Abstract
Chlorinated paraffins (CP) are complex mixtures of several million theoretically possible individual compounds. Contrary to medium-chain CP (MCCP, C14–C17) and long-chain CP (LCCP, C18–C30), the third sub-group investigated, short-chain chlorinated paraffins (SCCP, C10–C13), have been listed in 2017 in Annex A (Elimination) of the Stockholm Convention on Persistent Organic Pollutants. The concentrations of CP were determined in 84 nation-wide pooled human milk samples collected between 2009 and 2019 in 57 countries participating in exposure studies coordinated by the World Health Organization and the United Nations Environment Programme. Until 2015, only total CP content was determined. In light of on-going efforts to also add other CP groups to the Annexes of the Stockholm Convention and the glaring lack of data on the general background contamination worldwide, later analysis determined SCCP and MCCP and investigated the presence of LCCP (C18–C20 only). CP were present in all 84 samples, ranging 8.7–700 ng/g lipid. A statistically significant increase rate of total CP concentrations in human milk of 30% over 10 years was found on a global level, with a considerable variation between UN Regional Groups. Homologue group patterns indicated higher shares of MCCP and LCCP in industrialized countries and economically dependent areas. Compared to all other POPs analysed in the samples, the concentration of the sum of SCCP and MCCP was in most cases only surpassed by DDT, except European countries with high shares of PCB. Considering the ubiquitous presence of CP in humans worldwide, further investigation into toxicological effects and human exposure seems more pressing than ever, so that regulatory action may follow.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Chlorinated paraffin
- Human milk
- Global WHO/UNEP studies
- Pooled sampling
- SCCP
- MCCP
- Homologue group pattern
- Persistent organic pollutant
1 Introduction
Chlorinated paraffins (CP) are complex substance mixtures often produced by radical chlorination of mixed alkane feedstocks, resulting in millions of theoretically possible isomers within this substance class (Vetter et al. 2022). For regulation purposes, these substances are often classified and grouped by carbon chain length, discerning between short-chain (SCCP, C10–C13), medium-chain (MCCP, C14–C17), and long-chain CP (LCCP, C>17) (PARCOM 1995; POPRC 2015). The U.S. Environmental Protection Agency additionally introduced a further subdivision between LCCP (C18–C20) and very long-chain CP (vLCCP, C21–C30), which is still rarely adapted in the literature (USEPA 2015, 2016). However, this subdivision is relevant from analytical point of view, because only LCCP (C18–C20) but not vLCCP (C21–C30) can be analysed by gas chromatography (Krätschmer et al. 2021b). Variations in chlorination degree and carbon chain length make the resulting products suitable for various industrial applications, some of which have a very high demand (USEPA 2009; Glüge et al. 2016; van Mourik et al. 2016).
1.1 Production and Environmental Fate
Large-scale industrial production and use of CP go back to the 1940s. As example, incidentally, the use as waterproofing and anti-mildew agent in uniforms and equipment led to the allocation of all produced CP in the United States of America to the US Army during World War II (Summary of War Regulations 1943). Today, CP are most commonly applied as plasticizers and flame retardants in a wide variety of products like PVC floors, paints, leather sealants or high-temperature lubricants (ECB 2005, 2008; Gallistl et al. 2018; Hahladakis et al. 2018). Although concrete production volumes are very hard to come by, it is generally accepted that the annual global production volume is extraordinarily high (Glüge et al. 2016). Coincidently, CP production surged at the same time when polychlorinated biphenyls (PCB) came under scrutiny and were later banned completely (OECD 1973; Breivik et al. 2007). Available data indicates a noticeable annual increase in the 1970s (Fig. 1) from less than 0.1 million tons/year (Muir et al. 2001) to an estimated 1.1 million tons/year in 2015 (Glüge et al. 2016). As a comparison, the estimated total production volume of PCB over six decades (1930–1993) was 1.0–1.5 million tons, with a peak annual production of <0.3 million tons/year reported in 1970 (Breivik et al. 2007; Stockholm Convention 2019).
