Abstract
The concentrations of polychlorinated naphthalenes (PCN) were determined in 40 pooled human milk samples from 39 countries covering all five of the United Nations regional groups. The samples were collected in the 2016–2019 exposure studies on persistent organic pollutants coordinated by the United Nations Environment Programme (UNEP).
The median concentration of the sum of 26 PCN was 55 pg/g lipid (range 27 pg/g to 170 pg/g). Human milk from European countries showed considerably higher levels than those found in milk from countries in the African, Asia-Pacific, and Latin America/Caribbean regions. The most abundant congeners were the congener pairs PCN 52/60 and PCN 66/67 (inseparable by conventional chromatography) and to a lesser extent PCN 28/36, PCN 42, PCN 46, PCN 48, PCN 59, and PCN 69.
Among other adverse biological effects, a critical response of many PCN congeners is dioxin-like toxicity. So, in addition to reporting concentrations of individual congeners, the toxic equivalents (TEQ) were also calculated in these samples, using two sets of relative effect potency (REP) values: a set that has been used in a number of human exposure studies and another set reported by Falandysz et al. (J Environ Sci Health, Part C: Environ Carcinogenesis Ecotoxicol Rev 32(3):239–272, 2014). The median PCN-TEQ concentration in human milk was 0.07 pg PCN-TEQ/g lipid (range 0.03 pg/g to 0.23 pg/g), when calculated using the human biomonitoring study REPs, and 0.03 pg PCN-TEQ/g lipid (range 0.01 pg/g to 0.10 pg/g), when calculated with other suggested REPs. The vast majority, about 90%, of this TEQ can be attributed to the PCN 66/67 congener pair. Individual REPs for PCN 66 and 67 from in vivo studies are quite different, but a chromatographic separation of these two congeners is not possible under routine GC conditions. Different approaches to estimate the uncertainties showed that the value of the REPs used is more important than the analytical problem to separate PCN 66 and PCN 67. PCN-TEQ based on the two sets of REPs differ approximately by a factor of 2.2, whereas the congener-specific determination was estimated to result in approximately 30% lower concentrations in comparison with the standard method.
The assessment of PCN 66 and PCN 67 in order to obtain confirmed TEF would be most important for calculations of the dioxin-like toxicity of PCN, followed by PCN 69. Minor contributions to PCN-TEQ concentrations in human milk come from PCN 52/60, PCN 64/68, PCN 70, and PCN 73.
On average, the contribution of PCN-TEQ to the cumulative TEQ (including the overall sum of toxic equivalents of PCDD, PCDF, and dioxin-like PCB [WHO2005-TEQ]) is between 1% and 2%, with a wider range of up to 5% for the 39 countries of this study. This is about an order of magnitude lower than the contribution of dioxin-like PCB to the cumulative TEQ (median 26%). In line with the observed higher total PCN concentrations, European countries also showed considerably higher levels of PCN-TEQ than found in the other regions. PCN-TEQ calculated with REPs used in human biomonitoring studies add on average about 2% to the cumulative TEQ of dioxin-like contaminants in Africa, the Asia-Pacific region, and Latin American and Caribbean countries and about 4% in European countries. The corresponding contribution of PCN-TEQ calculated using the other set would be 1% in non-European countries and 2% in European countries.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Human milk biomonitoring
- Stockholm Convention on Persistent Organic Pollutants
- Polychlorinated naphthalenes (PCN)
- Dioxin-like toxicity
- Toxic equivalents (TEQ)
- Global WHO/UNEP human milk studies
1 Introduction
Polychlorinated naphthalenes (PCN) were produced during much of the last century, principally from the 1930s to the 1980s. They were used in multiple applications such as adhesives, dielectrics, flame (fire) retardant, fuel additives, fungicides, impregnating agents, insecticides, lubricants, plasticizers, solvents, stabilizers, wood preservatives, etc. The combined total global production volumes of technical formulations were estimated to range from 150,000 to 400,000 tons (Falandysz 1998; Falandysz and Fernandes 2020). In addition to this industrial production, PCN are also formed and released unintentionally during combustion processes such as the incineration of waste, and during metal and cement production as well as during fires of any kind including large-scale bush and forest fires. The magnitude of such sources and implication for human exposure is largely unknown. They were also formed as by-products during the manufacture of other large-volume industrial chemicals such as polychlorinated biphenyls (PCB) (Taniyasu et al. 2003, 2005; Yamashita et al. 2000). The production, properties, applications, environmental distribution, contamination of food, occurrence in human tissues and other relevant background information on PCN have been extensively described in earlier reviews (Jakobsson and Asplund 2000; Falandysz 2003; Fernandes et al. 2017).
As chemically and physiologically stable substances, PCN are ubiquitous and persist in environmental and biological systems, including humans. They have been detected in significant quantities in sediments, biota and water and even from remote regions such as the Arctic (Bidleman et al. 2010). These compounds are also lipophilic, which results in their biomagnification in the food chain and bioaccumulation in fatty tissues of animals and humans. These properties together with concerns about their toxicity have resulted in them being classified as persistent organic pollutants (POPs). In order to protect human health and the environment, the Stockholm Convention on POPs has listed certain chemicals with the intention of eliminating production or restricting their release into the environment (UNEP 2001). In 2015, PCN were listed to Annexes A (Elimination) and C (Unintentional release) under this Convention (UNEP 2020). A draft guidance on preparing PCN inventories provides information on production and possible sources. Furthermore, it enables the collection of information for the development of effective strategies for the elimination of PCN and the environmentally sound management of products, stockpiles, and wastes containing PCN (UNEP 2017). All listed POPs, including PCN are subject to Article 16 of this Convention, which requires that they should be monitored in order to evaluate the effectiveness of the remedial actions applied. The analysis of these POPs in human milk as one of the core monitoring matrices has been recommended within the framework of the Global Monitoring Plan on POPs (GMP) (UNEP 2019).
Exposure to PCN reportedly provokes a number of toxic responses ranging from hepatotoxicity, neurotoxicity, and immune response suppression along with endocrine disruption (Fernandes et al. 2022a). Historically, these effects were seen in workers occupationally exposed to high level levels of PCN (Hayward 1998) which is now unlikely following the end of widescale PCN production and use. Some of these effects have been studied more recently in test animals (Kilanowicz et al. 2015; Klimczak et al. 2018; Stragierowicz et al. 2015, 2018) at doses of sub- to low-mg kg−1 of bodyweight. In current times, human exposure to PCN is likely to be lower and arise through non-occupational pathways (environmental and dietary intake) with more subtle and sensitive endpoints (Fernandes et al. 2022a). Exposure to some PCN congeners results in toxicological responses that are similar to 2,3,7,8-TCDD (dioxin-like toxicity) (Engwall et al. 1994; Villeneuve et al. 2000; Suzuki et al. 2020; Fernandes et al. 2022a). The most studied of these PCN congeners have the ability to bind with varying degrees of potency to the aryl hydrocarbon receptor (AhR) in line with their PCDD/PCDF analogs, the most potent of which include congeners such as 2,3,7,8-TetraCDD, 1,2,3,7,8-PentaCDD, etc. The preliminary stage of the biochemical mechanism that initiates many of the potent long-term toxic effects of these compounds on vertebrate species is through activation of the AhR (Denison et al. 2002; Falandysz et al. 2014; Fernandes et al. 2022b). Therefore, the inclusion of PCN in the Toxicity Equivalency Factor (TEF) concept for dioxin-like compounds has been suggested (Van den Berg et al. 2006).
