 in Eastern Europe). Globally, a decrease per 10 years by 84% was found for beta-HCH (calculated by the Theil–Sen method). For more details, see Malisch et al. (2023e).
1.2.2 HCB
In general, concentrations of HCB show a downward trend globally: The median of all samples decreases from the 2000–2003 period (16 countries, 16.4 μg/kg lipid) to the 2016–2019 period (43 countries, 3.3 μg/kg lipid) by about 80%. Of particular interest are time trends in regions with higher initial concentrations. In the Eastern European Group, the median HCB concentration falls continuously from the 2000–2003 period (58.3 μg/kg lipid) to the 2012–2015 period (13.3 μg/kg lipid) and then levels out around 13 μg/kg lipid in the 2016–2019 period. In countries of the Western European and Others Group, a downward trend is also observed from the 2000–2003 period (19.5 μg/kg lipid) to the 2008–2011 period (9.0 μg/kg lipid) and then leveling out at about 10 μg/kg lipid. In other groups, the background contamination levels out at concentrations up to 5 μg HCB/kg lipid (limit of quantification: 0.5 μg/kg lipid). The picture using the mean concentrations instead of median looks as if there was a maximum in the Eastern European Group in the 2008–2011 period. However, this is caused by a high HCB concentration found in the pooled sample of one country submitted at that time (sample from Moldova, 2009, 154 μg/kg lipid) (Malisch et al., 2023c).
The evaluation only of countries with repeated participation shows decreasing tendencies in nearly all countries. Figure 32 illustrates the temporal tendencies in 44 countries of the five UN regional groups that participated two or more times. In most countries, decreasing tendencies are observed, with a median of 27% decrease over the years between first and last participation. However, as explained above, also for HCB a variation at low background levels (<5 μg/kg) over time is observed when concentrations tend to level out.
In the African Group with all pooled samples collected over time being in the background range of or below 5 μg HCB/kg lipid, the overall decrease within 10 years was 11% calculated by the Theil–Sen method. Overall decrease rates between 30% and 67% were calculated by the Theil–Sen method for the other UN Regional Groups, and 57% on a global level (Table 9). For more details, see Malisch et al. (2023e).
1.2.3 PBDE
As shown above (Sect. 7.1.5), a considerable variation of the PBDE concentrations in human milk from 80 countries in the five periods between 2000 and 2019 was observed, depending on the inclusion of individual countries in a certain round. Of all samples, 80% were in a range below 5 ng ∑ PBDE6/g lipid; the highest concentration of 223 ng ∑ PBDE6/g lipid was found in the 2000–2003 period in the Western European and Others Group, followed by 107 ng ∑ PBDE6/g lipid in the Asia-Pacific Group in the 2016–2019 period. A general estimation of time trends is quite difficult. In particular in the Asia-Pacific Group, the Group of Latin American and Caribbean Countries and in the Western European and Others Group, median or maximal concentrations don’t appear to have a continuous downward time trend. The median of all country results shows a decrease of about 50% from the 2.62 ng ∑ PBDE6/g lipid in 2000–2003 period to 1.38 ng ∑ PBDE6/g lipid in the 2016–2019 period. These are seen as current background levels (Schächtele et al., 2023b).
Figure 33 illustrates the temporal tendencies in 36 countries of the five UN regional groups that participated two or more times. In most countries, a fluctuation at background levels (<5 ng/g lipid) over time is observed when concentrations tend to level out. However, decreasing trends were seen in all eight countries with ∑ PBDE6 concentrations above 5 ng/g lipid at times of their first participation (Fiji, Barbados, Jamaica, Mexico, Antigua-Barbuda, Haiti, Ireland, and Australia) with a median of about 50% decrease over the years between first and last participation (range 25–80%).
At the global level calculated from these 36 countries, the statistically significant overall decrease rate per 10 years was 32% calculated by the Theil–Sen method and 48% by the median method, respectively (Schächtele et al., 2023b).
1.2.4 PFOS
As PFAS analysis was initiated with samples submitted in 2008, time trends for PFOS can be derived beginning with the 2008–2011 period. As starting point, descriptive parameter summarizing the results of 86 national pools from 59 countries was used as basis for the first general estimation of temporal trends with differentiation into three equal four-year periods between 2008 and 2019. The median of the ∑ PFOS concentrations increased from 24.6 ng/L in the 2008–2011 period (N = 27) to 36.0 ng/L in the 2012–2015 period (N = 15) and then decreased to 13.6 ng/L in the 2016–2019 period (N = 44). The highest maximum level (218 ng/L) was found in the 2016–2019 period.
However, the regional distribution of samples and the regional distribution within each of the three periods varied considerably, with most samples originating from the African region. There was no sample from Western European and Others Group in the first period (2008–2011) and no country from this region with repeated participation in these three periods between 2008 and 2019. The second period (2012–2015) had the lowest number of samples in all UN Regions except the Eastern European Group. Note that two countries submitted two samples in different years of the same period: Chile (2008, 2011) and Niue (2017, 2019). The short period between the two samplings in these two countries should not be used for calculation of country-specific temporal trends.
The summarizing descriptive parameters seem to indicate an increase of the PFOS concentrations after listing in 2009 until the 2012–2015 period. Therefore, on basis of countries with repeated participation it was checked whether these fluctuations of the median were likely due to the result of participation of different countries in different rounds of the WHO/UNEP-coordinated exposure studies, as described above for other POPs.
Figure 34 illustrates the temporal tendencies in 24 countries of the four UN regional groups that participated two or more times. In 19 of the 22 countries with repeated participation in different periods, downward tendencies were observed over the years between first and last participation (median decrease: 46%; range 8–78%). In two countries, the PFOS concentrations remained quite constant; in one country, increasing tendencies were seen (Côte d’Ivoire, from 2010 to 2015).
Statistically significant time trends for the UN Regional Groups and worldwide were derived by pooling of two sets of data: (1) using the data from all countries; (2) using the data only from countries with repeated participation (not possible for Asia-Pacific Regional Group and the Western European and Others Group). Overall decrease rates over 10 years between 50% and 80% were achieved in the regions except the Asia-Pacific Group (with an increase of 25% based on the data of all countries) and worldwide computed using all countries or only countries with repeated participation (Table 10).
For details and the temporal trends for PFOA and PFHxS, see Malisch et al. (2023f).
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Malisch, R. et al. (2023). Overall Conclusions and Key Messages of the WHO/UNEP-Coordinated Human Milk Studies on Persistent Organic Pollutants. In: Malisch, R., Fürst, P., Šebková, K. (eds) Persistent Organic Pollutants in Human Milk. Springer, Cham. https://doi.org/10.1007/978-3-031-34087-1_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-34087-1_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34085-7
Online ISBN: 978-3-031-34087-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)