Discussing production volumes of specifically SCCP and MCCP is practically impossible—China is currently thought to be the largest producer of CP having increased production from 0.6 million tons/year in 2007 (Fiedler 2010; Glüge et al. 2016) to 1.05 million tons/year in 2013 (POPRC 2015; van Mourik et al. 2016), but technical CP products are only defined by overall chlorination degree, not carbon chain length or CP group. Industry reports identified besides China also the Russian Federation, India, Japan, Brazil and several European countries as active CP producing countries, of which several (including China, India, the Russian Federation, Italy and France) have not ratified the Stockholm Convention decision to ban SCCP (ECHA 2008; POPRC 2010, 2015; Euro Chlor 2021). In contrast, at least SCCP production has been phased out in the USA (POPRC 2015), Canada (Environment and Climate Change Canada 2008), Sweden (Kemikalieinspektionen 2012, 2013), Japan (Tsunemi 2010) and the European Union (Commission Regulation (EU) No. 519/2012). Current reports and research indicate rapid growth of CP production in the Asia-Pacific (ASPAC) economic sector, with production volumes of primary CP applications like the production of polyvinyl chloride (PVC) reaching 1.3 million tons/year in India alone (Chaemfa et al. 2014; Zhang et al. 2017; Persistence Market Research 2021). Such uses as secondary plasticizers in soft plastics, paints and adhesives and flame retardants in many other products overshadow the direct use of CP as lubricants and metalworking fluids (Tomy 2010; POPRC 2015; Zhang et al. 2017; Hahladakis et al. 2018).
Chlorinated paraffins can be released into the environment at every stage of their product life cycle (Fig. 2), starting with emissions from productions sites or in urban areas (Niu et al. 2021), abrasion during the use of recycled rubber floors (ECB 2005; Cao et al. 2018), to volatilization from waste storage, treatment or incineration (Nilsson et al. 2001; Feo et al. 2009; van Mourik et al. 2015; Brandsma et al. 2017; Matsukami and Kajiwara 2019; Matsukami et al. 2020). Not only the products, but also the recycling process of plastic itself is suspected of releasing CP (ECHA 2014; POPRC 2015). In the last decade, CP have been reported in the nature (air, sediment, water, biota) and domestic environment (indoor air, dust, household appliances, window coatings) and especially sediment is assumed to help distribute CP into benthic fauna and, consequently, into the food chain (Bayen et al. 2006; Feo et al. 2009; Wei et al. 2016; Glüge et al. 2018).
Schematic representation of the main exposure ways to chlorinated paraffins (figure based on Krätschmer 2022)
Besides CP found in (unprocessed) food, input from multiple sources during food processing, storage and preparation also has the potential to contribute to the total dietary intake of CP. CP have been found migrating from packaging materials into food simulants (Hahladakis et al. 2018; Wang et al. 2019), leaking from hand blenders (Yuan et al. 2017) and migrating into baked goods from oven isolation materials (Perkons et al. 2019). Using dishcloths (Gallistl et al. 2017) and fume hood filters (Bendig et al. 2013) as passive samplers and specifically targeting oiled hinges (Sprengel and Vetter 2021) and isolation coatings (Gallistl et al. 2018) of kitchen appliances, a strong presence of SCCP and MCCP (in addition to several other polyhalogenated compounds) in German households could be shown.
Interestingly, studies of air samples found that emissions from CP productions plants only influence the immediate area, whereas CP levels in the urban environment seem to be caused by the numerous consumer products containing CP present in every household (Niu et al. 2021). Therefore, to decrease CP emissions, the use of CP in mainly plastic products needs to be decreased first. Because of this unique situation for such a class of high production volume chemicals, production bans and restrictions would serve more immediately to protect consumers than any maximum levels in food or the environment.