The World Health Organization (WHO)- and United Nations Environment Programme (UNEP)-coordinated exposure studies on POPs in human milk are reviewed in the different parts of this compendium covering a variety of aspects of analysis, exposure, and potential adverse health effects for the breastfed infant. The general introduction (Part I) gives an overview of the WHO/UNEP-coordinated exposure studies performed between 1987 and 2019 (Malisch et al. 2023a), a review of human milk surveys on POPs (Fürst 2023) and a review of the Stockholm Convention on POPs and its implementation summarized by regional and global monitoring reports (Šebková 2023). Analytical aspects, including methods used for determination of PCN in samples of the WHO/UNEP-coordinated exposure studies and their validation (Schächtele et al. 2023), are presented in Part II. In various articles in Part III, the findings for chlorinated and brominated POPs between 2000 and 2019 are reported and discussed, including here for PCN. Part IV presents an assessment of time trends and a risk–benefit analysis for the breastfed infant deriving from dioxin-like compounds and Part V provides a summary of all findings and conclusions.
All substance-specific data of the WHO/UNEP-coordinated exposure studies are deposited at the Global Monitoring Plan Data Warehouse (GMP DWH), which can be publicly accessed. This serves as the source of information for the regional and global reports of the GMP and for the evaluation of the effectiveness of the Convention to eliminate or reduce emissions of selected POPs (Global Monitoring Plan Data Warehouse 2020).
In this article, the PCN results for 40 pooled samples from 39 countries collected between 2016 and 2019 are discussed.
2 General Aspects
2.1 Link to the General Introduction (Countries, UN Regions, Protocol, Samples)
An overview of the scope, protocols for collection of samples, expansion of analytes of interest over time in terms of inclusion in the Stockholm Convention, participation of countries with classification in UN regional groups and temporal differentiation are given in the general introduction in Part I (Malisch et al. 2023a). In brief, the collection of a number of individual samples and preparation of representative pooled samples in all rounds were supervised by a national coordinator in each country following the WHO/UNEP-standardized protocols. Equal aliquots of the individual samples collected within a country were combined to produce a composite, which was considered representative to provide data on the average levels of POPs for that country or a subpopulation at the time of sampling. The pooled samples were then sent to the WHO/UNEP Reference Laboratories for analysis.
40 pooled samples from 39 countries collected between 2016 and 2019 were analyzed for PCN. The detailed data for all samples is contained at the POPs GMP Data Warehouse and can be publicly retrieved (Global Monitoring Plan Data Warehouse 2020).
In accordance with the implementation of the GMP, parties report flexibly through one of the five United Nations regional organization groups. Therefore, countries are classified according to one of these five UN geopolitical groups: (1) African Group, (2) Asia-Pacific Group, (3) Eastern European Group, (4) Group of Latin American and Caribbean Countries (GRULAC), and (5) Western European and Others Group (WEOG). For participating countries and regional distribution, see Malisch et al. 2023a. It should be noted that these quantitative results are not intended to be used for the ranking of individual countries.
2.2 Analysis
There are theoretically 75 PCN congeners (mono-chlorinated to octa-chlorinated) and in practice all occur at varying concentrations in technical products or are formed during thermal reactions (Hanari et al. 2013, 2015; Horii et al. 2004; Ieda et al. 2011). The GMP guidance (UNEP 2019) does not specify any specific congeners for analysis yet (“congeners to be decided”). For the UNEP-coordinated human milk survey 2016–2019, a set of 26 PCN congeners (Table 1) were used for establishment and performance indication of the analytical method that was developed and used. These congeners were chosen based on the toxicological characteristics, reported levels of occurrence, congener patterns, and the availability of analytical standards at times of method development and validation.
A full description of the internally standardized method, which uses eight 13Carbon labeled PCN of the homologue groups TetraCN to OctaCN, and the results of the method validation have been presented earlier in Part II (Schächtele et al. 2023). In brief, samples were centrifuged and the upper cream layer was removed. After addition of sodium sulfate, the cream was extracted using hexane and purified on a multi-adsorbent column system on a DEXTechPlus sample preparation system using a standard silica column impregnated with sulfuric acid for fat degradation and thus for lipid separation. Purification and fractionation of the PCN from other contaminants such as PCB were achieved using alumina and activated carbon columns. The PCN congeners were measured on a high-resolution gas chromatograph coupled to a high-resolution mass spectrometer (HRGC/HRMS at a resolution of 10,000 at 5% peak height; Thermofisher DFS) using a DB-5 MS (60 m) column to separate the PCN congeners. Analytical confirmation measurement was carried out using GC-Orbitrap Q Exactive at a resolution of 60,000. All concentrations are reported on a lipid basis.
3 Results and Discussion
3.1 Sum of Measured PCN Congeners (∑ 26 PCN)
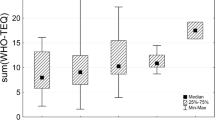
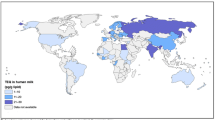
The median concentration of ∑26 PCN in 40 pooled human milk samples from 39 countries was 55 pg/g lipid (range 27 pg/g to 170 pg/g). The concentrations in samples from European countries were considerably higher than those found in the other regions included in this study (Africa, Asia-Pacific, and Latin America and Caribbean). The median concentration for Europe, at 152 pg/g, was three-fold higher than found in the other regions (Table 2). This higher PCN contamination in European human milk is reflected in the minimum concentration which at 86 pg/g is very close to the mean maximum level (93 pg/g) for the other three regions. This finding is not unexpected as the European region saw high levels of manufacture and use of PCN during the last century, as was reported earlier for North America (Hayward 1998; Falandysz et al. 2008; Noma et al. 2004). In the non-European countries included in this study (which do not include the North American region), the concentrations of ∑26 PCN were relatively low, ranging from 27 pg/g to 66 pg/g, apart from the Solomon Islands and Jamaica. Figures 1, 2, 3 and 4 illustrate the findings in countries of these regions.
3.2 PCN Patterns
Concurring with observations reported for human tissue samples as summarized in a review article (Fernandes et al. 2017), the most abundant congeners that were observed in the human milk samples were two penta-chlorinated and two hexa-chlorinated congeners, namely PCN 52/60 and PCN 66/67, and to a lesser extent, tetra-chlorinated congeners (PCN 28/36, PCN 42, PCN 46, and PCN 48), penta-chlorinated PCN 59 and hexa-chlorinated PCN 69. In Africa, the Pacific Islands and European countries, the abundance of PCN 52/60 is higher than that of PCN 66/67, which is in contrast to the Asian and Latin American countries that were included. Furthermore, in some countries of the Pacific Islands and Latin America and the Caribbean higher proportions of PCN 28/36 were found than in other countries/regions (Table 3; Figs. 5, 6, 7, 8, and 9). It is important to note that the patterns are at best an indication of the congener distribution in human milk for the different studied regions and may not be representative, as some countries where PCN were manufactured, e.g., UK, France, Italy, etc. (Hayward 1998; Falandysz et al. 2008; Noma et al. 2004) and may have seen higher levels of usage due to earlier industrialization, were not participants in this study.
