Abstract
Temporal trends of polychlorinated biphenyls (PCB), polychlorinated dibenzo-p-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF) in human milk were assessed by consideration only of countries with repeated participation in WHO/UNEP-coordinated exposure studies performed between 1987 and 2019. In contrast to a general estimation of time trends from all participating countries, this is a more precise approach because levels among countries are often highly variable. Studies on time trends for contaminants in human milk are important components of the effectiveness evaluation of the Stockholm Convention on Persistent Organic Pollutants (POPs). There is no stipulation of a quantitative goal for the rate of reduction/decrease in POPs levels, however, as a quantitative objective for studies, these should have the ability to detect a 50% decrease in the levels of POPs within a 10-year period.
For non-dioxin-like PCB (calculated as the sum of six indicator PCB), a decrease of about 50% to 60% over 10 years was achieved in most of the five UN Regional Groups. Considerable decreases in concentrations with reductions of up to 95% over three decades were observed in European countries with high concentrations at the end of the 1980s, compared to slower decreases in less polluted ones. For the toxic equivalents (TEQ) of PCDD/PCDF and the total TEQ of PCDD/PCDF and dioxin-like PCB, a decrease of about 50% over 10 years was found mainly in Western European and some other countries with initially relatively high concentrations. TEQ concentrations of PCDD/PCDF decreased by up to 90% over three decades. Lower decreases observed in many countries have to be seen in the context of the quite low levels in these countries in comparison with other countries.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Time trends
- Human milk biomonitoring
- Stockholm Convention on Persistent Organic Pollutants
- Non-dioxin-like PCB
- Dioxin-like PCB
- PCDD/PCDF
- Dioxins
- Global WHO/UNEP studies
- UN Regional Groups
1 Introduction
In this compendium, global human milk surveys for chemicals of health and environmental concerns are reviewed. In five parts, specific papers address various aspects: Part I gives a review of human milk surveys on persistent organic pollutants (POPs) from a historical perspective (Fürst 2023), an overview of the seven exposure studies coordinated by the World Health Organization (WHO) and the United Nations Environment Programme (UNEP) performed between 1987 and 2019 (Malisch et al. 2023a) and a review on the Stockholm Convention and its implementation by regional and global monitoring reports (Šebková 2023). Part II presents analytical methods and their validation for determination of chlorinated and brominated POPs between 2000 and 2019, including for polychlorinated biphenyls (PCB), polychlorinated dibenzo-p-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF) (Malisch and Schächtele 2023). In Part III the findings for chlorinated and brominated POPs in samples collected between 2000 and 2019 are presented in various formats, among them for PCB and PCDD/PCDF (Malisch et al. 2023b). Part IV presents two kinds of assessments: of risk–benefit analysis for the breast-fed infant from dioxin-like compounds (van den Berg et al. 2023) and of time trends derived from countries with repeated participation in the WHO- and UNEP-coordinated studies in three chapters for (1) PCB and PCDD/PCDF (in this chapter); (2) for DDT, beta-HCH and HCB and (3) perfluorinated alkyl substances. The assessment of temporal trends for polybrominated diphenylethers (PBDE) is included in the discussion of findings for polybrominated substances in Part III (Schächtele et al. 2023). Part V presents conclusions and key messages of these surveys.
A review of scientific publications between 1995 and 2011 on the spatial and temporal trends of Stockholm Convention POPs in breast milk compiles data on PCB and PCDD/PCDF levels providing a global overview (Fång et al. 2015). Furthermore, the regional and global monitoring reports for the Global Monitoring Plan assess datasets in the core media—ambient air, human tissues (human breast milk or blood), and water for hydrophilic POPs, but also other media such as soil, biota, plants are used to support interpretation of observed levels and their trends (Šebková 2023). These reports are available at the homepage of the Stockholm Convention (>Implementation>Global Monitoring Plan>Monitoring Reports).
One of the objectives of the WHO/UNEP-coordinated exposure studies was to generate comparable and consistent monitoring data on the presence of these contaminants in order to identify trends in levels. Adopted by the parties to the Stockholm Convention on POPs, The Guidance Document on the Global Monitoring Plan (GMP) considers such data on the presence of POPs in the environment and in human tissues necessary for the evaluation of the effectiveness of the Convention to eliminate or reduce emissions of specific POPs as required under Article 16 of the Convention. To provide reliable monitoring information for the Parties to the Stockholm Convention, as a quantitative objective for temporal studies the GMP guidance document proposed the ability to detect a 50% decrease in the levels of POPs within a 10-year period. This corresponds to an annual decrease of 7%. However, there is no stipulation of a quantitative goal for the rate of reduction/decrease in POPs levels. The Convention’s objectives are either to eliminate or to reduce production, use and releases, depending on the annex where a chemical is listed, but the rate of decline is nowhere specified or required (UNEP 2015, 2019).
The presentation and discussion of the 2000–2019 results in Part III include a first general estimation of time trends for all 82 countries participating in these five rounds over these 20 years, comparing the temporal tendencies of the average concentrations of PCB and PCDD/PCDF in the studies over time (Malisch et al. 2023b). However, this chapter presents a more precise approach for the assessment of temporal trends by consideration of results only from those 50 of the 82 countries with repeated participation in the studies. Because levels correlate within countries more closely than among countries, this allows more certainty in drawing conclusions on time trends which are not potentially influenced by individual results from countries submitted just for a single round and seems optimal for the evaluation of the effectiveness for the purpose of Article 16 of the Convention. However, it should be noted that typically only very few time points from most individual countries are available which prevents from deriving statistically significant temporal trends in these cases. Yet, the existing data can indicate decreasing or increasing tendencies in POP concentrations. Furthermore, pooling of data in regions allows to derive statistically significant time trends in the UN Regional Groups and globally. To minimize possible sources of variation for time trend analysis of POPs, including PCB, PCDD and PCDF, the concept of the WHO/UNEP-coordinated exposure studies has two basic elements: (1) preparation of pooled samples from a number of individual samples considered to be representative for a country or region/subgroup; (2) analysis by a reference laboratory (see subsection 2.1).
With regard to length of time-series, the GMP guidance document considers it naïve to expect monitoring of POPs to reveal temporal trends with any confidence within a sampling period of five years unless the changes are very large. More likely would be a period of at least 10–15 years to detect significant changes of moderate size (5%/year) (UNEP 2019). This assessment comprises much longer periods: The first WHO-coordinated exposure study with the aim to determine concentrations of non-dioxin-like PCB (expressed as the sum of 6 indicator PCB [ΣPCB6]), PCDD and PCDF took place in 1987–1988 (WHO 1989). The second study (1992–1993) not only included the 6 non-dioxin-like indicator PCB, but also dioxin-like PCB (WHO 1996). With inclusion of these two studies, data from a total of 57 countries with repeated participation between 1987 and 2019 are available for assessment of temporal trends for PCB, PCDD and PCDF. In this article, this comprehensive database is evaluated to derive time trends for non-dioxin-like PCB and the toxic equivalents (TEQ) of PCDD and PCDF for a period of 32 years starting with the period 1987–1988 and for the total TEQ comprising PCDD, PCDF and dioxin-like PCB for a period of 27 years starting with 1992–1993.
2 General Aspects
2.1 Minimization of Sources of Variation
Numerous factors might affect the measured concentration of POPs in human milk samples. Sampling design is considered to be the most important factor. For most POPs, the precision of chemical analysis is generally believed to constitute only a minor part of the total variance in monitoring time-series of environmental data where sample variation is expected to be large, much larger compared to laboratory precision. This is especially true when the same accredited laboratory is used throughout the whole series as in this case. However, if, from year to year, different laboratories carry out the analysis, it could seriously decrease or disable the possibility to evaluate time-series of, for example, POPs. The same is true if the same laboratory changes its methodology (UNEP 2007, 2015, 2019).
To minimize possible sources of variation for time trend analysis of POPs, including PCB, PCDD and PCDF covered by this article, the concept of the WHO/UNEP-coordinated exposure studies has the following basic elements:
-
1.
For minimization of possible sources of variation from the sampling design:
-
(a)
collection of a large number of individual samples from mothers based on the standardized WHO/UNEP protocol;
-
(b)
from equal amounts of the individual samples, preparation of pooled samples that are considered to represent the average levels of POPs in human milk for a country or subgroup/region of that country at the time of sampling (for more information on the sampling design, see subsection 2.2);
-
(a)
-
2.
For minimization of the variation from chemical analysis: determination of chlorinated and brominated POPs in the pooled samples by the WHO/UNEP Reference Laboratory for the 2000–2019 studies applying long-term analytical quality control (see subsection 2.3).
2.2 Samples and UN Regional Groups
An overview of the scope, protocols for collection of samples and participation of countries is given in Part I (Malisch et al. 2023a). In brief, in all rounds, the design was based on collection of a number of individual samples and preparation of pooled samples following a standardized protocol that was supervised by national coordinators. Equal aliquots of individual samples were combined to give composite samples, which are considered representative of the average levels of the analytes of interest in human milk for a certain country or subpopulation of a country at the time of sampling.
During the five studies conducted from 2000 to 2019, a total of 232 pooled samples were submitted for analysis by 82 countries. 50 countries participated in more than one of these studies. The detailed data for all 232 pooled samples is contained at the POPs Global Monitoring Plan (GMP) Data Warehouse and can be publicly viewed and retrieved (GMP DWH 2020).
Furthermore, results of countries that participated in the first (1987–1988) and second rounds of WHO-coordinated exposure studies on concentrations of PCB and PCDD/PCDF in human milk (WHO 1989; WHO 1996) were included resulting in a total of 57 countries with repeated participation for these compounds.
In accordance with the implementation of the GMP, parties report through one of the five United Nations Regional Groups. Therefore, countries are classified according to one of these five UN Regional Groups (geopolitical groups), namely the African Group, the Asia-Pacific Group, the Group of Latin American and Caribbean Countries (GRULAC), the Eastern European Group and the Western European and Others Group (WEOG). Note that Australia, New Zealand and the USA (being informally a member) are included as “Others” in the WEOG category (for participating countries and regional distribution, see Malisch et al. 2023a).
2.3 Sum Parameters and Long-term Quality Control
The following sum parameters are used: (1) sum of six Indicator PCB (ΣPCB6) for non-dioxin-like PCB, (2) sum of toxic equivalents (TEQ) of PCDD/PCDF (WHO-PCDD/PCDF-TEQ) and (3) total sum of toxic equivalents (“Total TEQ”) of mixtures of PCDD/PCDF and dioxin-like PCB (WHO2005-TEQ). For calculation, see Malisch et al. 2023b.