1.2 Toxicological Aspects
Regrettably, a large share of published toxicological studies on effects of SCCP and MCCP date back more than a decade and were often conducted using either complex technical mixtures or very few individual compounds for lack of alternatives (El-Sayed Ali and Legler 2010). In addition to that, analytical methods for CP were less reliable—any results should therefore be viewed as indicative rather than absolute. Available data suggest that CP exposure primarily targets the liver (Cooley et al. 2001; Du et al. 2019), kidneys (EFSA CONTAM Panel et al. 2020) and the thyroid and parathyroid glands (Cooley et al. 2001; Qiao et al. 2018), causing non-lethal effects in these organs. A feeding study in hens however did not report adverse effects of high CP doses, most likely caused by the quick excretion over several pathways (Ueberschär et al. 2007). Additionally, a study in Sprague-Dawley rats indicated that toxic effects of CP are inversely related to the alkyl chain length and directly to the CP′ mean degree of chlorination of CP (Geng et al. 2016). A physiologically-based pharmacokinetic (PBPK) model calculated for Sprague-Dawley rats and extrapolated to humans (Dong et al. 2019) found the half-life of SCCP with 5.2 years to be comparable to half-lives of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, 3.2–11.3 years (Milbrath et al. 2009)) and perfluorooctanesulfonic acid (PFOS, 3.9–6.9 years (Olsen et al. 2007; Thompson et al. 2010)). The half-lives calculated for MCCP and LCCP however are much shorter (1.2 and 0.6 years, respectively). A recent review of available toxicological studies concluded that while it is reasonably sure that CP are absorbed in the gastro-intestinal tract and probably various other organs, the metabolic pathways and transformations are still poorly studied, leaving a wide gap in the knowledge on these compounds (Darnerud and Bergman 2022).
Based on reported neoplastic effects in male mice, the World Health Organization (WHO) recommended a tolerable daily intake (TDI) of 11 μg SCCP per kg bodyweight and day in 1996 (WHO et al. 1996). This TDI value already included a security factor of 1000. The European Food Safety Authority EFSA however stated in 2020 in their Scientific Opinion that available toxicological data is insufficient for concluding on a TDI for any of the CP groups (EFSA CONTAM Panel et al. 2020). Instead, EFSA modelled a benchmark dose lower confidence limit 10% value (BMDL10), representing the lowest dose that causes no more than 10% incidences of the chosen limiting effect (SCCP: increased liver weight, MCCP: nephritis) in rats (EFSA CONTAM Panel et al. 2020). The BMDL10 have been set to 2.3 mg/kg bodyweight/day (SCCP) and 36 mg/kg bodyweight/day (MCCP) with a margin of exposure of 1000, although there have been protests in the scientific community stating that these values are too high, especially for MCCP (Zellmer et al. 2020). No limiting value of any kind could be established for LCCP, although kidneys were identified as likely target organs (EFSA CONTAM Panel et al. 2020).
Concerning infant’s exposure to POPs and CP in particular, studies suggest that POPs found in human milk are likely to be present in the infant’s blood as well (Chakraborty and Das 2016). Besides this lactational exposure, a study comparing paired maternal and umbilical cord serum samples was able to show evidence of placental transfer to the infant in utero (Qiao et al. 2018). This emphasizes the need for conservative and updated risk assessments in the interest of protecting vulnerable population groups and consequently the need for more occurrence and toxicological data.
1.3 Classification as POP and Regulatory Situation
Due to the difference in the regulatory status, it is important to distinguish between SCCP (listed as POPs) and MCCP (unregulated, candidate POPs) when discussing CP results. Both SCCP and MCCP have been under scrutiny for their persistent (Muir et al. 2001; ECHA 2008), bioaccumulative (Fisk et al. 2000; Houde et al. 2008; Yuan et al. 2019) and toxic (Cooley et al. 2001; El-Sayed Ali and Legler 2010; Geng et al. 2016) properties, but only production and worldwide use of SCCP was severely restricted by the parties that ratified this part of the United Nations’ (UN) Stockholm Convention in 2017 (Conference of the Parties of the Stockholm Convention 2017).
Shortly after classifying SCCP as POPs, the need for more occurrence data and toxicological studies to expand on existing data (Fisk et al. 2000; ECB 2005; Thompson and Vaughan 2014; Yuan et al. 2019) came into focus of research groups and official control bodies. Especially since MCCP but not SCCP are classified as ‘may cause harm to breastfed children’ under the harmonized classification of the EU Classification, Labelling and Packaging (CLP) Regulation (ECHA 2019), an in-depth assessment of potential health risks of MCCP seems prudent (Swedish Chemicals Agency 2018; EFSA CONTAM Panel et al. 2020; Zellmer et al. 2020).