Overall, the combined global data from these human milk WHO surveys indicate that PCN showing dioxin-like toxicity represent a significant part of the total amount of PCN in this matrix. Especially, 1,2,3,4,6,7-HexaCN/1,2,3,5,6,7-HexaCN (PCN 66/67) represents approximately 20–40% of the total amount of PCN. 2,3,6,7-TetraCN (PCN 48), a congener with lateral substitution like 2,3,7,8-TCDD, however smaller size and therefore lower AhR binding potency, is generally present between 1 and 4% of the total.
There may be other PCN congeners of toxicological interest such as 1,2,3,6,7-PentaCN (PCN 54) that was not included in the analysis. This congener resembles 1,2,3,7,8-PentaCDD that shows potent dioxin-like activity as described in Sect. 1 (introduction). PCN 54 was absent or found at extremely low concentrations in PCN technical mixtures (Hanari et al. 2013). A mass percent contribution of 0.05% in Halowax 1014 was found (Helm et al. 1999). It has not appeared to show significant presence in human serum samples from an industrial city in Eastern China when industrial technical products and industrial thermal processes are seen as a relevant pathway to human exposure (Jin et al. 2019). It was found in low concentrations when combustion related sources are dominant as observed in a study on pine needles across Poland, where the PCN 54 contribution was about 0.2% of the total PCN (as median; for comparison, the median of the PCN 66/67 contribution: 0.6%) (Orlikowska et al. 2009). These findings might have implications depending on possible regional PCN sources (e.g., technical mixtures or combustion processes), and it would be informative to know if PCN 54 actually occurs in human milk. Thus, for completion of the database for human risk assessment, PCN 54 could be considered in future monitoring, as well.
Furthermore, the effect of metabolism of some dietary PCN congeners in humans and higher order animals (Fernandes et al. 2017) is likely to result in further modification of the occurrence profiles as seen in human milk. The abundance of PCN 52/60 and PCN 66/67 could indicate a significant role of metabolism as these congeners do not contain two adjacent hydrogen atoms, which is well known to facilitate metabolism of PCB, PCDD, and PCDF. However, the abundance could also arise from selective retention in vertebrates including humans, eventually resulting in substantially higher exposure to these specific congeners as seen in human fat and milk (Kunisue et al. 2009; Pratt et al. 2013).
3.3 Dioxin-Like Properties
Dioxin-like compounds (DLC) comprise polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated dibenzofurans (PCDF), and certain polychlorinated biphenyls (PCB). Most of the most studied toxic and biological effects of these compounds are mediated through the aryl hydrocarbon receptor (AhR), a cytosolic receptor protein present in most vertebrate tissues. The AhR binds potently to a range of diverse compounds including contaminants such as 2,3,7,8–substituted PCDD/PCDF and some laterally substituted PCB. Different toxic potencies are expressed as Toxic Equivalency Factors (TEF) for DLC—a concept that allows for a combined expression of toxicity that represents all the relevant congeners that occur in a sample. Criteria for inclusion of a compound in the TEF concept are (1) structural similarity to PCDD/PCDF, (2) ability to bind to AhR; (3) ability to elicit AhR-mediated biochemical and toxic responses; (4) to be persistent and accumulate in the food chain (Van den Berg et al. 1998). The reported information on PCN shows an ability to meet all these criteria and there are a number of studies that report AhR binding and the relative potencies of this action (Engwall et al. 1994; Blankenship et al. 2000; Falandysz et al. 2014; Fernandes et al. 2017, 2022b). It is clear therefore that PCN should be considered in the TEF concept (Van den Berg et al. 2006; Fernandes et al. 2022b).
In terms of human exposure, the expression of toxicity-normalized concentrations for dietary PCN content, which mirrors the approach used for PCDD/PCDF and PCB, has a number of advantages. In general, a significant part of PCN toxicity is considered dioxin-like and therefore it allows an association and comparison with the toxic content arising from PCDD/PCDF and PCB. Moreover, it also allows a more holistic consideration of the total dioxin-like toxicity of, e.g., human milk (Fernandes et al. 2022b) as the effects of individual congeners are considered additive. Practically, for risk assessment and comparative purposes, the expression of the total dioxin-like toxicity as a single value (rather than a set of individual congener concentrations) allows more efficient handling and comparison of the data. In earlier periods, when there was very little occurrence data on PCN (or it was presented as homologue group concentrations) there was understandably a reluctance to apply a TEF approach to PCN. However, during the last two decades more data on the occurrence in food and human tissues has emerged including frequently individual congener concentrations.
Although the current volume of such studies may be insufficient to establish robust TEFs for PCN, relative potency values (REPs) can be used as an interim conservative approach for the cumulative risk assessment of PCN. The approach can also be used to determine the relative contribution of these PCN compared with PCDD/PCDF and PCB (COT 2009; Fernandes et al. 2010, 2017, 2022b).
3.3.1 Relative Potency of PCN
At present, there is one in vivo study available (Hooth et al. 2012). Therefore, most of these REPs were derived from in vitro studies. A review of in vitro and in silico REPs for selected individual PCN congeners derived from five studies (Hanberg et al. 1990; Blankenship et al. 2000; Villeneuve et al. 2000; Behnisch et al. 2003; Puzyn et al. 2007) shows a range of potencies (Fernandes et al. 2017) (Table 4). Another study on 42 PCN using DR-CALUX showed dioxin-like activities of 31 congeners. REPs determined in previous studies were comparable to REP values obtained in this study (Suzuki et al. 2020).
The choice of congeners covered in this study was based principally on the toxicological characteristics of individual PCN congeners and the levels and patterns of their occurrence in foods. Thus, this study included tetra- to octa-chlorinated compounds, and generally those that were reported to show the highest REP values, e.g., PCN 66, 67, 70, etc. In order to provide an indication of the dioxin-like toxicity arising from the presence of PCN, the data were calculated as toxic equivalents (TEQ), computed using REPs (1) used for human exposure studies (Falandysz et al. 2019; 2020; Fernandes et al. 2010, 2011, 2017, 2022b; Pratt et al. 2013; Zhihua et al. 2019; Zacs et al. 2021) and (2) other suggested REPs (Falandysz et al. 2014), which were derived as reasonable approximation with regard to the wide range of in vitro and in silico REPs as listed in Table 4 and the in vivo REPs for PCN 66 and 67 (Hooth et al. 2012) (Table 5).