All concentrations are reported on a lipid basis.
As an accredited laboratory since 1998, a comprehensive quality control program has been applied by the reference laboratory to assure the long-time reliability of results of human milk samples received for WHO/UNEP-coordinated exposure studies between 2000 and 2019. This included procedural blanks, the use of fortified vegetable oil and numerous quality control samples as an in-house reference material, duplicate analyses, and successful participation in 32 proficiency tests (PTs) covering 81 samples of food of animal origin or human milk. Trueness was estimated from the PT samples in the relevant range for human milk above 1 pg WHO-TEQ/g lipid: The deviation was less than 10% from the assigned values for WHO-PCDD/PCDF-PCB-TEQ and WHO-PCDD/PCDF-TEQ and less than about 15% for WHO-PCB-TEQ for about 90% of the results. For the sum of six indicator PCBs (relevant occurrence range, 1–1000 ng/g lipid), approximately 90% of the results differed by less than 15% from the assigned values. A long-term precision of <15% (coefficient of variation of within-laboratory reproducibility) was achieved, based on quality control samples analysed during 2000 and 2019. The analytical methodology used, fulfilled the requirements of the general criteria for the operation of testing laboratories as laid down in EN ISO/IEC 17025:2018, the analytical criteria for PCDD/PCDF and PCB in feed and food specified in EU legislation and the criterion for monitoring information for Parties to the Stockholm Convention (Malisch and Schächtele 2023).
2.4 Methods of Statistical Data Treatment
Each pooled sample (considered to be representative for a certain country or subpopulation of a country at the time of sampling) was identified by country, UNEP region, year, and analyte and its concentration reported on lipid basis. In the first three studies, countries were encouraged to submit at least two pooled samples, whereas in the following rounds, in most cases, only one pooled sample was submitted by a country considered to represent the country in that period. If a country had sent two or more samples in a certain round, the median of these samples in this period has been used for the country in some summarizing figures and tables introducing into a section for a certain analyte. These are identified as “country results” although data result from aggregation of multiple sample results. However, for the time trend analysis, data were not aggregated, and values of all individual samples were used for time trend analysis.
Based on visually assessed statistical distributions of compound-specific data, the decadic log-transformation was applied before the trend analysis to accomplish two goals:
-
to bring the data distributions closer to the normal distribution, which yields less biased trend estimates even in case of non-parametric estimators,
-
to enable estimation of exponential trends using common methods for the linear trends (a linear trend becomes exponential after the reverse log-transformation).
Rather than (unrealistic) linear trends, exponential trends as commonly observed in cases after stop of production and application of a chemical were expected considering the first order kinetics of the decrease of the compounds concentration in the population (Sharma et al. 2021). This approach is in line with various elimination studies deriving half-lives for PCB and PCDD/PCDF (EU Scientific Committee on Food 2000; Canady et al. 2002; EFSA 2018).
The GMP guidance document recommends to apply simple linear regression or the Theil-Sen estimator for power analysis of statistical trends (UNEP 2019). Therefore, the non-parametric linear Theil-Sen trend estimator (Sen 1968; Theil 1992) was used on log-transformed data for derivation of the trends c = A ∙ 10−s ∙ t, where c denotes the compound concentration, t denotes time, A denotes a concentration coefficient and s denotes the speed of the decrease. R package ‘Median-based Linear Models (mblm)’ (Komsta 2013) was used for this regression.
Based on the trend characteristics, decreases (decrease rate constants) per 1 year, 4 years (average lengths of the WHO/UNEP-coordinated studies), 5 years and 10 years were computed: (1) in case of the exponential trends in a form of a percentage decrease per year, (2) in case of the trends with baselines in a form of percentage decrease of the concentration above the baseline. (A 10-year period is used by the GMP guidance document for stipulation of the quantitative objective for the ability of temporal studies to detect a 50% decrease in the levels of POPs [UNEP 2015; UNEP 2019]). Statistical significance of differences between the trends with and without the baseline was estimated to show whether it is advantageous to consider the baselines or not. Since we did not find any significant difference between the trend computed with and without the baseline and all the baselines found in the data were lower than 24 pg/g lipid (as maximum for ΣPCB6) with most of them under 1 pg/g lipid (mainly TEQ-based results), we considered the background concentrations negligible or zero and all other analyses were done without these baselines.
Trends can be derived if the trend test (significance of the Theil-Sen estimator) is positive on 95% confidence level of significance (i.e. p-values <0.05). As the p-value calculated by the Theil-Sen estimator is never below 0.05 for less than 5 data points and for most countries only less than 5 data points were available, statistically significant trends could be derived only for regions (combining data from countries) and few countries, showing on 95% confidence level whether the trend is not caused by random variance in the data. Then, the R2 value was used as an indicative measure of a goodness of fit. In addition, for some countries, based on statistically significant decreases (decrease rate constants) and participation also in the decade after 2010, a prognosis of the estimated concentrations in 2025 was derived. However, for many countries, only two or three data points are available. In these cases, the observed changes of the concentrations are statistically not significant and indicate tendencies.
Considering the data were in general not normally distributed and the non-parametric method was used for the trend estimation, several trends with high p-value and low R2 value were discussed case by case for conclusions.
Simpson’s paradox is a statistical phenomenon in which a trend appears in certain groups of data but disappears or reverses when the groups are combined (Simpson 1951). In the assessment of temporal trends, this paradox can cause improper results induced by use of samples from different countries in different times. To prevent this, two additional analyses were made. First, the annual decrease was computed not only over the whole period but also separately for three decades (before 2000, 2001–2010 and after 2011) whenever data were available. Slopes of trends in these three decades were then compared using z-scores (Fisher test of differences between trends) (Fisher 1915). Second, a method of deriving the regional trend as a median of trends in countries within the region was used (“median method”): For each country, one trend was estimated using the Theil-Sen estimator. Median slope and median intercept over all these trends within a UN region were then considered as the resulting regional trend. The z-score test was used to compare this “median trend” with the regional trend derived before using the Theil-Sen trend estimator.
2.5 Background Concentrations Versus High Concentrations after Exposure
For the purpose of these chapters, a (low) background concentration is defined as that portion of the measured human milk levels that is found in the absence of specific sources (e.g. use or emission) and, therefore, is not attributable to a known exposure source within the study area. In contrast, after use or emission of chemicals initially high concentrations of these substances may be found in human milk. However, after a sufficiently long phase-out period the levels of many POPs may decrease considerably and approach background levels. According to the Global Monitoring Plan background levels of POPs are found at locations not influenced by local sources.
Reduction rates should be seen in context with the concentration range (levels above or in the range of background contamination): If high levels are found, sources might be detected, which could be reduced or eliminated and would then result in decreasing trends. However, at low background levels, other factors, e.g., contamination of feed and food by air via long-range transport or from subsequent bioaccumulation, cannot be influenced locally, with the possible consequence of levelling out of the found concentration or some fluctuation of the calculated decreases (decrease rate constants).
An additional assessment was done considering a case with background concentration (i.e. not purely an exponential trend). Non-linear regression using the PORT method (Fox 1984) implemented in R package stats was used to fit trends of a formula c = A ∙ 10−s ∙ t + c0 where c0 denotes the background concentration corresponding to the time invariant persistent exposition to the compounds.
PCB are industrial chemicals that were manufactured for decades before their production and use was banned by many countries around 1985. Therefore, background levels of PCB in human milk are the result of implementation of a reduction and control policy for this chemical.
PCDD/PCDF are unintentional by-products formed in (1) a number of chemical processes and, therefore, found as contaminants in certain chemicals; (2) many combustion processes and (3) certain geological processes and are, therefore, present in certain clays. The UNEP toolkit for identification and quantification of releases of dioxins, furans and other unintentional POPs lists a wide range of possible sources (UNEP 2013). Although PCDD/PCDF are regarded as unintentionally produced POPs, their release is mainly caused by intentional human activities (Wang et al. 2016). Therefore, background concentration of PCDD/PCDF is understood here as what is found after implementation of a reduction policy for sources from anthropogenic activities and a sufficiently long phase-out period.
However, the term “background” does not imply per se any level of safety. With respect to potential adverse effects, risk assessments need to consider many factors, including the toxicity of the chemical of interest and the measured concentration range. For human milk, potential adverse effects have to be balanced against its many known positive health aspects for (breast-fed) infants. The risk–benefit evaluation of breastfeeding for dioxin-like compounds is part of the assessment chapters in Part IV.
3 Non-Dioxin-Like Polychlorinated Biphenyls (Indicator PCB, ΣPCB6)
Time trends for six non-dioxin-like PCB (Indicator PCB, ΣPCB6) can be derived beginning with the 1987–1988 period. In total, 140 country results (including data from aggregation of multiple pooled samples that were averaged to represent a country in a certain period) for 57 countries with repeated participation in the period 1987–2019 and 119 aggregated data for 50 countries for the period 2000–2019 were used as basis for the first general estimation of time trends. In 7 periods between 1987 and 2019, the median of the ΣPCB6 concentrations decreased considerably from 211 ng/g to 14 ng/g (= 93% decrease). The Stockholm Convention was adopted in 2001 and entered into force in 2004 (UNEP 2001). Therefore, if only the period 2000–2019 is taken into consideration, the median concentrations of ΣPCB6 went down by 89% from initially 123 ng/g in the period 2000–2003 (comprising the year 2001) and by 71% from initially 66 ng/g in the period 2004–2007 (comprising the year 2004) (Table 1).
These summarizing descriptive parameters seem to indicate fluctuations between decreasing and increasing periods, e.g. an increase of the median concentrations from the first to the second and from the fifth to the sixth round. However, these changes are likely due to the result of participation of different countries in different rounds. Therefore, for more precise time trends a country-specific evaluation is necessary.