Despite EFSA’s findings seemingly indicating low risk from MCCP, the European Chemicals Agency ECHA declared them candidate Substances of Very High Concern (SVHC) in 2021 with proven toxic, persistent and bioaccumulative properties (ECHA 2021a, b). Upon conclusion of the assessment period, a restriction of MCCP to 0.1% in products and other chemicals is expected within the European Union (EU). Additionally, MCCP are scheduled to be assessed for addition to the Annexes of the Stockholm Convention starting early 2022 (Stockholm Convention 2021).
1.4 CP as Target Analytes in the WHO/UNEP-Coordinated Human Milk Studies on Persistent Organic Pollutants
Analysing human tissue allows for monitoring of the total exposure levels of target contaminants in the population. Of the possible tissue samples, human milk presents the preferable option as the sampling process is non-invasive and yields a much higher lipid content than blood serum.
The WHO and the United Nations Environment Programme (UNEP) performed seven global human exposure studies for certain persistent organic pollutants (POPs) between 1987 and 2019. Initially, these studies were conducted by WHO for polychlorinated biphenyls (PCB), polychlorinated dibenzo-p-dioxins (PCDD) and polychlorinated dibenzofurans (PCDF). After a sufficient number of countries ratified the Stockholm Convention on POPs in 2004, WHO and UNEP agreed to collaborate in joint studies to support the implementation of the convention. The number of POPs initially covered by the convention was expanded considerably since its adoption (UNEP 2020). SCCP were added in 2017 to Annex A to the Convention for elimination of production and use of chemicals. The Article 16 requires the periodic evaluation of the effectiveness of the convention in reducing emissions of POPs. One of the pillars of this evaluation is to be based on comparable and consistent monitoring data on the presence of POPs in the environment and in humans. Therefore, four more rounds were organized by WHO and UNEP between 2005 and 2019.
As such, human milk samples are often used to report occurrence levels of lipophilic contaminants such as polychlorinated biphenyls (PCB) (Mamontova et al. 2017; Müller et al. 2017; Bawa et al. 2018), polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/F) (Abballe et al. 2008; Fång et al. 2015), brominated flame retardants (BFR) (Antignac et al. 2016), organochlorine pesticides (OCP) (Al Antary et al. 2017; Polanco Rodríguez et al. 2017; Bawa et al. 2018; Chen et al. 2018) and CP (Cao et al. 2017; Xia et al. 2017a, b; Yang et al. 2018)¸ see also review in Part I of this book (Fürst 2023). Cross-comparisons of these studies are however exceedingly difficult, as the necessary ethical clearances and budget constraints seem to result in small studies with mixed parity of the mothers, only rarely screening for contamination sources and mixtures of individual, city-wide grouped or regionally grouped samples. Especially individual samples often show high variability and hinder comparisons on an international level (Krätschmer et al. 2021a).
The sampling protocol implemented by the WHO and UNEP in their human milk studies however results in cost-effective nation-wide pools which are considered to represent countries or subpopulations at the time of sampling (Malisch et al. 2023c). The gradual addition of new persistent organic pollutants to the analysis portfolio of these studies since entry into force of the Stockholm Convention in 2004 (Stockholm Convention 2013) led to the addition of SCCP as optional parameter in 2012 in these studies.
In accordance with the implementation of the Global Monitoring Plan (GMP), parties report through one of the five UN Regional Groups (Malisch et al. 2023c). Therefore, countries are classified according to one of the following five geopolitical groups (DGACM 2019) for the purposes of this article: the African Group (AFR), the Asia-Pacific Group (ASPAC), the Group of Latin American and Caribbean Countries (GRULAC), the Central and Eastern Europe (CEE) and the Western European and Others Group (WEOG). Notably, Australia is a member of the WEOG instead of the geographically closer ASPAC group (Fig. 3).
The method used for analysis of samples collected between 2009 and 2015 at the State Institute for Chemical and Veterinary Analysis (CVUA) Freiburg produced results which can be discussed as total CP values only (Krätschmer and Schächtele 2019). With regard to plans to add specifically SCCP (and not MCCP or LCCP) to the listed compounds, a new method using high-resolution mass spectrometry with Orbitrap technology was established and applied to the human milk samples starting 2016. This method allowed for the distinction between SCCP and MCCP as well as qualitative detection of some LCCP (Mézière et al. 2020; Krätschmer et al. 2021a, b; Schächtele et al. 2023); it was used also for re-analysis of samples arriving after 2011, if sufficient sample amount was left. Suitability of this method was shown through participation in several interlaboratory studies and proficiency tests on food and biota samples containing SCCP and MCCP (Krätschmer and Schächtele 2019; Schächtele et al. 2023).