As these REP for PCN have been derived from mostly in vitro studies, the question arises as to which extent these REPs are actually realistic for in vivo conditions, especially in those situations when prolonged exposure takes places, e.g., a breastfed infant. At present only one in vivo study has been done which included direct comparison with 2,3,7,8-TCDD and covered biological as well as toxicological endpoints after a two-week oral exposure (Hooth et al. 2012). This study included both PCN 66 and 67 that both are structural analogs with 1,2,3,4,7,8 and 1,2,3,6,7,8-HxCDD that both have a relatively high WHO TEF of 0.1 (Van den Berg et al. 2006). In view of the high contribution of PCN 66 and 67 to the total amount of PCN, it is relevant to determine if the REPs based on in vitro experiments and listed in Table 5 are close to or similar to those obtained in vivo. The in vivo study with PCN 66 and 67 determined REPs of, respectively, 0.0015–0.0072 and 0.00029–0.00067 for CYP1A1, CYP1A2, and thymic atrophy.
Both sets of REPs, those used in human exposure studies and those proposed by Falandysz et al. (2014) were used for the TEQ calculations of the WHO/UNEP human milk samples. For PCN 66, the REPs of 0.004, respectively, 0.002 are in comparison with the results from the in vivo study in good agreement. However, the REP for PCN 67 used in human exposure studies is approximately one order of magnitude higher than that obtained in the in vivo study (Hooth et al. 2012), the proposed REP half of that.
In vitro REPs for PCN may overestimate the actual in vivo effect which is in line with similar comparisons made for REPs of some PCDD/PCDF and dl-PCB. The most likely reason for this discrepancy is caused by the lack of metabolism in vitro (Van den Berg et al. 1998, 2006). This may lead to overestimation of the calculated PCN-TEQ in human milk depending on the extent of the contribution of PCN 67 to the summed PCN-TEQ.
Therefore, the individual contribution of PCN 66 and PCN 67 to TEQ could be calculated by use of different REPs for these two congeners with adjustments for PCN 67 closer to the range determined in the in vivo study, if the individual concentrations were known. However, a chromatographic separation of PCN 66 and 67 is not possible under routine GC conditions, and these two congeners can be determined routinely only as the sum of the two congeners (see Subsection 3.3.3 in the following). The relative proportions of PCN 66 and PCN 67 in the milk samples are therefore not known, so the precautionary principle was applied, using the higher REP to allow a higher margin of safety, as seen similarly in other PCN studies on food and human milk (Pratt et al. 2013; Fernandes et al. 2017; Zacs et al. 2021). While this is conservative, it may also be a health protective approach. Therefore, for the sum parameter of the co-eluting pair, the REP applied to PCN 66 is also applied to PCN 67.
As conclusion, in addition to the discussion of scientifically sound REPs for these congeners, there is the analytical issue of determining individual concentrations for PCN 66 and 67 in samples. Only a special GC column with considerable limitations under routine conditions could separate PCN 66 and 67 (Helm 2002; see Subsection 3.3.3 in the following) and was used for determination of the mass percent contribution of these congeners in Halowax 1014: PCN 66 contributed 0.47%, PCN 67 0.29% (determined as single congeners), resulting in a ratio of 1.62 to 1. Determined by negative ion mass spectrometry, the peak area ratio in Halowax 1014 was 1.87, in Halowax 1013 1.43 and in Halowax 1099 1.36, in air of the Lake Ontario 1.3 (Helm et al. 1999). Thus, about 55% to 60% of the sum of PCN 66 and 67 came from PCN 66. According to our best of knowledge, these are the only available quantitative data for the ratio between PCN 66 and 67. Both congeners do not contain two adjacent hydrogen atoms, which is well known to facilitate metabolism in PCB, PCDD, and PCDF. Therefore, it can be assumed that the ratio is not changed due to metabolic processes during bioaccumulation to human matrices. As a result with regard to the first step (determination of individual concentrations) for a more precise estimation with the aim of a congener-specific assessment, it could be assumed that about 55% to 60% of the PCN 66/67 concentration comes from PCN 66. Then, as second step, these estimated individual concentrations could be multiplied by the REPs for PCN 66 as used in human exposure studies or as proposed by Falandysz et al. (2014), and by an REP of 0.0005 for PCN 67, which is in close agreement with the in vivo study. For more details and comparison of results of different approaches, see Subsection 3.3.3.
These PCN-TEQ values provide an interim indication of the PCN-associated dioxin-like toxicity in the human milk samples of the 2016–2019 study. To the best of our knowledge these analyses of PCN in human milk and their estimated contribution to dioxin-like toxicity are the first study covering this aspect so thoroughly.
3.3.2 Calculation of PCN-TEQ, Part 1: Lower–Upper Bound Results; Co-Eluting Congeners PCN 52/60 and 64/68
Different imputation approaches for handling “non-detects” (more exactly: not quantified congeners) in PCDD/PCDF analysis were tested using the limit of detection (LOD), among them: (1) calculation of the contribution of each non-detected congener to the TEQ as zero (lower bound concentrations); (2) calculation of the contribution of each non-detected congener to the TEQ as the limit of detection (upper bound concentrations); (3) calculation of the contribution of each non-detected congener to the TEQ as half of the limit of detection (middle bound concentrations) (Hoogerbrugge and Liem 2000). Later, these proposed definitions of lower and upper bound concentrations were used as pillars of the analytical criteria, but they were based on the limit of quantification (LOQ) rather than the LOD: An important criterion for assessing the reliability of estimated TEQ that are derived from PCDD/PCDF and dioxin-like PCB concentrations is the difference between the lower bound and upper bound TEQ result (Malisch et al. 2023b, Malisch and Schächtele 2023). As a measure of satisfactory analytical quality assurance and quality control (QA/QC), this difference should be less than 20% (UNEP 2019).
The median of the differences between lower and upper bound PCN-TEQ in all 40 samples was 0.3%. The range was between 0% to 2%, if REPs as used for human biomonitoring were applied, and between 0% and 3%, if other suggested REPs were used (Table 6). Therefore, all samples analyzed in this study fulfilled this QA/QC criterion. These differences were considered negligible. Thus, as for PCDD/PCDF and dioxin-like PCB, only the upper bound PCN-TEQ are used for discussion of the results.
With regard to the REPs used in human exposure studies and the other suggested REPs as listed in Table 5, two co-eluting pairs had different REPs for the individual PCN: (1) PCN 52/60 applying REPs used in human biomonitoring; (2) PCN 64/68 using other suggested REPs. The results discussed in this article are based on the assumption that, where congeners co-eluted, the content comprised only the congener with the higher REP. Use of the higher REP for the co-eluting congeners increases the PCN-TEQ by 2.1% as median (range 0.9% to 3.6%), if 0.0001 for PCN 60 is used instead of 0.000025 for PCN 52; by 0.3% as median (range 0.1% to 4.3%), if 0.0005 is used for PCN 68 instead of 0.00001 for PCN 64 (Table 6). Also these differences are considered negligible.