3.1 European and Non-European Countries
For PCB, as a first step, the differentiation between European and non-European countries is necessary. As the total results for 232 pooled human milk samples of 82 countries (regardless of the number of participations in the WHO/UNEP-coordinated exposure studies) for the period 2000–2019 showed, European countries used to have higher PCB concentrations than non-European countries with the greatest difference in the initial rounds (Malisch et al. 2023b): The highest concentrations of the sum of six Indicator PCB were found in the Eastern European Group (median about 120 ng ΣPCB6/g lipid, maximum about 1000 ng ΣPCB6/g lipid), followed by the group of Western European and Other States (median about 75 ng ΣPCB6/g lipid, maximum 467 ng ΣPCB6/g lipid). In all other groups, considerably lower ΣPCB6 levels were found (median approximately between 8 and 22 ng/g, maximum slightly lower than 100 ng/g lipid). The minimum in 24 European countries (14.6 ng ΣPCB6/g lipid) equated approximately the median (16.4 ng ΣPCB6/g lipid) in 58 non-European countries (with a minimum of 0.9 ng ΣPCB6/g lipid). Thus, within the wide range of concentrations varying approximately between 1 ng/g lipid and 1000 ng/g lipid, the lower end between 1 ng/g lipid and 10 ng/g lipid might be seen as background contamination in non-European countries, whereas the upper part of the frequency distribution of ΣPCB6 concentrations is an indication for former use.
This wide range is also illustrated by the summarizing overviews (using aggregated data) of the results for ΣPCB6 of 25 countries from the Eastern European and Western European and Others groups (Fig. 1) in comparison with 32 countries from the African, Asia-Pacific and Latin American and the Caribbean regional groups (Fig. 2), all of them with repeated participation between 1987 and 2019: In European countries, ΣPCB6 were at the end of the 1980s and early 1990s in a range up to 800 ng/g lipid and decreased to 2019 to concentrations mostly below 100 ng/g lipid. These levels are approximately the maximum concentrations found in other UN Regional Groups over the whole period 1987–2019. Note that these two figures are normalized to 900 ng/g lipid as maximum value allowing a direct visual comparison of the ΣPCB6 concentrations among the different collection periods as an indication of time trends.
Overview of Indicator PCB concentrations in human milk in seven periods between 1987 and 2019 with indication of temporal tendencies as median in the respective studies for 25 countries from the Eastern European Group and the Western European and Others Group with two or more participations (ng ΣPCB6/g lipid; aggregated data; red line for the median)
Overview of Indicator PCB concentrations in human milk in seven periods between 1987 and 2019 for 32 countries from the African, Asia-Pacific and Latin America and the Caribbean regional groups with two or more participations (ng ΣPCB6/g lipid; aggregated data). (This figure is normalized to 900 ng/g lipid as maximum value as in Fig. 1 allowing a direct visual comparison of the ΣPCB6 concentrations among the different collection periods in European and non-European countries)
Figure 3 illustrates the time trends of ΣPCB6 concentrations (aggregated data) between 1987 and 2019 in the 25 individual countries of the Eastern European and Western European and Others groups. Significant reductions were already achieved in the 1990s. After detection of PCB in environmental samples in 1966 (Jensen 1966; Jensen 1972), PCB were detected for the first time in human tissue, including human milk, in 1970 (Acker and Schulte 1970a; Acker and Schulte 1970b; Schulte 1971; Schulte and Acker 1974a; Schulte and Acker 1974b). As a result, during 1971/2 the PCB producers of the Western world (at that time only 4 remained) introduced voluntary restrictions on use. In 1973 the OECD recommended the use of PCB only in closed systems, which were adopted into national regulations, e.g., by Germany in 1978. Therefore, in Germany, a decrease of ΣPCB6 concentrations in human milk by 95% was achieved between the end of the 1980s and culminated in low background levels in 2019. Similarly successful were Belgium, the Netherlands, the Czech Republic (Czechia), the Slovak Republic (Slovakia), Austria and Lithuania with reductions in the range of 85% to 95% between the end of the 1980s/early 1990s and the period between 2012 and 2019. Also, all other countries had substantially reduced ΣPCB6 concentrations in human milk, but mostly in a shorter period, at least in cases for which data are available.
Overview of the development of Indicator PCB concentrations in human milk (ng ΣPCB6/g lipid; aggregated data) over time between 1987 and 2019 for 25 countries of the Eastern European and Western European and Others regional groups that participated two or more times in the WHO/UNEP-coordinated surveys
Also, in most countries from the regions of Africa, the Asia-Pacific and Latin American and Caribbean, a decrease between different periods was observed, but these decreases began at considerably lower concentrations. These results are discussed in more detail in the following sections.
3.2 Global Level and Comparison Between UN Regional Groups
For time trend analysis of ΣPCB6 between 1987 and 2019, results of 247 pooled samples were available. In two Asian and many European countries PCB were monitored over three decades, whereas the studies in African, Latin American and Caribbean countries comprise two decades with most of these countries participating for the first time in the 2008–2011 period. Basic results of the exponential trends comprise the overall decrease per 1 year and 10 years (Table 2). For the country-specific results in the following subsections, also the decrease per 5 years is shown, which is about 20% higher than the decrease per 4 years representing the average lengths of WHO/UNEP-coordinated exposure studies.
In nearly all UN regions and at a global level, a 50% decrease within a 10-year period (corresponding to an annual decrease of 7%) was achieved for the levels of ΣPCB6. The Latin American and Caribbean countries had lower ΣPCB6 concentrations in comparable periods, obviously resulting in lower decrease rates. This is an indication that the decrease might be faster in regions with higher concentration, compared to a slower decrease in less polluted regions. All trends were statistically significant (p-value <0.001).
Statistical differences between the Theil-Sen method and the median method to derive time trends in different UNEP Regional Groups were insignificant on 95% confidence level, which shows that the Simpson paradox caused by different sampling periods is weak in these cases. On a global level calculated as the median of all samples, the annual decrease was 11.5% (i.e. half-life of 5.7 years) computed by the Theil-Sen method, compared with an annual decrease of 7.3% in the case of the median method.
The exponential trends of indicator PCB derived by the Theil-Sen method in the five UN Regional Groups and worldwide are illustrated in Fig. 4a–f.
(a–f) Theil-Sen exponential trends of indicator PCB concentrations in human milk (ng ΣPCB6/g lipid) in the five UN Regional Groups and worldwide. The shaded area shows the 95% confidence interval of the trend; the thick black lines in the middle of the frequency distribution in a certain year show median concentrations in individual years, whiskers show ranges between fifth and 95th percentiles
3.3 African Group
Figures 5 (for aggregated data) and 6 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of ΣPCB6 concentrations in 13 countries from Africa with repeated participation between 2000 and 2019. In Egypt, a reduction of approximately 85% was observed from the median of 9 pooled samples of various regions submitted in the 2000–2003 period to the one pooled sample submitted in the 2016–2019 period, which is considered to represent the country at that time. Most countries participated for the first time in the 2008–2011 period; in these countries, ΣPCB6 concentrations fell on average by about 50% until the period 2016 to 2019 (range between decrease by 71% and increase by 37%). Ethiopia also had the lowest levels of all countries in the 2000–2019 studies for this parameter: 2.15 ng ΣPCB6/g lipid in 2012 with a downward trend even of this low background level to 0.90 ng/g lipid in 2019. In Côte d’Ivoire, the ΣPCB6 concentrations remained unchanged over the observed shorter period (between 2008–2011 and 2012–2015). An increase was observed in Senegal.
The overall decreases per 1 year, 5 years and 10 years are given in Table 3. In nearly all African countries concentrations tend to decrease. A decrease around 50%–60% was observed, even with low background levels (median for 11 countries with downward trends for the decrease per 10 years: 60%; range 43%–88%). ΣPCB6 concentrations remained quite constant in Côte d’Ivoire and increased by 42% in Senegal within a 10-year period. A statistically significant decrease of 88% over 10 years was found in Egypt (p < 0.001). In the other countries, the limited number of samples did not allow to determine a statistically significant decrease (p ~ 1.000) (for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). However, for all African countries on average, the decrease over 10 years of 50% (calculated by the Theil-Sen method and use of all individual samples) was statistically significant (p < 0.001) (see Table 2 in subsection 3.2).
3.4 Asia-Pacific Group
Figures 7 (for aggregated data) and 8 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of ΣPCB6 concentrations in 10 countries from the Asia-Pacific Group with repeated participation between 1987 and 2019. As in European countries, Vietnam and Thailand also revealed a decrease of 85% to 95% between the end of the 1980s and 2016–2019, however, at considerably lower concentrations in both periods. In Fiji, a reduction of approximately 75% was observed from 2000–2003 to 2008–2011, which then remained stable until 2016–2019. In Hong Kong SAR of China, ΣPCB6 concentrations fell approximately 50% from 2000–2003 to 2008–2011. Most countries of the Pacific subregion participated for the first time in the period 2008–2011; Kiribati in 2004–2007. In nearly all Pacific Island countries, a decrease was observed in the following years even with the low initial background levels.
The overall decreases per 1 year, 5 years and 10 years are given in Table 4. A decrease in the levels of ΣPCB6 within a 10-year period around 50% was achieved by most countries even with the low range of background contamination (median for 9 countries with downward trends for decreasing rates per 10 years: 55%; range 35%–64%). Statistically significant was the decrease of 64% over 10 years in Hong Kong SAR of China (p < 0.001) and of 55% in Fiji (p = 0.008). In the other countries, the limited number of samples did not allow determination of a statistically significant decrease (p = 0.250 and 1.000, respectively) (for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4); however, for the Asia-Pacific Group on average, the decrease over 10 years of 65% (calculated by the Theil-Sen method and use of all individual pooled samples) was statistically significant (p < 0.001) (see Table 2 in subsection 3.2). For Fiji with a statistically significant decrease and participation also in the 2016–2019 period, also a prognosis of the estimated concentrations in 2025 was derived.
3.5 Group of Latin American and Caribbean Countries (GRULAC)
Figures 9 (for aggregated data) and 10 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of ΣPCB6 concentrations in 9 Latin American and Caribbean countries with repeated participation between 2000 and 2019. In Brazil a reduction of 62% was found from the median of 10 pooled samples of the 2000–2003 period to the median of 3 pooled samples of the 2012–2015 period. Most other countries participated for the first time in the period 2008–2011 with Haiti in 2004–2007. In nearly all countries, a decrease was observed in the following years. In Mexico, the background levels remained unchanged.
The overall decreases per 1 year, 5 years and 10 years are given in Table 5. A decrease in the levels of ΣPCB6 around 50% within a 10-year period was achieved by most countries (median for 8 countries with decreasing rates per 10 years: 51%; range 30%–81%). ΣPCB6 concentrations remained stable at the background levels found in Mexico. Statistically significant was the decrease of 56% over 10 years in Brazil (p = 0.022). In the other countries, the limited number of samples did not allow to determine a statistically significant decrease (for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). However, for all participating Latin American and Caribbean countries on average, the decrease over 10 years of 34% (calculated by the Theil-Sen method and use of all individual samples) was statistically significant (p < 0.001) (see Table 2 in subsection 3.2).