All substance-specific data were deposited at the Global Monitoring Plan Data Warehouse (GMP DWH), which can be publicly accessed. This serves as the source of information for the regional and global reports of the GMP and for the evaluation of the effectiveness of the convention to eliminate or reduce emissions of selected POPs (Global Monitoring Plan Data Warehouse 2020).
2 Materials and Methods
2.1 Sample Collection
The detailed study design, rationale and sample collection procedure are described elsewhere (Krätschmer et al. 2021b; Schächtele et al. 2023). To summarize, national coordinators in each participating country organized representative sampling campaigns, with the eligibility criteria applied to the mothers including willingness to breastfeed, age of the mother, primiparity, expectation of a singleton, healthy pregnancy, a minimum time of 10 years residency in the area and the absence of known POP hotspots near the place of residence. Samples were collected 3–8 weeks after birth with informed consent of the mothers, of which 25 mL was analysed locally for basic POPs (e.g., indicator PCB or pesticides). The rest of each sample from at least 50 mothers was added to one pooled milk sample per country and shipped frozen to CVUA Freiburg (Germany) and Örebro University (Sweden) for analysis. Lower numbers of contributors (at least 25 mothers) to the pooled samples were accepted in the case of small countries. The present report solely includes results produced in Freiburg.
2.2 Sample Preparation
Sample preparation of the pooled samples was performed as described elsewhere (Krätschmer et al. 2019, 2021a). In brief, 50 g of the hand warm, homogenized sample was treated with cooled centrifugation (4 °C, 3000 rpm, 10 min) in baked-out glass centrifuge tubes to separate the cream from the hydrogenous phase. After adding the recovery standard (13C10-1,5,5,6,6,10-hexachlorodecane, Cambridge Isotope Laboratories, Tewksbury, MA, USA), the cream was then dried by grinding with sodium sulphate until a powdery consistency was reached. Cold extraction with dichloromethane/n-hexane (1:1, v/v) was performed and the filtered solvent evaporated to dryness.
Further sample clean-up was performed using glass column chromatography using an acidified silica column and fractionation on a Florisil column (magnesium silicate primed with 1.5% water). The second fraction containing CP was then concentrated before the addition of the syringe standard ε-hexachlorocyclohexane (ε-HCH, Dr. Ehrenstorfer, Augsburg, Germany) for analysis.
2.3 Measurement of SCCP and MCCP
Given the major developments in this field of analysis in recent years, the method applied to human milk samples at CVUA Freiburg has also changed over the years. Until 2016, CP samples were analysed by GC-EI-MS/MS (Reth et al. 2005), using the average results of three monitored mass transitions (m/z 102 → 67, m/z 102 → 65, m/z 91 → 53). This allowed for a reliable and comparable quantification of the total CP amount in the sample, but not for a distinction between SCCP and MCCP. The new method introduced 2017 used GC-ECNI-Orbitrap-HRMS, with a high enough mass resolution and full scan mode able to discern between SCCP and MCCP on the homologue group level (Krätschmer et al. 2018).
While high-resolution mass spectrometry often allows for quantification of the full spectrum of homologue groups in order to quantify CP of a certain alkyl chain length, a reduction of the number of mass traces to be monitored would also help speed up establishing CP analysis more widely in environmental and food laboratories. It needs to be pointed out though that the chlorination patterns shown here were originally determined by GC-ECNI-HRMS and later corrected for their known shift toward higher chlorination degrees with a set of correction factors (Mézière et al. 2020).