3.3.3 Calculation of PCN-TEQ, Part 2: Co-Eluting Congeners PCN 66/67
A problem for calculation of the individual contribution of PCN 66 and 67 to PCN-TEQ is the lack of the individual concentrations for these congeners. Chromatographic separation of PCN 66 and 67 is not possible under routine GC conditions: For this study, six different kinds of GC columns as commonly used for confirmation analyses of pesticides or contaminants were not able to separate this co-eluting pair. Therefore, for routine analysis only the sum of the two congeners was determined. This was also the only determinable parameter for PCN 66 and 67 in an interlaboratory study on PCN performed by the EU Reference Laboratory for POPs with 10 laboratories participating in 2021 (Schächtele et al. 2023).
Nine different GC columns were checked for separation of otherwise co-eluting PCB and PCN congeners. The Rxi-17SilMS demonstrated the most drastic difference in PCB selectivity and, to a lesser extent, PCN when compared with the other eight columns and could work as a confirmatory column or as a second dimension column for GC × GC separations. However, the very close retention times (41.79 min for PCN 66, 41.81 min for PCN 67) show that in practice these two congeners could not be separated (Stultz and Dorman 2020).
Only a special GC column (Rt-βDEXcs) could separate PCN 66 and 67, however, with considerable limitations under routine conditions (long GC run times of about 100 min, high column bleeding and therefore increased maintenance of the MS ion source) (Helm 2002). This column was used for determination of the mass percent contribution of these congeners in technical products and air (Helm et al. 1999). Furthermore, this special GC column was used for separation of closely eluting PCN congeners including PCN 66 and 67 in technical mixtures by two-dimensional GC/quadrupole mass spectrometric detection (GC × GC/qMS) on Rt-βDEXcst and DB-Wax phases. However, no quantitative data were given on the composition, and neither PCN 66 nor 67 was shown in the figures on relative abundance (Hanari et al. 2013). As a conclusion, the determination of the concentration of the individual congeners PCN 66 and 67 in human milk is not possible under routine conditions and requires research for development of a practical method with sufficient sensitivity and its validation. Therefore, as derived above (Subsection 3.3.1) the assumption that about 55% to 60% of the PCN 66/67 concentration comes from PCN 66 is presently the most practical option and was used for estimation of specific PCN 66 and 67 concentrations, to be multiplied by the respective REPs for calculation of the separate contributions to PCN-TEQ. Then, 0.0005 was used as REP for PCN 67 in good agreement with the in vivo study.
As a result, the PCN-TEQ calculated by the two sets of REPs (used in human exposure studies with identical REPs for PCN 66 and 67 [0.004 for both congeners] or those proposed by Falandysz et al. 2014 also with identical REPs for PCN 66 and 67 [0.002 for both congeners]) can be compared with the PCN-TEQ based on these specifically estimated contributions of PCN 66 and 67.
Furthermore, a kind of «middle bound» TEQ can be calculated. The lower/middle/upper bound concept for TEQ calculations (see Subsection 3.3.2) was developed for handling of «non-detects» and thus a question related to the analytical sensitivity and the LOQ. As conservative approach and for protection of the consumer, maximum levels for TEQ deriving from PCDD/PCDF and dioxin-like PCB are based on upper bound concentrations, and the analytical criteria with acceptable differences between lower and upper bound results below 20% make sure that reliable analytical results are obtained. In contrast, at times before introduction of these criteria, relatively large differences between lower and upper bound values were seen. Then, for risk assessment, the application of the upper bound concentrations leads to an overestimation of the intake, the application of the lower bound concentrations to an underestimation of the intake. For these cases, the application of a «middle bound» TEQ concentration was recommended by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (Canady et al. 2002). However, the PCN 66/67 issue and possible consequences for over- or underestimation of TEQ is not a question of handling of LOQs (all results were well above LOQ), but of use of too high or too low REPs. Therefore, in analogy, a «middle bound» TEQ was calculated as mean of the lower and upper range for both standard procedures (applying REPs used in human exposure studies or those proposed by Falandysz et al. 2014) by multiplying the sum parameter PCN 66/67 (1) with the lowest REP derived from in vivo studies (0.00029) for the lower range, and (2) with the highest REP derived from in vivo studies (0.0072) for the upper range.
Table 7 shows the results of these six approaches to calculate PCN-TEQ. The key question was whether the PCN-TEQ calculated by the standard methods would be too high and their contribution to the total amount of TEQ in human milk overestimated, depending on the extent of the contribution of PCN 67 to PCN-TEQ as a result of its concentration and the applied REP. If for calculation of PCN-TEQ the REPs used in human exposure studies are applied, the results of the «middle bound approach» are similar to the standard method. The PCN-TEQ based on the congener-specific estimations are about 30% lower than the standard method. PCN-TEQ calculated by REPs as suggested by Falandysz et al. (2014) are about 60% lower than by REPs used in human exposure studies. The PCN-TEQ based on the congener-specific estimations are again about 30% lower than the standard method. The «middle bound approach» leads to higher PCN-TEQ values, closer to the values based on REPs used in human exposure studies.
As a conclusion, harmonization of the value of the REPs used is more important than the separate determination of PCN 66 and 67. PCN-TEQ based on the two sets of REPs differ by a factor of 2.2 (as median of the factors for the individual country results obtained by the standard method), whereas the congener-specific determination is expected to result in differences of approximately 30% to the standard method. These uncertainties can only be reduced by (1) assessment of the wide range of reported REPs and assignment of one set of proposed REPs or TEFs, at least for PCN 66 and 67 as most important congeners, and (2) analytical research and method development allowing the determination of PCN 66 and 67 in human milk samples separately.
3.4 PCN-TEQ Results
The median PCN-TEQ concentration in 40 pooled human milk samples from 39 countries was 0.07 pg PCN-TEQ/g lipid (range 0.03 pg/g to 0.23 pg/g), if calculated with the REPs as applied in human biomonitoring studies, and 0.03 pg PCN-TEQ/g lipid (range 0.01 pg/g to 0.10 pg/g), if calculated with other suggested REPs. Human milk from European countries had considerably higher TEQ levels than those found in the African region, the Asia-Pacific region, and the Latin American and Caribbean region (Table 8). Figures 10, 11, 12, and 13 illustrate the findings in countries of these regions. In all regions and all countries, the PCN-TEQ concentrations were higher by a factor of about 2.2 when calculated by REPs as applied in human biomonitoring studies in comparison with other suggested REPs.