3.6 Eastern European Group
Figure 11 (for aggregated data) and Fig. 12 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of ΣPCB6 concentrations in 11 countries of the Eastern European Group with repeated participation between 1987 and 2019. A continuous decrease is observed in all countries over all periods from the high concentrations found in the 1992–1993 period and decreasing considerably during the following rounds and later levelling out. The highest concentrations were found in 1992–1993 in Czechia (median of three pooled samples: 800 ng ΣPCB6/g lipid) and in Slovakia (median of three pooled samples: 753 ng ΣPCB6/g lipid). These decreased until 2019 in Czechia by 86% and in Slovakia by 90%. In 6 countries participating in the 1992–1993 study, the ΣPCB6 concentrations decreased by approximately 40% until the 2000–2003 period (range 16%–41%, based on the median of submitted pooled samples).
The overall decreases per 1 year, 5 years and 10 years are given in Table 6. A decrease in the levels of ΣPCB6 within a 10-year period of approximately 50% to 60% was achieved by most countries. ΣPCB6 concentrations slightly decreased in Georgia between the periods 2008–2011 and 2012–2015. Whereas in Russia the median of two pooled samples collected in the 1987–1988 period decreased to the median of 7 pooled samples submitted in the 2000–2003 period, the Theil-Sen method using all individual pooled samples identified a statistically insignificant increase as a result of the frequency distribution of the 7 samples of the 2000–2003 period in comparison with the 1987–1988 period. Statistically significant (p < 0.05) were the decreasing rates between 50% and 63% over 10 years in 6 other countries. For all countries of the Eastern European Group on average, the decrease over 10 years of 53% (calculated by the Theil-Sen method and use of all individual samples) was statistically significant (p < 0.001) (see Table 2 in subsection 3.2). Based on a statistically significant decrease and participation also in the decade after 2010, a prognosis of the estimated concentrations in 2025 was also derived for 4 countries.
3.7 Western European and Others Group (WEOG)
Figures 13 (for aggregated data) and 14 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of ΣPCB6 concentrations in 14 countries of the Western European and Others Group with repeated participation between 1987 and 2019. A continuous decrease is observed in all countries over all periods, with high concentrations found in the 1987–1988 period decreasing over the following rounds and later levelling out. In Germany, which covers the whole period between 1987 and 2019, a decrease of ΣPCB6 concentrations in human milk by 95% was achieved. Belgium and the Netherlands were similarly successful with reductions in the range of 90% to 94% between the end of the 1980s and the 2012–2015 period.
The median of the decreases for all countries per 10 years was 62% (range 47%–79%). The range between 47% and 68% was statistically significant (p < 0.05) in 8 countries (Table 7). For all WEOG countries on average, the decrease over 10 years of 63% (calculated by the Theil-Sen method and use of all individual samples) was statistically significant (p < 0.001) (see Table 2 in subsection 3.2). Based on a statistically significant decrease with participation also after 2010, a prognosis of the estimated concentrations in 2025 was derived for 5 countries.
4 Toxic Equivalents of PCDD and PCDF (WHO-PCDD/PCDF-TEQ)
Throughout this publication, all TEQ-related evaluations are based on the WHO-TEFs as proposed in 2005 (Van den Berg et al. 2006). The term “WHO-PCDD/PCDF-TEQ” is used for TEQ of PCDD/PCDF. Results are given as upper bound concentrations and expressed on a lipid basis (Malisch et al. 2023b).
Time trends for WHO-PCDD/PCDF-TEQ can be derived beginning with the 1987–1988 period. For a first general estimation of time trends, 146 aggregated values from data for 57 countries with repeated participation in the period 1987–2019 and 119 aggregated data for 50 countries for the period 2000–2019 were used as the basis. In 7 periods between 1987 and 2019, the median of the concentrations decreased considerably from 16.9 pg/g WHO-PCDD/PCDF-TEQ to 2.68 pg/g (= 84% decrease). The Stockholm Convention was adopted in 2001 and entered into force in 2004. Therefore, if only the period 2000–2019 is taken into consideration, the median concentrations went down by 63% from initially 7.34 pg WHO-PCDD/PCDF-TEQ/g in the period 2000–2003. Other than observed for non-dioxin-like-PCB, the WHO-PCDD/PCDF-TEQ concentrations decreased continuously without fluctuations between periods (Table 8).
Figure 15 illustrates the overall picture for temporal trends of concentrations of WHO-PCDD/PCDF-TEQ for 57 countries (using the above-mentioned aggregated data) participating repeatedly in the seven periods between 1987 and 2019.
Overview of WHO-PCDD/PCDF-TEQ concentrations in human milk in seven periods between 1987 and 2019 with indication of temporal tendencies as median in the respective studies for 57 countries with two or more participations (pg WHO-PCDD/PCDF-TEQ/g lipid; red line for the median in a certain round; aggregated data using the median if two or more samples were submitted by a country in a certain period)
For 17 countries of the Eastern European and Western European and Others groups and two countries from Asia with repeated participation in the 7 periods between 1987 and 2019, data for WHO-PCDD/PCDF-TEQ are available starting with the first (1987–1988) and second (1992–1993) round of WHO-coordinated exposure studies. Figure 16 illustrates the country-specific results (using aggregated data). A great falling trend of PCDD/PCDF levels in human milk was found with largest declines in countries with the highest initial levels: In the period 1987–1988, the Netherlands, Belgium and Germany had the highest WHO-PCDD/PCDF-TEQ concentrations (ranging 32–42 pg/g as median of two pooled samples from the Netherlands, of three pooled samples from Belgium and of ten pooled samples from Germany). These countries were highly industrialized and made serious efforts in the 1990s to detect and eliminate PCDD/PCDF sources. For example, at the 1990 symposium on dioxins in Germany, new health-based guidance values on proposed acceptable daily intake were derived. To meet these values over time, dioxin sources needed to be detected and eliminated. A major contributor, municipal waste incineration, was identified and a maximum limit of 0.1 ng TEQ/m3 air was fixed. In addition, regulations on hazardous materials were expanded (Umweltbundesamt und Bundesgesundheitsamt 1990). These efforts were very successful so that annual emissions of PCDD/PCDF in Germany dropped 95% from about 750 g I-TEQ in 1990 to about 70 g I-TEQ in 2004 and stabilized around this level until the end of the observation period in 2011 (Umweltbundesamt 2014). PCDD/PCDF were also released from production of organochlorine chemicals (e.g. PCB, PCP, 2,4,5-T, 2,4-D, chlornitrofen, chloranil) between the 1950s and 1980s, before PCDD/PCDF concentrations in these products as a result of the Seveso accident and regulatory measures were limited (Umweltbundesamt und Bundesgesundheitsamt 1990; Weber et al. 2008).
Over time, similar measures were introduced in other countries and at the EU level. As a result, a substantial decline of about 90% was observed for WHO-PCDD/PCDF-TEQ concentrations in human milk to 4.48 pg/g found in the Netherlands in 2014, 4.03 pg/g found in Belgium in 2015 and 2.90 pg/g found in Germany in 2019. Belgium is the country with the highest number of participations (n = 6); its results show that the most substantial decrease occurred between 1987–1988 (36.7 pg WHO-PCDD/PCDF-TEQ/g) and 2004–2007 (8.49 pg WHO-PCDD/PCDF-TEQ/g) with lower decreases afterwards (to 4.03 pg WHO-PCDD/PCDF-TEQ/g in 2015).
In comparison with the PCDD/PCDF sources relevant in these highly industrialized countries, a different situation was observed in Vietnam. The database for the 1987–1988 period comprises 10 pooled samples from various regions (WHO 1989) showing a wide range of WHO-PCDD/PCDF-TEQ concentrations (median 18.6 pg WHO-PCDD/PCDF-TEQ2005/g, range 6.08 pg/g to 33.9 pg/g). Whereas the lowest equivalent levels outside the European region were reported for the Hanoi area of Vietnam, the highest values were found in certain areas of southern Vietnam. These findings are discussed with regard to the use of the herbicide and defoliant chemical “Agent Orange” during the war in Vietnam in the following subsection “4.3 Asia-Pacific Group”. The pooled sample submitted by Vietnam in 2019 and considered to represent the country at that time had 86% lower WHO-PCDD/PCDF-TEQ concentrations than the median of the 1987–1988 concentrations.
4.1 Global Level and Comparison Between UN Regional Groups
For time trend analysis of WHO-PCDD/PCDF-TEQ, results of 274 pooled samples were available. Monitoring of PCDD/PCDF concentrations in two Asian and many European countries PCDD/PCDF covers three decades. The studies in Africa and Latin America and the Caribbean comprised the period 2000–2019 for some countries, whereas most countries participated for the first time in the 2008–2011 period.
In Western European countries with relatively high WHO-PCDD/PCDF-TEQ concentrations in human milk in the first (1987–1988) and second (1992–1993) rounds, a decrease of 51% over 10 years was achieved. Most Eastern European countries participated for the first time in the 1992–1993 period with on average lower WHO-PCDD/PCDF-TEQ concentrations in human milk in this period (median 11.5 pg WHO-PCDD/PCDF-TEQ/g lipid; range 8.3–14.1 pg WHO-PCDD/PCDF-TEQ/g lipid; N = 7) than in Western European countries (median 17.1 pg WHO-PCDD/PCDF-TEQ/g lipid; range 10.1–26.0 pg WHO-PCDD/PCDF-TEQ/g lipid; N = 8); for the Eastern European group, the decrease within a 10-year-period was 35% (Table 9).
As shown in the following subsections, in comparison with the Western European and Others Group and the Eastern European Group, a higher variation of decrease rates between countries was observed in other UN Regional Groups. Lower decrease rates were observed in some countries, but this has to be seen in context with the quite low levels in these countries. Therefore, these regional trends have also to be seen in context with the variation among participating countries. The country-specific aspects are discussed in the following subsections.