2.4 Quality Control
Each sample batch additionally included a procedural blank (sodium sulphate prepared like a sample) as well as different quality control (QC) samples. To match the pooled human milk samples of the present study, these QC samples consisted of raw cow’s milk with and without the fortification with SCCP and MCCP standards on different levels. Recoveries ranged 83–110% (SCCP) and 84–119% (MCCP) for the GC-ECNI-Orbitrap method and 84–101% (CP) for the GC-EI-MS/MS method, respectively. Procedural blanks were 0.166 ± 0.023 ng/g sample CP for the samples from 2012 to 2017 and 0.098 ± 0.016 ng/g sample SCCP and 0.046 ± 0.038 ng/g sample MCCP for samples from 2017 to 2021. Limits of quantification (LOQs) were determined based on validation studies and the procedural blank levels.
As reported elsewhere, LOQs for human milk samples using the GC-ECNI-Orbitrap-HRMS method were 7.1 ng/g lipid SCCP and 12 ng/g lipid MCCP (Krätschmer et al. 2021a). All samples investigated with this method were above the LOQ. The LOQ for human samples using the GC-EI-MS/MS method was 38 ng/g lipid CP. Total CP results for three of the 27 pooled samples analysed using this method were below LOQ. More detailed description of the initial and ongoing validation process, interlaboratory exercise results and the different quantification approaches can be found in a different part of this book (Schächtele et al. 2023).
2.5 Assessment of Temporal Trends
General temporal trends were assessed for total CP content (all 84 samples, collected 2009–2019 in 57 countries) and for SCCP and MCCP concentrations (57 samples/countries collected 2015–2019; GC-Orbitrap-HRMS). Evaluation was based on the non-parametric linear Theil-Sen trend estimator (Sen 1968; Theil 1992) in order to address that exponential trends were expected (as commonly observed in cases after stop of production and application of a chemical rather than unrealistic linear trends (Sharma et al. 2021)). The R package ‘Median-based Linear Models (mblm)’ (Komsta 2013) was used for this regression.
Trends were positively assigned if the trend test (significance of the Theil-Sen estimator) was positive on 95% confidence level of significance (i.e., p-values < 0.05). Simulations showed that the Theil-Sen p-values are never <0.05 for less than 5 data points. Yet, the required five data points were not available for any country. This prevented that statistically significant temporal trends could be derived for individual countries. However, pooling of data in regions allowed us to investigate statistically significant time trends for several UN Regional Groups and at the global level (Sect. 3.1).
2.6 Grouping of Countries into UN Regional Groups
For the purpose of this article, participating countries are not grouped by geographical aspects, by according to the United Nations Regional Groups, although including the non-member state Kiribati in the Asia-Pacific Group.
3 Results for Samples Collected 2009–2019
3.1 Global Overview of Sum of CP Levels
As mentioned in the previous section, samples of the 2009–2011 period and some samples of the 2012–2013 period were only analysed on the sum of CP. Only a few samples collected in 2012–2013 and all samples arriving after 2014 were (re-) analysed on both SCCP and MCCP and on homologue group patterns. For a better comparison with older samples, the new samples were also discussed by means of their sum of CP values.
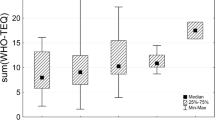
Remarkably, average CP levels in the Asia-Pacific Group (ASPAC) and Central and Eastern Europe (CEE) were lower in 2009–2014 compared to the period 2015–2019 (Fig. 4). By contrast, average CP levels in the Western European and Others Group (WEOG) seem to have decreased in the more recent time period, with a comparatively close grouping of the results. The median results of the African Group (AFR) and the Group of Latin America and Caribbean Countries (GRULAC) were very similar in both time spans so that no trend could be observed. It needs to be pointed out though that two data points are by no means sufficient to indicate an overall time trend for a region or country. Still, an overall indication of increasing CP levels in the background contamination of human milk in two out of five UN Regional Groups is cause for concern.
Range of sum of CP determined in pooled human milk samples from the 2009 to 2019 period of WHO/UNEP-coordinated human milk studies. AFR, Africa; ASPAC, Asia-Pacific Group; CEE, Central and Eastern Europe; GRULAC, Group of Latin American and Caribbean Countries; WEOG, Western European and Others Group (including Australia)
Further evaluation of temporal trends of the CP levels in the five UN Regional Groups was carried out with the Theil-Sen method to derive changes over a 1- and 10-year period (Fig. 5). Again, no significant changes of CP levels were observed in the AFR and GRULAC groups as a whole, while a decrease by 63% was calculated in the WEOG group over 10 years (p = 0.001). In contrast, in the ASPAC and CEE groups CP increased by up to 200% over the decade (p < 0.010). The 10-year-trend worldwide, based on all 84 results of 57 countries covering the period between 2009 and 2019 indicated an increase of total CP in human milk by 30% (p < 0.001) (Table 1, Fig. 5).