Results of the 2016–2019 survey for PCN-TEQ concentrations in human milk in countries from Africa (pg PCN-TEQ/g lipid), if calculated with (1) REPs as used in human biomonitoring studies and (2) other suggested REPs (Falandysz et al. 2014)
Results of the 2016–2019 survey for PCN-TEQ concentrations in human milk in countries from the Asia-Pacific region (pg PCN-TEQ/g lipid), if calculated with (1) REPs as used in human biomonitoring studies and (2) other suggested REPs (Falandysz et al. 2014)
Results of the 2016–2019 survey for PCN-TEQ concentrations in human milk in countries from Latin American and Caribbean countries (pg PCN-TEQ/g lipid), if calculated with (1) REPs as used in human biomonitoring studies and (2) other suggested REPs (Falandysz et al. 2014)
Results of the 2016–2019 survey for PCN-TEQ concentrations in human milk in countries from European countries (pg PCN-TEQ/g lipid), if calculated with (1) REPs as used in human biomonitoring studies and (2) other suggested REPs (Falandysz et al. 2014)
Among the PCN congeners included in this study, by far the highest contribution (90%) to the dioxin-like toxicity was attributed to PCN 66/67. This contribution is a result of relatively high concentrations within the group of 26 PCN (see Subsection 3.2 «PCN patterns») combined with the relatively high REPs used, notwithstanding whether the values used were those taken from the human biomonitoring studies or from other suggested REPs (Falandysz et al. 2014). Differences between these two REP groups for PCN 69 result in differences between the contribution to PCN-TEQ (median 7%, maximum 37%, if REPs used for human biomonitoring studies are applied; median 1%, maximum 7%, if the other suggested REPs are applied). Although PCN 52/60 occurs at relatively high levels in all samples the low REP value results in a contribution of about 3% (range 1% to 5%) to PCN-TEQ, when REPs used for human biomonitoring studies are applied, and about 0.1%, if other suggested REPs (Falandysz et al. 2014) are applied. Other congeners with minor TEQ contributions were PCN 64/68, PCN 70, and PCN 73 (Figs. 14 and 15). As a result, the assessment of PCN 66 and PCN 67 in order to obtain confirmed TEF would be most important for calculations of the dioxin-like toxicity of PCN, followed by PCN 69.
Contribution (%) of PCN congeners to PCN-TEQ calculated with other suggested REPs (Falandysz et al. 2014)
3.5 Contribution of PCN-TEQ to the Cumulative TEQ (Including the Overall Sum of PCDD, PCDF, and Dioxin-like PCB Toxic Equivalents [WHO2005-TEQ])
Finally, the contribution of PCN to the cumulative TEQ (including the overall sum of toxic equivalents with PCDD, PCDF, and dioxin-like PCB [WHO-PCDD/PCDF-PCB-TEQ = WHO2005-TEQ, see Malisch et al. 2023b]) is of interest. In Subsection 3.3.1, the problem to determine exactly the contribution of PCN 66 and 67 to PCN-TEQ was discussed, and in Subsection 3.3.3 options to answer the key question whether the PCN-TEQ calculated by the standard methods would be too high and their contribution to the total amount of TEQ in human milk overestimated, depending on the extent of the contribution of PCN 67 to PCN-TEQ as a result of its concentration and the applied REP. The results of the six approaches discussed in Subsection 3.3.3 were assessed for their contribution to the cumulative TEQ as sum of WHO2005-TEQ and PCN-TEQ. On average, the contribution of PCN-TEQ to the cumulative TEQ is between 1% and 2% calculated by these six approaches, with a wider range up to 5% for the 39 countries of this study within each of these approaches (Table 9).
This is about an order of magnitude lower than the contribution of dioxin-like PCB to the cumulated TEQ (median 26.4%, range 8.1% to 54.9%). These differences between the contribution of PCN and DL-PCB reflect the differences between estimated production rates between PCN (estimated range 150,000 to 400,000 tons (Falandysz 1998, Falandysz and Fernandes 2020)) and PCB (more than one million tons of technical PCB mixtures (Breivik et al. 2002; EFSA 2005)).
PCN-TEQ calculated by the standard method with REPs used in human biomonitoring studies add on average in Africa, the Asia-Pacific region, and Latin American and Caribbean countries, about 2% to the cumulative TEQ, and in European countries about 4%. If results are calculated by the standard method using the other suggested REPs (Falandysz et al. 2014), the average contribution in Africa, the Asia-Pacific region, and Latin American and Caribbean countries is about 1% and in European countries about 2% (Table 10).
References
Behnisch PA, Hosoe K, Sakai S (2003) Brominated dioxin-like compounds: in vitro assessment in comparison to classical dioxin-like compounds and other polyaromatic compounds. Environ Int 29:861–877
Bidleman T, Helm P, Braune B, Gabrielsen G (2010) Polychlorinated naphthalenes in polar environments - a review. Sci Total Environ 408(15):2919–2935
Blankenship A, Kannan K, Villalobos S, Villeneuve D, Falandysz J, Imagawa T, Jakobsson E, Giesy JP (2000) Relative potencies of Halowax mixtures and individual polychlorinated naphthalenes (PCNs) to induce ah receptor-mediated responses in the rat hepatoma H4IIE-Luc cell bioassay. Environ Sci Technol 34(15):3153–3158
Breivik K, Sweetman A, Pacyna JM, Jones KC (2002) Towards a global historical emission inventory for selected PCB congeners — a mass balance approach: 1. Global production and consumption. Sci Total Environ 290(1–3):181–198. https://doi.org/10.1016/S0048-9697(01)01075-0
Canady R, Crump K, Feeley M, Freujer J, Kogevinas M, Malisch R, Verger P, Wilson J, Zeilmaker M (2002) Polychlorinated dibenzodioxins, polychlorinated dibenzofurans and coplanar biphenyls. WHO Food Additives Series 48 “Safety evaluation of certain food additives and contaminants, prepared by the fifty-seventh meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). IPCS (International Programme on Chemical Safety), World Health Organization, Geneva, p. 451–664
COT (Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment) (2009) Polychlorinated naphthalenes and chlorinated paraffins in Food. https://cot.food.gov.uk/sites/default/files/cot/tox200902pcnscp.pdf
Denison MS, Pandini A, Nagy SR, Baldwin EP, Bonati L (2002) Ligand binding and activation of the ah receptor. Chem Int 141(1–2):3–24
EFSA (2005) Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the presence of non dioxin-like polychlorinated biphenyls (PCB) in feed and food
Engwall M, Brundstrom B, Jakobsson E (1994) Ethoxyresorufin O-deethylase (EROD) and arylhydrocarbon hydroxylase (AHH) – inducing potency and lethality of chlorinated naphthalenes in chicken (Gallus domesticus) and eider duck (Somateria mollissima) embryos. Arch Toxicol 68:37–42
Falandysz J (1998) Polychlorinated naphthalenes: an environmental update. Environ Pollut 101:77–90
Falandysz J (2003) Chloronaphthalenes as food-chain contaminants: a review. Food Add Contam 20:995–1014
Falandysz J, Fernandes A (2020) Compositional profiles, persistency and toxicity of polychlorinated naphthalene (PCN) congeners in edible cod liver products from 1972–2017. Environ Pollut 260:114035