Large differences between the decreases calculated by the Theil-Sen method or the median method indicate a considerable variation between countries in a region, e.g. in Africa as a result of the high decrease in Egypt after initially comparably high WHO-PCDD/PCDF-TEQ concentrations in the 2000–2003 period. Furthermore, other African countries participated for the first time in the 2008–2011 period. Therefore, in addition to the overall decrease (decrease rate constant) in Africa, also the decrease for African countries without Egypt is given indicating a closer agreement between the Theil-Sen method and the median method. Similarly, for the Asia-Pacific region, the decrease without Hong Kong (excluding a possible influence of the numerous pooled samples sent in two periods) and without Vietnam (excluding a possible influence of the numerous pooled samples in the first period) and in the Group of Latin American and Caribbean Countries the decrease without Brazil (excluding a possible influence of numerous pooled samples sent by Brazil in two sampling periods) were calculated.
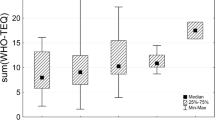
The global decrease over 10 years was estimated to be 48% computed by the Theil-Sen method, compared with 46% in the case of the median method. All trends were statistically significant (p-value <0.001).
The exponential trends of WHO-PCDD/PCDF-TEQ concentrations derived by the Theil-Sen method in the five UN Regional Groups and worldwide are illustrated in Fig. 17a–f.
(a-f) Theil-Sen exponential trends of WHO-PCDD/PCDF-TEQ concentrations in human milk (pg WHO-PCDD/PCDF-TEQ2005/g lipid) in the 5 UN regions and worldwide. The shaded area shows the 95% confidence interval of the trend; the thick black lines in the middle of the frequency distribution in a certain year show median concentrations in individual years, whiskers show ranges between fifth and 95th percentiles
4.2 African Group
Figures 18 (for aggregated data) and 19 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO-PCDD/PCDF-TEQ concentrations in 13 countries from Africa with repeated participation between 2000 and 2019. In Egypt, a reduction of approximately 70% was observed from the median of 9 pooled samples of various regions submitted in the 2000–2003 period to the one pooled sample submitted in the 2016–2019 period, which is considered to represent the country at that time. Most countries participated for the first time in the 2008–2011 period; in these countries, WHO-PCDD/PCDF-TEQ concentrations fell on average by about 10% until the period 2016 to 2019. In seven of these countries with comparably low WHO-PCDD/PCDF-TEQ concentrations (below 3 pg/g lipid), the concentrations remained quite constant over this period. In Ethiopia, the lowest WHO-PCDD/PCDF-TEQ concentrations of all countries in the 2000–2019 studies were found, which remained constant over 7 years (1.01 pg/g in 2012 and 1.02 pg/g in 2019).
The calculated decrease for WHO-PCDD/PCDF-TEQ (Table 10; for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4) was higher in case of elevated concentrations as in Egypt, where possible PCDD/PCDF sources for findings in the 2001–2002 samples were discussed (Malisch et al. 2023b). However, downward tendencies seem to level out at ranges below 3 pg WHO-PCDD/PCDF-TEQ/g lipid, which reflects the background contamination.
4.3 Asia-Pacific Group
Figures 20 (for aggregated data) and 21 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO-PCDD/PCDF-TEQ concentrations in 10 countries from the Asia-Pacific Group with repeated participation between 1987 and 2019.
For two Asian countries, data are available already starting in the 1987–1988 round. Since then, the WHO-PCDD/PCDF-TEQ concentration decreased in Thailand by 67% until 2018. As explained above, the database for Vietnam in the 1987–1988 period comprises 10 pooled samples from various provinces (WHO 1989) showing a wide range of WHO-PCDD/PCDF-TEQ concentrations (median 18.6 pg WHO-PCDD/PCDF-TEQ2005/g, range 6.08 pg/g to 33.9 pg/g). Whereas the lowest equivalent levels outside the European region were reported for the Hanoi area of Vietnam, the highest values were found in certain areas of southern Vietnam. The contribution of 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) to the WHO-PCDD/PCDF-TEQ levels in these human milk samples from southern Vietnam was in most cases between about 30% and 66%. This congener was contained in traces in “Agent Orange”, a mixture of two phenoxy herbicides (2,4-dichlorophenoxyacetic acid [2,4-D] and 2,4,5-trichlorophenoxyacetic acid [2,4,5-T]) used by US military as a defoliant chemical during the war in Vietnam (Baughman and Meselson 1973; Schecter et al. 1987; Institute of Medicine (US) 1994; Stellmann et al. 2003). The WHO-PCDD/PCDF-TEQ concentration of pooled samples submitted by Vietnam in 2019, which is considered to represent the country at that time, was 86% lower than the median of the 1987–1988 concentration. The contribution of 2,3,7,8-TCDD to the WHO-PCDD/PCDF-TEQ was 15%—this is within the usual range of all human milk samples of the 2000–2019 periods.
Decreasing temporal trends were observed in nearly all other countries of the Asia-Pacific region. In Hong Kong SAR of China, the median of 13 pooled samples from different subgroups collected in 2002 decreased by 15% to the median of four pooled samples collected in 2009. In this range, the WHO-PCDD/PCDF-TEQ concentrations also decreased in the Pacific countries, which generally had quite low concentrations in the range below 4 pg WHO-PCDD/PCDF-TEQ/g lipid in the period 2008–2011 and below 3 pg WHO-PCDD/PCDF-TEQ/g lipid in the period 2016–2019. Fiji participated in four rounds between 2000 and 2019. Over this period, the WHO-PCDD/PCDF-TEQ concentration decreased by 30% from 3.08 pg/g as an already comparably low level in 2002 to 2.20 pg/g in 2019. Only in the Marshall Islands did the WHO-PCDD/PCDF-TEQ concentration increase from 3.98 pg/g to 9.32 pg/g. For this country, the congener pattern and possible sources for the increased levels were discussed separately (Malisch et al. 2023b).
The overall decreases per 1 year, 5 years and 10 years are given in Table 11 (for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). As concluded above for the decrease in African countries with Egypt as example for elevated concentrations related to known sources, a considerable decrease in the levels of POPs within a 10-year period was achieved in Vietnam in comparison with the 1987–1988 samples. Downward tendencies seem to level out at ranges below 3 pg WHO-PCDD/PCDF-TEQ/g lipid, which reflects the background contamination.
4.4 Group of Latin American and Caribbean Countries (GRULAC)
Figures 22 (for aggregated data) and 23 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO-PCDD/PCDF-TEQ concentrations in 9 Latin American and Caribbean countries with repeated participation between 2000 and 2019. In Brazil a reduction of 35% was found from the median of 10 pooled samples of the 2000–2003 period to the median of 3 pooled samples of the 2012–2015 period. Most other countries participated for the first time in the period 2008–2011 with Haiti in 2004–2007. In nearly all countries, a decrease was observed in the following years. In Jamaica, the low levels remained unchanged.
The two samples from Chile apparently showed considerable differences of the WHO-PCDD/PCDF-TEQ concentrations between 2008 (8.44 pg/g) and 2011 (3.92 pg/g). Neither the contribution of PCDD, PCDF and dioxin-like PCB to the TEQ nor the PCDD/PCDF pattern changed during this relatively short time period of 3 years. Thus, sampling differences might explain these findings (Malisch et al. 2023b).
Overall decreases per 1 year, 5 years and 10 years are given in Table 12 (for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4).
4.5 Eastern European Group
Figures 24 (for aggregated data) and 25 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO-PCDD/PCDF-TEQ concentrations in 11 countries of the Eastern European Group with repeated participation between 1987 and 2019.
One country (Hungary) already participated in the 1987–1988 round. Here, the WHO-PCDD/PCDF-TEQ concentrations decreased by 56% until 2004–2007. In countries which participated for the first time in the 1992–1993 period, the concentrations (as aggregated data, calculated as median if two or more samples were submitted in a certain period) decreased by 77% in Croatia until 2014, by 76% in the Czech Republic until 2019, by 70% in Lithuania until 2015, by 79% in the Slovak Republic until 2019, by 25% in the Ukraine until 2001, and by 22% in Russia until 2001–2002. In two countries participating for the first time in the 2000–2003 period, WHO-PCDD/PCDF-TEQ concentrations decreased by 18% (Bulgaria, 2014) and 22% (Romania, 2014). Finally, two countries (Georgia and Moldova) participated for the first time in the 2008–2011 period. Here, concentrations decreased by 21% (Georgia) and 42% (Moldova) until 2015.
The overall decreases per 1 year, 5 years and 10 years calculated on all individual countries are given in Table 13 (for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). For all Eastern European countries on average the decrease over 10 years of 35% (calculated by the Theil-Sen method and use of all individual samples) was statistically significant (p < 0.001) (see Table 9 in subsection 4.1). Based on a statistically significant decrease and participation also after 2010, a prognosis of the estimated concentrations in 2025 was also derived for 4 countries.
4.6 Western European and Others Group (WEOG)
Figures 26 (for aggregated data) and 27 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO-PCDD/PCDF-TEQ concentrations in 14 countries of the Western European and Others Group with repeated participation between 1987 and 2019. A continuous decrease is observed in all countries over all periods, with high concentrations found in the 1987–1988 period decreasing over the following rounds and later levelling out: Whereas the overall decrease (%) per 10 years before 2000 was 62% and between 2001 and 2010 58%, it was 8% in the decade after 2011 (Table 14).
In Germany, which covers the whole period between 1987 and 2019, based on aggregated data a decrease of WHO-PCDD/PCDF-TEQ concentrations in human milk by 91% was achieved. Similarly successful were Belgium and the Netherlands both with reductions of 89% between the end of the 1980s and the 2012–2015 period.
The median of the decreases for all countries per 10 years was 48% (range 29%–58%). The range between 28% and 54% was statistically significant in 10 countries (Table 15; for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). For all WEOG countries on average the decrease over 10 years of 51% was statistically significant (p < 0.001) (see Table 9 in subsection 4.1). Based on a statistically significant decrease and participation also after 2010, also a prognosis of the estimated concentrations in 2025 was derived for 7 countries.
5 Total Toxic Equivalents of PCDD, PCDF and Dioxin-Like PCB (WHO2005-TEQ)
All TEQ-related evaluations are based on the WHO-TEFs as proposed in 2005 (Van den Berg et al. 2006). The term “WHO-PCDD/PCDF-TEQ” is used for TEQ of PCDD/PCDF, “WHO-PCB-TEQ” for TEQ of dioxin-like PCB and “WHO2005-TEQ” for the total TEQ as sum of the contribution of PCDD/PCDF and dioxin-like PCB. Results are given as upper bound concentrations and expressed on a lipid basis (Malisch et al. 2023b).