Temporal trends of the sum of CP concentrations in pooled human milk samples in ASPAC, CEE and WEOG UN Regional Groups and globally (ng total CP/g lipid). Decrease rates and uncertainty were determined using the Theil-Sen method. Negative decrease rates are to be read as increase in that time period
Whereas for the assessment of temporal trends (over a decade) of total CP concentrations could be based on a sufficient number of samples for nearly all UN Regional Groups and globally, the available SCCP and MCCP data covered only a much shorter periods in most groups (AFR: 2015–2019; ASPAC: 2018–2019; GRULAC: 2012–2019; EEC: 2014–2019; WEOG: 2013–2019). Therefore, temporal trends in individual UN Regional Groups could not be examined. Yet, on a global level (using all available data) indicated an increasing trend for both MCCP and especially SCCP (Table 2), although only the observation for SCCP is statistically significant (p < 0.001).
This overall increasing trend was in line with predicted increase of the CP production (Glüge et al. 2016) and diametrically opposed to the overall decreasing trends for most of the other POPs monitored within the UNEP human milk survey (Malisch et al. 2023a, d).
3.2 A Closer Look by Geographical Area
Within each geographical area, a wide range of CP levels was found (Figs. 6 and 7). Most notably, the samples from Mongolia (2018, 700 ng/g lipid CP) and Niger (2015, 680 ng/g lipid CP) were higher than other samples from their regional groups. However, an earlier sample from Niger (2011, 240 ng/g lipid CP) was markedly lower in its CP levels, indicating a considerable increase in comparison with the 2015 pooled sample. The sum of CP increased between two sampling periods by more than a factor of 2 also in Mauritius, Czech Republic, Peru and Uruguay.
SCCP, MCCP and sum of CP results for pooled country samples of human milk from member states of the Central and Eastern Europe (CEE), Western European and Others Group (WEOG) and Group of Latin American and Caribbean Countries (GRULAC). Brazil submitted three samples from different regions in the same year, see Sect. 3.2
On average, African and Asia/Pacific pooled samples had the highest combined SCCP and MCCP content of the five UN Regional Groups: with a median of 160 ng/g lipid each and mean results of 190 ng/g lipid (AFR) and 200 ng/g lipid (ASPAC), respectively, they surpassed mean and median levels of the other three groups by a factor of 2–3. Looking at the standard deviation of samples from the same UN region, AFR and ASPAC again surpass the other Regional Groups with 120 and 170 ng/g lipid or 63–84%, respectively. These high standard deviations are mostly caused by a few countries with remarkably high CP levels, but also based on a higher general variation between the different countries.
Interestingly, variations between country samples in Europe, Western countries and South/Latin America were much smaller in absolute numbers with only ~40–50 ng/g lipid, but comparable to the African groups’ relative standard deviation with a range of 51–59%. This is especially notable since WEOG also includes Australia as a geographically separated member, drastically widening the area covered by the otherwise very continent- or region-specific groupings. However, looking solely at Pacific Island States within the ASPAC group, a much lower standard deviation and more uniform distribution of SCCP and MCCP was observed, with the exception of Palau (2018). The standard deviation of the total CP amount for these countries was 87 ng/g lipid or 36%. While the overall CP levels seem more comparable in this area, available specific data on SCCP and MCCP levels show a higher variation of MCCP between these countries (56 ng/g lipid or 50%).
Unfortunately, the available population data on these pooled samples does not allow for more specific investigations of reasons for the observed differences between different countries in the same UN Regional Group. Especially the considerable number and diffuse nature of possible contamination sources causing exposure to CP means that despite careful planning and canvassing participants for the human milk studies in order to avoid known hotspots for other POPs, some individuals with markedly higher CP exposure levels might have been included as one of the 25–50 donors contributing to the pooled samples. Such outliers might lead to a bias in the pooled sample, more so if the overall observed concentrations are on the lower end of the concentration range (Gewurtz et al. 2011).