Falandysz J, Chudzyński K, Takekuma M, Yamamoto T, Noma Y, Hanari N, Yamashita N (2008) Multivariate analysis of identity of imported technical PCN formulation. J Environ Sci Health A Toxic Hazard Subst Environ Eng 43(12):1381–1390
Falandysz J, Fernandes A, Gregoraszczuk E, Rose M (2014) The toxicological effects of halogenated naphthalenes: a review of aryl-hydrocarbon receptor-mediated (dioxin-like) relative potency factors. J Environ Sci Health, Part C: Environ Carcinogenesis Ecotoxicol Rev 32(3):239–272. https://doi.org/10.1080/10590501.2014.938945
Falandysz J, Smith F, Panton S, Fernandes A (2019) A retrospective investigation into the occurrence and human exposure to polychlorinated naphthalenes (PCNs), dibenzo-p-dioxins and furans (PCDD/fs) and PCBs through cod liver products (1972–2017). Chemosphere 231:240–248
Fernandes A, Mortimer D, Gem M, Smith F, Rose M, Panton S, Carr M (2010) Polychlorinated naphthalenes (PCNs): congener specific analysis, occurrence in food and dietary exposure in the UK. Environ Sci Technol 44:3533–3538
Fernandes A, Tlustos C, Rose M, Smith F, Carr M, Panton S (2011) Polychlorinated naphthalenes (PCNs) in Irish foods: occurrence and human exposure. Chemosphere 85:322–328
Fernandes A, Rose M, Falandysz J (2017) Polychlorinated naphthalenes (PCNs) in food and humans (review article). Environ Int 104:1–13
Fernandes A, Falandysz J, Yuan B (2022a) Widening knowledge horizons on legacy POPs: chlorinated paraffins and polychlorinated naphthalenes. Chemosphere 289:133131
Fernandes A, Kilanowicz A, Stragierowicz J, Klimczak M, Falandysz J (2022b) The toxicological profile of polychlorinated naphthalenes (PCNs). Sci Total Environ 837:155764
Fürst P (2023) Human milk surveys on persistent organic pollutants from a historical perspective. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Global Monitoring Plan Data Warehouse (2020) Hůlek, R., Borůvková, J., Kalina, J., Bednářová, Z., Šebková, K., Hruban, T., Novotný, V., Ismael, M., Klánová J. Global monitoring plan data warehouse of the stockholm convention on persistent organic pollutants: visualisation platform and on-line tool for the analysis of global levels of POPs in air, water, breast milk and blood [online]. Masaryk University, 2020. Available from: https://www.pops-gmp.org
Hanari N, Falandysz J, Nakano T, Petrick G, Yand Amashita N (2013) Separation of closely eluting chloronaphthalene congeners by two-dimensional gas chromatography/quadrupole mass spectrometry: an advanced tool in the study and risk analysis of dioxin-like chloronaphthalenes. J Chromatogr A 1301:209–214
Hanari N, Falandysz J, Petrick G, Yamashita N (2015) Congener-specific determination of mono- to trichlorinated naphthalenes by comprehensive 2D gas chromatography/mass spectrometry. Pacifichem. 2015 International chemical congress of Pacific Basin societies, December 15–20, 2015 Honolulu, Hawaii, USA. Poster 1207
Hanberg A, Waern F, Asplund L, Haglund E, Safe S (1990) Swedish dioxin survey: determination of 2,3,7,8-TCDD toxic equivalent factors for some polychlorinated biphenyls and naphthalenes using biological tests. Chemosphere 20:1161–1164
Hayward D (1998) Identification of bioaccumulating polychlorinated naphthalenes and their toxicological significance. Environ Res 76:1–18
Helm PA (2002) The influence of sources, source regions, and fate and transport process on the occurrence of polychlorinated naphthalenes and coplanar polychlorinated biphenyls in urban and arctic environments. Thesis, University of Toronto
Helm PA, Jantunen LMM, Bidleman TF, Dorman FL (1999) Complete separation of isomeric penta- and hexachloronaphthalenes by capillary gas chromatography. J High Resol Chromatogr 22(11):639–643
Hoogerbrugge R, Liem AKD (2000) How to handle non-detects? Organohalogen Compd 45:13–16
Hooth MJ, Nyska A, Fomby LM, Vasconcelos DY, Vallant M, DeVito MJ, Walker NJ (2012) Repeated dose toxicity and relative potency of 1,2,3,4,6,7-hexachloronaphthalene (PCN 66) 1,2,3,5,6,7-hexachloronaphthalene (PCN 67) compared to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) for induction of CYP1A1, CYP1A2 and thymic atrophy in female Harlan Sprague-Dawley rats. Toxicology 301(1–3):85–93
Horii Y, Falandysz J, Hanari N, Rostkowski P, Puzyn T, Okada M, Amano K, Naya T, Taniyasu S, Yamashita N (2004) Concentrations and fluxes of chloronaphthalenes in sediments from the lake Kitaura in Japan in recent 15 centuries. J Environ Sci Health A 39:587–609
Ieda T, Ochiai N, Miyawaki T, Ohurac T, Horii Y (2011) Environmental analysis of chlorinated and brominated polycyclic aromatic hydrcarbons by comprehensive two-dimensional gas chromatography coupled to high-resolution time-of-flight mass spectrometry. J Chromatogr A 1218:3224–3232
Jakobsson E, Asplund L (2000) Polychlorinated napthalenes (PCNs). In: Paasivirta J, Hutzinger O (eds) Chapter 5 in the handbook of environmental chemistry, vol 3 Part K, new types of persistent halogenated compounds. Springer, Berlin
Jin J, Wang S, Hu J, Wu J, Li M, Wang Y, Jin J (2019) Polychlorinated naphthalenes in human serum samples from an industrial city in eastern China: levels, sources, and sex difference. Ecotoxicol Environ Saf 177:86–92
Kilanowicz A, Czech E, Plewka D, Wiaderkiewicz A, Sitarek K (2015) Developmental toxicity of hexachloronaphthalene in Wistar rats. A role of CYP1A1 expression. Reprod Toxicol 58:93–103
Klimczak M, Daragó A, Bruchajzer E, Domeradzka-Gajda K, Stepnik M, Kuzajska K, Kilanowicz A (2018) The effects of hexachloronaphthalene on selected parameters of heme biosynthesis and systemic toxicity in female Wistar rats after 90-day oral exposure. Environ Toxicol 33(6):695–705
Kunisue T, Johnson-Restrepo B, Hilker D, Aldous K, Kannan K (2009) Polychlorinated naphthalenes in human adipose tissue from New York, USA. Environ, Poll 157:910–915
Malisch R, Schächtele A (2023) Analysis and quality control of WHO- and UNEP-coordinated human milk studies 2016-2019 - polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part II)
Malisch R, Malisch K, van Leeuwen FXR, Moy G, Tritscher A, Witt A, Alvarez J (2023a) Overview of the WHO and UNEP-coordinated exposure studies on persistent organic pollutants in human milk and link to the Stockholm convention. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Malisch R, Schächtele A, van Leeuwen FXR, Moy G, Tritscher A (2023b) WHO- and UNEP-coordinated exposure studies 2000-2019: findings of polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
Noma Y, Yamamoto T, Sakai S (2004) Congener-specific composition of polychlorinated naphthalenes, coplanar PCBs, dibenzo-p-dioxins and dibenzofurans in the Halowax series. Environ Sci Technol 38:1675–1680
Orlikowska A, Hanari N, Wyrzykowska B, Bochentin I, Horii Y, Yamashita N, Falandysz J (2009) Airborne chloronaphthalenes in scots pine needles of Poland. Chemosphere 75:1196–1205