As dioxin-like PCB were of interest starting from the second WHO round (1992–1993), time trends for total TEQ (WHO2005-TEQ) can be derived only beginning with this period. For a first general estimation of time trends, 130 aggregated data for 54 countries with repeated participation in the period 1992–2019 and 116 aggregated data for 50 countries for the period 2000–2019 were used as the basis. In 6 periods between 1992 and 2019, the median of the concentrations decreased considerably from 21.7 pg WHO2005-TEQ/g to 3.91 pg/g (= 82% decrease). The Stockholm Convention was adopted in 2001 and entered into force in 2004. Therefore, if only the period 2000–2019 is taken into consideration, the median concentrations went down by 70% from initially 13.1 pg WHO2005-TEQ/g in the period 2000–2003. Other than observed for the continuous decrease of WHO-PCDD/PCDF-TEQ levels, the WHO2005-TEQ concentrations seemed to decrease with a fluctuation by an increase from the fifth to the sixth round (Table 16). As explained above for non-dioxin-like-PCB, also these changes are the result of participation of different countries in different rounds. Therefore, for more precise time trends, a country-specific evaluation is necessary.
5.1 Global Level and Comparison Between UN Regional Groups
Figure 28 illustrates the developments in 54 countries from all UN regions with repeated participation between 1992 and 2019 for 130 aggregated data. Similar temporal trends as described for WHO-PCDD/PCDF-TEQ were observed. Discrepancies depend on dioxin-like PCB and their contribution to total TEQ in comparison with the PCDD/PCDF contribution (Malisch et al. 2023b).
For time trend analysis of total TEQ concentrations, results of 226 individual pooled samples were available. Monitoring of WHO2005-TEQ concentrations in many European countries PCDD/PCDF covers three decades, whereas the studies in Africa, the Asia-Pacific Region, Latin America and the Caribbean comprised the period 2000–2019, with most countries participating for the first time in the 2008–2011 period.
In nearly all countries, WHO2005-TEQ concentrations were consistently decreasing over the whole monitored period. A 50% decrease in the levels of total TEQ within a 10-year period was achieved in regions with countries with initially relatively high WHO2005-TEQ concentrations in human milk, as found, e.g., in Western European in the second (1992–1993) or third (2000–2003) round or in an African country in the 2000–2003 period. In comparison with the initial levels found in Western European countries, most Eastern European countries had on average lower WHO2005-TEQ concentrations in human milk; for this group, the decrease within a 10-year-period was nearly 40%. The lower decrease rates observed in some African, Pacific, Latin American and Caribbean and Eastern European countries have to be seen in context with the quite low initial levels in these countries in comparison with some countries of other regions and their periods of participation.
The regional trends (Table 17) have also to be seen in context with the variation among participating countries. Large differences between the decreases calculated by the Theil-Sen method or the median method indicate a considerable variation between countries in a region, e.g., in Africa as result of the high decrease in Egypt after initially comparatively high WHO-PCDD/PCDF-TEQ concentrations in the 2000–2003 period. The decreases calculated by the Theil-Sen method for the African Group without Egypt are in better agreement with the median method. Similarly, in Latin America and the Caribbean, a differentiation between Brazil (with participation already in the 2000–2003 period and submission of 10 pooled samples at that time) and other countries (mostly participating for the first time in the period 2008–2011 with submission of one pooled sample per period) was introduced. In the Asia-Pacific Group, the decrease (decrease rate constant) without Hong Kong was also calculated to exclude a possible influence of the comparably high number of pooled samples from different subgroups collected in 2002 and 2009. The country-specific aspects are discussed in the following sections.
The annual global decrease was estimated at 6.6% (i.e. half-life of 10.1 years) computed by the Theil-Sen method, which is in agreement with an annual decrease of 6.4% in the case of the median method. All trends were statistically significant (p-value <0.001).
The exponential trends of WHO2005-TEQ concentrations derived by the Theil-Sen method in the five UN Regional Groups and worldwide are illustrated in Fig. 29a–f.
(a-f) Theil-Sen exponential trends of total TEQ concentrations in human milk (pg WHO2005-TEQ/g lipid) in the 5 UN regions and worldwide. The shaded area shows the 95% confidence interval of the trend; the thick black lines in the middle of the frequency distribution in a certain year show median concentrations in individual years, whiskers show ranges between fifth and 95th percentiles
5.2 African Group
Figures 30 (for aggregated data) and 31 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO2005-TEQ concentrations in 13 countries from the Africa Group with repeated participation between 2000 and 2019.
Most countries participated for the first time in the 2008–2011 period; in these countries, WHO2005-TEQ concentrations fell on average by about 23% (range 14%–40%) until the period 2016 to 2019. The calculated decrease for WHO2005-TEQ within a 10-year period is in a range between 14% and 61% (median 28%) (Table 18; for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). The highest rate was observed in Egypt, where PCDD/PCDF sources were discussed for elevated findings in the 2001–2002 samples (Malisch et al. 2023b).
For the decrease of total TEQ concentrations, the relative contribution of dioxin-like PCB and PCDD/PCDF has an influence. As an example for changes of the contribution of dioxin-like PCB to the total TEQ over time, in Togo the relatively low background concentrations for WHO-PCDD/PCDF-TEQ increased from 2008–2011 to 2016–2019 by 22%, whereas the WHO2005-TEQ concentration decreased by 26%. In this period, the contribution of dioxin-like PCB to the total TEQ decreased in Togo from 55% in 2010 to 25% in 2017 (Malisch et al. 2023b). Whereas the WHO-PCDD/PCDF-TEQ concentrations decreased in African countries from 2008–2011 to 2016–2019 on average by 10% (range 38% decrease to 22% increase), the WHO-PCB-TEQ concentrations decreased on average by 47% (range 23% to 67%).
5.3 Asia-Pacific Group
Figures 32 (for aggregated data) and 33 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO2005-TEQ concentrations in 8 countries from the Asia-Pacific Group with repeated participation between 1992 and 2019.
Decreasing temporal trends were observed in nearly all other countries of the Asia-Pacific region. In Hong Kong SAR of China, the median of 13 pooled samples from different subgroups collected in 2002 decreased by 12% to the median of four pooled samples collected in 2009. Fiji participated in four rounds between 2000 and 2019. Over this period, the WHO2005-TEQ concentration decreased by 34% from 4.42 pg/g (as the median of two pooled samples) as an already comparably low level in 2002 to 2.90 pg/g in 2019. Only in the Marshall Islands the WHO2005-TEQ concentration increased from 6.32 pg/g in 2011 to 11.6 pg/g in 2019. For this country, the congener pattern and possible sources for the increased levels were discussed separately (Malisch et al. 2023b).
The overall decreases per 1 year, 5 years and 10 years are given in Table 19 (for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). The decrease in the levels of WHO2005-TEQ within a 10-year period was in nearly all countries between 13% and 81% showing a continuous decrease also in the range of low background concentrations.
5.4 Group of Latin American and Caribbean Countries (GRULAC)
Figures 34 (for aggregated data) and 35 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO2005-TEQ concentrations in 9 Latin American and Caribbean countries with repeated participation between 2000 and 2019. In Brazil a reduction of 40% was found from the median of 10 pooled samples of the 2000–2003 period to the median of 3 pooled samples of the 2012–2015 period. Most other countries participated for the first time in the period 2008–2011, Haiti in 2004–2007. In all countries, a decrease was observed in the following years.
The overall decreases per 1 year, 5 years and 10 years are given in Table 20 (for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). The decrease in the levels of WHO2005-TEQ within a 10-year period was in all countries between 20% and 93% showing a continuous decrease also in the range of low background concentrations. The high decrease found in Chile for samples collected over a relatively short period (2008 and 2011) has to be seen in context with discussion of PCDD/PCDF patterns and the assumption that differences in the regional origin might explain these findings (Malisch et al. 2023b).
5.5 Eastern European Group
Figures 36 (for aggregated data) and 37 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO2005-TEQ concentrations in 11 countries of the Eastern European Group with repeated participation between 1987 and 2019.
In countries which participated for the first time in the 1992–1993 period, the concentrations (as aggregated data, calculated as median if two or more samples were submitted in a certain period) decreased by 71% in Croatia until 2014, by 74% in the Czech Republic until 2019, by 40% in Hungary until 2006, by 76% in Lithuania until 2015, by 80% in the Slovak Republic until 2019, by 13% in the Ukraine until 2001, and increased by 11% in Russia until 2001–2002. In two countries participating for the first time in the 2000–2003 period, WHO2005-TEQ concentrations decreased by 26% (Bulgaria, 2014) and 27% (Romania, 2014). Finally, two countries (Georgia and Moldova) participated for the first time in the 2008–2011 period, where concentrations decreased in both by 39% until 2015.
The overall decreases per 1 year, 4 years, 5 years and 10 years calculated for all individual countries are given in Table 21 (for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). Statistically significant overall decreases per 10 years for countries between 14% and 47% were found in 6 countries. For four of these with participation also after 2010, also a prognosis of the estimated concentrations in 2025 was derived.
5.6 Western European and Others Group (WEOG)
Figures 38 (for aggregated data) and 39 (comprising all individual pooled samples and assuming exponential trends, see subsection 2.4) illustrate the time trends of WHO2005-TEQ concentrations in 13 countries of the Western European and Others Group with repeated participation between 1992 and 2019. A continuous decrease is observed in all countries over all periods, with high concentrations found in the 1987–1988 period decreasing faster over the following rounds and later at lower concentration ranges slower: Whereas the overall decrease (%) per 10 years before 2000 was 75% and 58% between 2001 and 2010, it was 8% in the decade after 2011 (Table 22).
Based on aggregated data, in Germany, which covers the whole period between 1992 and 2019, a decrease of WHO2005-TEQ concentrations in human milk by 84% was achieved. Similarly successful were Belgium and the Netherlands with reductions of nearly 80% between 1992 and the 2012–2015 period.
The median of the decreases (decrease rate constants) for all countries per 10 years was 48% (range 34–56%) (Table 23; for statistical significance of trends requiring p-values <0.05 and changes of concentrations indicating tendencies, see subsection 2.4). Based on statistically significant decrease rates, estimated concentrations in 2025 were derived for 4 countries with participation also after 2010.
6 Summary and Conclusions
One of the objectives of the WHO/UNEP-coordinated exposure studies in human milk was to generate comparable and consistent monitoring data to estimate time trends on levels of POPs included in the Stockholm Convention on Persistent Organic Pollutants as required under Article 16 of the Convention. The Convention parties adopted the Guidance Document on the Global Monitoring Plan that considers necessary data on the levels of these POPs in certain environmental compartments and human tissues (blood and milk) to estimate time trends to measure the effectiveness of the Convention in eliminating or reducing emissions of POPs.
The presentation and discussion of the 2000–2019 results in Part III of this compendium includes a general estimation of time trends during the five rounds for all participating countries over these 20 years using data gathered from various rounds. However, a more precise approach for determining time trends is the evaluation of results from only countries with repeated participation in these studies: This allows more certainty in drawing of conclusions on temporal trends, which are not potentially influenced by single results of a countries submitted for a single round, and seemed optimal for the evaluation of the effectiveness for the purpose of Article 16.
For statistically significant trends, a minimum of five data points have to be available. However, for most countries only less than five data points are available. This prevents deriving statistically significant temporal trends in these cases. Yet, the existing data can indicate decreasing or increasing tendencies in POP concentrations. Furthermore, pooling of data in regions allows to derive statistically significant time trends for the UN Regional Groups. To minimize possible sources of variation for time trend analysis of POPs, including PCB, PCDD and PCDF, the concept of the WHO/UNEP-coordinated exposure studies has two basic elements (preparation of pooled samples from a number of individual samples considered to be representative for a country or region/subgroup; analysis by a reference laboratory).
This chapter presents temporal trends derived for the (1) sum of six Indicator PCB (ΣPCB6), (2) sum of toxic equivalents (TEQ) of PCDD/PCDF (WHO-PCDD/PCDF-TEQ) and (3) total sum of toxic equivalents (“Total TEQ”) of mixtures of PCDD/PCDF and dioxin-like PCB (WHO2005-TEQ). All concentrations are reported on a lipid basis.
Five rounds of WHO/UNEP-coordinated exposure studies from 2000–2019 as well as two WHO-coordinated exposure studies carried out in 1987–1988 and 1992–1993 are used as the basis of this evaluation. Therefore, data for human milk samples from 57 countries with repeated participation from all United Nations Regional Groups between 1987 and 2019 were available to derive time trends.
Decreases (decrease rate constants) per 1 year, 5 years (approximately 20% higher than the decrease over 4 years as average lengths of the WHO/UNEP-coordinated studies) and 10 years were computed using the Theil-Sen trend estimator including values of all individual samples for the trend analysis. For confirmation, a method of deriving the regional trend as a median of trends in countries within the region was used (“median method”).
The reduction rates should be seen also in context with the concentration range (differentiation of levels above or in the range of the background contamination). If high levels are found, sources might be detected which could be eliminated. However, at low background levels, other factors, e.g. contamination of feed and food by air via long-range transport and subsequent bioaccumulation, cannot be influenced locally.
Indicator PCB
In European countries, the sum of 6 non-dioxin-like Indicator PCB was in a range up to 800 ng/g at the end of the 1980s and early 1990s and decreased to mostly below 100 ng/g until 2019. This was approximately the maximum concentration found in non-European regions over the whole period 1987–2019.
A 50% decrease within a 10-year period was achieved for the levels of ΣPCB6 in nearly all UN regions (range 34% to 65% calculated by the Theil-Sen method; range 45% to 62% calculated by the median method) and at a global level (71% calculated by the Theil-Sen method; 56% calculated by the median method). The Latin American and Caribbean countries had lower ΣPCB6 concentrations in comparable periods, obviously resulting in lower decreases (decrease rate constants). This is an indication that the decrease might be faster in regions with higher concentration, compared to a slower decrease in less polluted regions.
As an example for European countries, in Germany a decrease of ΣPCB6 concentrations in human milk by 95% was achieved between the end of the 1980s and 2019. Similarly, Belgium, The Netherlands, the Czech Republic, the Slovak Republic, Austria and Lithuania were successful with reductions in the range of 85% to 95% between the end of the 1980s/early 1990s and the period between 2012 and 2019. Most other European countries also reduced ΣPCB6 concentrations in human milk substantially, although often over a shorter period. This trend is largely influenced by the stop of PCB production in the 1980s and a restriction of PCB use in open application in the 1970s with improved PCB waste management from 1990s on including Stockholm Convention measures from 2001 on (Weber et al. 2018).
As in European countries, Vietnam and Thailand also saw a decrease of ΣPCB6 concentrations in human milk by 85% to 95% between the end of the 1980s and 2016–2019, however, starting at considerably lower concentrations in both periods. In Egypt and Fiji, a reduction between approximately 85% and 75% was observed from 2000–2003 to 2016–2019. In Hong Kong SAR of China, ΣPCB6 concentrations fell approximately 50% from 2000–2003 to 2008–2011, and in Brazil about 60% from 2000–2003 to 2012–2015. Most other countries participated for the first time in the period 2008–2011. In nearly all countries, decreases were observed in the following years but there is not enough data to derive the time trends. In Mexico, the concentration remained unchanged at low background levels. Tendencies for increase were seen in Senegal and the Marshall Islands.
WHO-PCDD/PCDF-TEQ
At the global level, the decrease in the levels of PCDD/PCDF levels within a 10-year period was 48% calculated by the Theil-Sen method and 34% calculated by the median method. A decrease over 10 years of 51% was achieved in the Western European and Others Group with initially relatively high WHO-PCDD/PCDF-TEQ concentrations in the first (1987–1988) and second (1992–1993) rounds. Most Eastern European countries participated for the first time in the 1992–1993 period with on average lower WHO-PCDD/PCDF-TEQ concentrations in human milk than in Western European countries; for this group, the decrease within a 10-year-period was 35%.
In comparison with the Western European and Others Group and the Eastern European Group, a higher variation of decreases (decrease rate constants) between countries was observed in other UN Regional Groups. Lower decreases were observed in some countries, but this has to be seen in context with the quite low levels in these countries.
Overall in 7 periods between 1987 and 2019, the median of concentrations of WHO-PCDD/PCDF-TEQ decreased considerably from 16.9 pg/g in 1987–1988 as averaged for 13 countries to 2.68 pg/g in 2016–2019 as averaged for 33 countries, which is a reduction of 84%. Because the Stockholm Convention was adopted in 2001 and entered into force in 2004, the levels from period 2000–2019 may be taken at the base. In this case, the initial concentration would be 7.34 pg/g in the period 2000–2003, which still shows a significant reduction of 63% until 2016–2019.
As an example for European countries, the Netherlands, Belgium and Germany had the highest WHO-PCDD/PCDF-TEQ concentrations in the period 1987–1988 (ranging from 32–42 pg/g). As a result of numerous measures taken already in the 1990s to detect and eliminate dioxin sources, a substantial decline of about 90% was observed in human milk to 4.48 pg/g found in the Netherlands in 2014, 4.03 pg/g found in Belgium in 2015 and 2.90 pg/g found in Germany in 2019. Belgium is the country with the highest number of participations (n = 6); its results show that the most substantial decrease occurred between 1987–1988 and 2004–2007 with slight decreases afterwards.
Two Asian countries also participated in the 1987–1988 round. Significant decreases in WHO-PCDD/PCDF-TEQ concentration were observed in Vietnam (by 86% until 2019) and Thailand (by 67% until 2018). Increasing concentrations were found in the Marshall Islands. In all other countries the concentrations decreased by smaller amounts or remained stable in the range of levels of background contamination.
Total TEQ (WHO2005-TEQ)
As dioxin-like PCB were of interest starting from the second WHO round (1992–1993), time trends for total TEQ (WHO2005-TEQ) can be derived only from this period. Similar temporal trends as described for WHO-PCDD/PCDF-TEQ were observed. Discrepancies depend on dioxin-like PCB and their contribution to total TEQ in comparison with the PCDD/PCDF contribution.
In nearly all countries, WHO2005-TEQ concentrations were consistently decreasing over the whole monitored period. A decrease around 50% was achieved in regions with countries with initially relatively high WHO2005-TEQ concentrations in human milk, as found, e.g., in Western Europe in the second (1992–1993) or third (2000–2003) round or in an African country in the 2000–2003 period. Most Eastern European countries had on average lower WHO2005-TEQ concentrations in human milk than Western European countries; for the Eastern European group, the decrease within a 10-year-period was nearly 40%. The lower decreases (decrease rate constants) observed in some African, Pacific, Latin American and Caribbean and Eastern European countries are in line with the quite low levels in these countries in comparison with some countries of other regions and their periods of participation.
Overall Conclusions
The concept of WHO/UNEP-coordinated exposure studies with standardized protocols for preparation of pooled samples considered to be representative for a country or subpopulation in a country and analysis in a reference laboratory provides reliable data for human milk samples. The consideration only of countries with repeated participation over a decade or more provides the best possible database assessment of temporal trends. Statistically significant decreasing trends for PCB and PCDD/PCDF were observed for all parameters in the five UN Regional Groups and at a global level. However, for the majority of individual countries the limited available data did not allow for the statistically significant assessment of time trends, but decreasing tendencies were observed for many of them and constant or increasing levels for few of them. It is highly recommended to continue this monitoring effort to secure enough data for appropriate time trend assessments in the future.