Pratt I, Anderson W, Crowley D, Daly S, Evans R, Keane D, Fernandes A, Fitzgerald M, Geary M, Morrison J, Reilly A, Tlustos C (2013) Brominated and fluorinated organic pollutants in the breast milk of first-time Irish mothers: is there a relationship to levels in food? Food Addit Contam 30(10):1788–1798
Puzyn T, Falandysz J, Jones PD, Giesy JP (2007) Quantitative structure – activity relationships to predict of relative in vitro potencies (REPs) for chloronaphthalenes. J Environ Sci Health A Toxic Hazard Subst Environ Eng 42(5):573–590
Schächtele A, Hardebusch B, Krätschmer K, Tschiggfrei K, Zwickel T, Malisch R (2023) Analysis and quality control of WHO- and UNEP-coordinated exposure studies 2000-2019 - polybrominated diphenylethers, hexabromocyclododecane, chlorinated paraffins and polychlorinated naphthalenes. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part II)
Šebková K (2023) The Stockholm convention and its implementation by regional and global monitoring reports. In: persistent organic pollutants in human milk. In: Malisch R, Fürst P, Šebková K (eds). Springer, Cham (in this volume, Part I)
Stragierowicz J, Daragó A, Klimczak M, Galoch A, Duda-Szymańska J, Skrzypińska-Gawrysiak M, Kilanowicz A (2015) Histopathological changes in rat liver after exposure to polychlorinated naphthalenes - complementary research. Bromat Chem Toksykol 4:773–782
Stragierowicz J et al (2018) Hexachloronaphthalene (HxCN) as a potential endocrine disruptor in female rats. Environ Pollut 243:1026–1035
Stultz C, Dorman FL (2020) Characterization of 9 gas chromatography columns by linear and lee retention indices for polychlorinated biphenyls and polychlorinated naphthalenes. Separations 2020(7):38. https://doi.org/10.3390/separations7030038
Suzuki G, Michinaka C, Matsukami K, Noma Y, Kajiwara N (2020) Validity of using a relative potency factor approach for the risk management of dioxin-like polychlorinated naphthalenes. Chemosphere 244:125448
Taniyasu S, Kannan K, Holoubek I, Ansorgova A, Horii Y, Hanari N, Yamashita N, Aldous KM (2003) Isomer-specific analysis of chlorinated biphenyls, naphthalenes and dibenzofurans in Delor: polychlorinated biphenyl preparations from the former Czechoslovakia. Environ Pollut 126:169–178
Taniyasu S, Falandysz J, Świętojańska A, Flisak M, Horii Y, Hanari N, Yamashita N (2005) Clophen a 60 content of CBs, CNs, CDFs and CDDs after 2D-HPLC plus HRGC/LRMS and HRGC/HRMS separation and quantification. J Environ Sci Health A Toxic Hazard Subst Environ Eng 40:43–61
UNEP (2001). Stockholm convention on Persistent Organic Pollutants (POPs), text and annexes. http://chm.pops.int
UNEP (2017). Draft guidance on preparing inventories for polychlorinated naphthalenes. UNEP/POPS/COP.8/INF/19
UNEP (2019). Guidance on the global monitoring plan for persistent organic pollutants. UNEP/POPS/COP.9/INF/36. http://chm.pops.int/TheConvention/ConferenceoftheParties/Meetings/COP9/tabid/7521/Default.aspx
UNEP (2020) All POPs listed in the Stockholm convention. http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx
Van den Berg M, Birnbaum L, Bosveld ATC, Brunström B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, Kubiak T, Larsen JC, van Leeuwen FXR, Liem AKD, Nolt C, Peterson RE, Poellinger L, Safe S, Schrenk D, Tillitt D, Tysklind M, Younes M, Waern F, Zacharewski T (1998) Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 106:775–792
Van den Berg M, Birnbaum L, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson E (2006) The 2005 World Health Organization re-evaluation of human and mammalian toxic equivalency factors (TEFs) for dioxins and dioxin-like compounds. Toxicol Sci 93(2):223–241
Villeneuve DL, Khim JS, Kannan K, Falandysz J, Blankenship AL, Nikiforov V, Giesy JP (2000) Relative potencies of individual polychlorinated naphthalenes to induce dioxin-like response in fish and mammalian in vitro bioassays. Arch Environ Contam Toxicol 39:273–281
Yamashita N, Kannan K, Imagawa T, Miyazaki A, Giesy JP (2000) Concentrations and profiles of polychlorinated naphthalene congeners in eighteen technical polychlorinated biphenyl preparations. Environ Sci Technol 34:4236–4241
Zacs D, Perkons I, Pasecnaja E, Bartkevics V (2021) Polychlorinated naphthalenes (PCNs) in food products in Latvia: congener-specific analysis, occurrence, and dietary exposure of the general population. Chemosphere 264:128460
Zhihua L, Gong Y, Holmes M, Pan X, Xu Y, Zou X, Fernandes AR (2019) Geospatial visualisation of food contaminant distributions: polychlorinated naphthalenes (PCNs), potentially toxic elements (PTEs) and aflatoxins. Chemosphere 230:559–566
Acknowledgments
This publication was developed in the framework of the projects titled “Implementation of the POPs Monitoring Plan in the Asian Region” and “Continuing regional Support for the POPs Global Monitoring Plan under the Stockholm Convention in the Africa, Pacific and Latin-American and Caribbean Region”, funded by the Global Environment Facility and in close collaboration with and support of CVUA Freiburg.
The worldwide implementation of the Global Monitoring Plan for POPs, including that of the UNEP/WHO global human milk studies, is made possible thanks to the generous contributions to the Stockholm Convention Voluntary Trust Fund by the Governments of Japan, Norway, and Sweden and through the European Union’s Global Public Goods and Challenges Programme (GPGC). Further, the substantial contributions made by the Global Environment Facility to support POPs monitoring activities in regions implemented by UNEP, in close collaboration with WHO, particularly for the global human milk surveys, are greatly appreciated.
The authors express their gratitude to the National Coordinators of the WHO- and UNEP-coordinated exposure studies for their excellent work to collect the human milk samples and to prepare and send the pooled samples to the WHO/UNEP Reference Laboratories, which included great efforts to plan and implement the national studies with the assistance of the health, environment, laboratory, and administrative staff. The continuous exchange of information between the National Coordinators and WHO, UNEP and the Reference Laboratories was an important aspect for the successful organization of these studies on a global level.
We also thank the team at CVUA Freiburg for the performance of the analyses of the POPs presented in this publication.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
The authors alone are responsible for the views expressed in this publication, which do not necessarily represent the decisions, policy, or views of the World Health Organization and the United Nations Environment Programme.
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Tschiggfrei, K. et al. (2023). WHO- and UNEP-Coordinated Exposure Studies 2000–2019: Findings of Polychlorinated Naphthalenes. In: Malisch, R., Fürst, P., Šebková, K. (eds) Persistent Organic Pollutants in Human Milk. Springer, Cham. https://doi.org/10.1007/978-3-031-34087-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-34087-1_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34085-7
Online ISBN: 978-3-031-34087-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)