References
Acker L, Schulte E (1970a) Dtsch Lebensmittel-Rdsch 66:385–390
Acker L, Schulte E (1970b) Über das Vorkommen von chlorierten Biphenylen und Hexachlorbenzol neben chlorierten Insektiziden in Humanmilch und menschlichem Fettgewebe. Naturwissenschaften 57:497
Baughman R, Meselson M (1973) An analytical method for detecting TCDD (dioxin): levels of TCDD in samples from Vietnam. Environ Health Perspect 5:27–35
Canady R, Crump K, Feeley M, Freujer J, Kogevinas M, Malisch R, Verger P, Wilson J, Zeilmaker M (2002) Polychlorinated dibenzodioxins, polychlorinated dibenzofurans and coplanar biphenyls. WHO Food Additives Series 48 “Safety evaluation of certain food additives and contaminants, prepared by the fifty-seventh meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). IPCS (International Programme on Chemical Safety), World Health Organization, Geneva, p. 451–664
EFSA (2018) Risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J 16(11):5333. https://doi.org/10.2903/j.efsa.2018.5333
EU Scientific Committee on Food (2000) Opinion of the SCF on the risk assessment of dioxins and dioxin-like PCBs in Food
Fång J, Nyberg E, Winnberg U, Bignert A, Bergman A (2015) Spatial and temporal trends of the Stockholm Convention POPs in mothers’ milk — a global review. Environ Sci Pollut Res 22:8989–9041
Fisher RA (1915) Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika 10:507. https://doi.org/10.2307/2331838
Fox PA (1984) The PORT mathematical subroutine library
Fürst P (2023) Human milk surveys on persistent organic pollutants from a historical perspective. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
GMP DWH (2020) Global monitoring plan data warehouse of the stockholm convention on persistent organic pollutants: visualisation platform and on-line tool for the analysis of global levels of POPs in air, water, breast milk and blood, online at http://www.pops-gmp.org (Hůlek, R., Borůvková, J., Kalina, J., Bednářová, Z., Šebková, K., Hruban, T., Novotný, V., Ismael, M. and Klánová J. [Masaryk University, 2020])
Institute of Medicine (US) (1994) Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. Veterans and Agent Orange: Health effects of herbicides used in Vietnam. , Washington, DC. National Academic Press (US)
Jensen S (1966) Report of a new chemical hazard. New Scientist 32:612
Jensen S (1972, August) The PCB story. Ambio 1(4):123–131
Komsta, Ł., 2013. Package ‘mblm’
Malisch R, Schächtele A (2023) Analysis and quality control of WHO- and UNEP- coordinated human milk studies 2000-2019: polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part II)
Malisch R, Malisch K, van Leeuwen FXR, Moy G, Tritscher A, Witt A, Alvarez J (2023a) Overview of WHO- and UNEP-coordinated human milk studies and their link to the Stockholm Convention on persistent organic pollutants. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Malisch R, Schächtele A, van Leeuwen FXR, Moy G, Tritscher A (2023b) WHO- and UNEP-coordinated exposure studies 2000-2019: Findings of polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
Schächtele A, Malisch R, Hardebusch B, van Leeuwen FXR, Moy G, Tritscher A, van Duursen M, van den Berg M, Šebková K, Klánová J, Kalina J (2023) WHO- and UNEP-coordinated exposure studies 2000-2019: Findings of polybrominated substances. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
Schecter A, Ryan JJ, Constable JD (1987) Polychlorinated dibenzo-p-dioxin and polychlorinated dibenzofuran levels in human breast milk from Vietnam compared with cow’s milk and human breast milk from the North American continent. Chemosphere 16:2003–2016
Schulte E (1971) Beitrag zur Analytik der Rückstände von chlorierten Kohlenwasserstoffen insbesondere von polychlorierten Biphenylen und über ihr Vorkommen im Fettgewebe des Menschen und in Humanmilch. Dissertation Universität Münster
Schulte E, Acker L (1974a) Identifizierung und Metabolisierbarkeit von polychlorierten Biphenylen. Naturwissenschaften 61:79–80
Schulte E, Acker L (1974b) Gaschromatographie mit Glascapillaren bei Temperaturen bis zu 320 °C und ihre Anwendung zur Trennung von Polychlorbiphenylen. Fresenius Z Anal Chem 268:260–267
Šebková K (2023) The Stockholm Convention and its implementation by regional and global monitoring reports. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Sen PK (1968) Estimates of the regression coefficient based on Kendall’s Tau. J Am Stat Assoc 63:1379–1389. https://doi.org/10.1080/01621459.1968.10480934
Sharma BM, Kalina J, Whaley P, Scheringer M (2021) Towards guidelines for time-trend reviews examining temporal variability in human biomonitoring data of pollutants. Environ Int 151:106437. https://doi.org/10.1016/j.envint.2021.106437
Simpson EH (1951) The Interpretation of Interaction in Contingency Tables. J R Stat Soc 13:238–241
Stellmann MJ, Stellmann SD, Christian R, Weber T, Tomasallo C (2003) The extent and patterns of usage of Agent Orange and other herbicides in Vietnam. Nature 422:681–687
Theil H (1992) A rank-invariant method of linear and polynomial regression analysis. In: Raj B, Koerts J (eds) Henri Theil’s contributions to economics and econometrics. Advanced studies in theoretical and applied econometrics, vol 23. Springer, Dordrecht, pp 345–381. https://doi.org/10.1007/978-94-011-2546-8_20
Umweltbundesamt (2014) Dioxine und dioxinähnliche PCB in Umwelt und Nahrungsketten, Januar 2014. https://www.umweltbundesamt.de/sites/default/files/medien/378/publikationen/hgp_dioxine_entwurf_25.04.2014_grau-ocker.pdf
Umweltbundesamt und Bundesgesundheitsamt (1990) Eintragsminimierung zur Reduzierung der Dioxinbelastung dringend erforderlich – erste Auswertung des Dioxinsymposiums resultierte in Maßnahmenkatalog von Umweltbundesamt und Bundesgesundheitsamt Bundesgesundheitsblatt 8/90
UNEP (2001). Stockholm convention on Persistent Organic Pollutants (POPs), text and annexes. http://chm.pops.int
UNEP (2007) Guidance on the global monitoring plan for persistent organic pollutants, preliminary version, February 2007
UNEP (2013) Toolkit for identification and quantification of releases of dioxins, furans and other unintentional POPs under Article 5 of the Stockholm Convention
UNEP (2015) Guidance on the global monitoring plan for persistent organic pollutants, January 2013. UNEP/POPS/COP.7/39 (Conference of the Parties to the Stockholm Convention on Persistent Organic Pollutants, Geneva, 4–15 May 2015)
UNEP (2019) Guidance on the global monitoring plan for persistent organic pollutants. UNEP/POPS/COP.9/INF/36. http://chm.pops.int/TheConvention/ConferenceoftheParties/Meetings/COP9/tabid/7521/Default.aspx
Van den Berg M, Birnbaum L, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson E (2006) The 2005 World Health Organization re-evaluation of human and mammalian Toxic Equivalency Factors (TEFs) for dioxins and dioxin-like compounds. Toxicol Sci 93(2):223–241
van den Berg M, van Duursen M, Tritscher A, Malisch R, Peterson RE (2023) Risk-benefit analysis for the breastfed infant based on the WHO- and UNEP human milk surveys for dioxin-like compounds. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part IV)
Wang B, Fiedler H, Huang J, Deng S, Wang Y, Yu G (2016) A primary estimate of global PCDD/F release based on the quantity and quality of national economic and social activities. Chemosphere 151:303–309
Weber R, Gaus C, Tysklind M, Johnston P, Forter M, Hollert H, Heinisch H, Holoubek I, Lloyd-Smith M, Masunaga S, Moccarelli P, Santillo D, Seike N, Symons R, Torres JPM, Verta M, Varbelow G, Vijgen J, Watson A, Costner P, Wölz J, Wycisk P, Zennegg M (2008) Dioxin- and POP-contaminated sites—contemporary and future relevance and challenges. Env Sci Pollut Res Int 15:363–393
Weber R, Herold C, Hollert H, Kamphues J, Ungemach L, Blepp M, Ballschmiter K (2018) Life cycle of PCBs and contamination of the environment and of food products from animal origin. Environ Sci Pollut Res Int 25(17):16325–16343. https://doi.org/10.1007/s11356-018-1811-y
WHO (1989) Levels of PCBs, PCDDs, and PCDFs in breast milk. Environmental Health Series 34, 1–92 (WHO Regional Office for Europe)
WHO (1996) Levels of PCBs, PCDDs and PCDFs in human milk. Environmental Health in Europe No. 3, 1–121 (WHO European Centre for Environment and Health)
Acknowledgements
This publication was developed in the framework of the projects titled “Implementation of the POPs Monitoring Plan in the Asian Region” and “Continuing Regional Support for the POPs Global Monitoring Plan Under the Stockholm Convention in the Africa, Pacific and Latin-American and Caribbean Region”, funded by the Global Environment Facility and in close collaboration with and support of CVUA Freiburg.
The worldwide implementation of the Global Monitoring Plan for POPs, including that of the UNEP/WHO global human milk survey, is made possible thanks to the generous contributions to the Stockholm Convention Voluntary Trust Fund by the Governments of Japan, Norway and Sweden and through the European Union’s Global Public Goods and Challenges Programme (GPGC). Further, the substantial contributions made by the Global Environment Facility to support POPs monitoring activities in regions implemented by UNEP, in close collaboration with WHO, particularly for the global human milk surveys, is greatly appreciated.
The authors express their gratitude to the National Coordinators of the WHO- and UNEP-coordinated exposure surveys for their excellent work to collect the human milk samples and to prepare and send the pooled samples to the Reference Laboratory, which included great efforts to plan and implement the national studies with the assistance of the health, environment, laboratory and administrative staff. The continuous exchange of information between the National Coordinators and WHO, UNEP and the Reference Laboratory was an important aspect for the successful organization of these studies on a global level.
Data analysis was supported by the RECETOX Research Infrastructure (No LM2018121) and the CETOCOEN EXCELLENCE Teaming Phase 2 project financed by the European Union’s Horizon 2020 (No 857560) and the European Structural and Investment Funds: No CZ.02.1.01/0.0/0.0/17_043/0009632).
Hae Jung Yoon, Seongsoo Park and Philippe Verger (Department of Food Safety and Zoonoses) are acknowledged for their coordinating support during their time at WHO, and Lawrence Grant (WHO) for the statistical analysis of the sampling protocols.
We thank Katarina Magulova and Ana Witt (Secretariat of the Basel, Rotterdam and Stockholm Conventions) and Jacqueline Alvarez, Haosong Jiao and Gamini Manuweera (United Nations Environment Programme, Economy Division, Chemicals and Health Branch) for their support and contributions to these surveys, furthermore Heidelore Fiedler for the conception and implementation of the GMP projects at her time at United Nations Environment Programme, Economy Division, Chemicals and Health Branch.
We also thank the team at CVUA Freiburg for performance of the analyses and for the scientific support and cooperation with the national coordinators.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors alone are responsible for the views expressed in this publication, which do not necessarily represent the decisions, policy or views of the World Health Organization and the United Nations Environment Programme.
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Malisch, R. et al. (2023). Time Trends in Human Milk Derived from WHO- and UNEP-Coordinated Exposure Studies, Chapter 1: Polychlorinated Biphenyls, Polychlorinated Dibenzo-p-Dioxins and Polychlorinated Dibenzofurans. In: Malisch, R., Fürst, P., Šebková, K. (eds) Persistent Organic Pollutants in Human Milk. Springer, Cham. https://doi.org/10.1007/978-3-031-34087-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-34087-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34085-7
Online ISBN: 978-3-031-34087-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)