Even without such a scenario, differences in consumption behaviour, underlying medical conditions, living environment or travel habits can lead to variations in the individual and pooled human milk samples. For example, the presence of many plastic (i.e., polyvinyl chloride, PVC) consumer products and building materials might cause higher daily CP exposure, for example through transfer into food from packaging material or through evaporation from electronic equipment or flooring (Schinkel et al. 2019; McGrath et al. 2021). Studies also suggest that rapid weight loss or (potentially undiagnosed) underlying medical conditions like type-2 diabetes mellitus can lead to an increased release of POPs into the blood circulation instead of accumulation in fatty tissue (e.g., Jansen et al. 2017; Berg et al. 2021), although no data on CP are available.
The larger differences in results observed between pooled samples from the same country and sampling year, i.e. three pooled samples from Brazil (2012), and two pooled samples from Australia (2013), but not between two pooled samples from Germany (2019), however are caused by different population groups and/or regions being sampled. The direct comparison of country-specific results also shows that samples belonging to CEE, GRULAC and WEOG were in most cases below 150 ng/g lipid CP, while AFR and ASPAC samples surpassed that mark in 15 of 27 (AFR) and in 8 of 15 (ASPAC) cases, respectively.
The comparison between Western and Eastern European countries is interesting in the context of the EU—even though most of the countries shown as part of CEE and WEOG in Fig. 7 have been subject to the same POPs regulations since the early 2010s (Commission Regulation (EU) No. 519/2012), there is a distinction between most Western European countries with equal or higher shares of SCCP and most Eastern European countries with equal or higher shares of MCCP.
As likely reason for these differences between countries that are in close proximity to each other, the locations of CP production sites might be considered, which included Germany, France, Italy, Spain, Slovakia, Romania and the United Kingdom (ECHA 2008; Euro Chlor 2021). However, studies of air samples found that emissions from CP productions plants only influence the immediate area, whereas CP levels in the urban environment seem to be caused by the numerous consumer products containing CP present in every household (Niu et al. 2021). Therefore, the wide use of CP, e.g., in mainly plastic products needs to be considered, as well. With the exception of Germany, available pooled human milk samples from these countries seem to have a slight dominance of MCCP, which fits with the predominant production and use of MCCP and LCCP even before the EU ban of SCCP production in 2012 (European Commission 2012) combined with the long product life of SCCP put on the market before 2012.
Regarding the relation between SCCP and MCCP, MCCP levels at least equalled SCCP levels in most pooled country samples of all UN Regional Groups, contributing 24–85% to the total CP levels reported here (Fig. 8). In 36 of the 53 countries where distinct data is available, MCCP even surpassed SCCP. Using the SCCP/MCCP ratio as an indication of this relation, it is interesting to see that African and Eastern European country samples had a tendency toward a higher dominance of MCCP, whereas in the other UN Regional Groups, median SCCP levels were closer to equal distribution compared to MCCP (AFR 58% MCCP, ASPAC 55%, GRULAC 53%, CEE 67%, WEOG 54%).
Range of SCCP/MCCP ratios, sorted by geographical area or grouping. The dotted horizontal line indicates ratio = 1, i.e. equal presence of SCCP and MCCP. AFR, Africa; ASPAC, Asia-Pacific Group; CEE, Central and Eastern Europe; GRULAC, Group of Latin America and Caribbean Countries; WEOG, Western European and Others Group
Interestingly, the pooled European samples seem to have a different SCCP/MCCP distribution in comparison with the samples of human milk reported for individual European countries: While one English and two Swedish studies on small regional pools or individual samples showed a slight to very strong dominance of SCCP over MCCP (in contrast to a slight dominance of MCCP in the Swedish sample of 2019), another study on individual samples from Germany in 2011 indicated the opposite (Thomas et al. 2006; Hilger et al. 2011; Darnerud et al. 2012; Zhou et al. 2020). Similar to the German pool sample of the present study (collected 2019, 65% of the determined CP were MCCP), the median of all individual samples from the 2011 study had 66% MCCP (Hilger et al. 2011). Further comparison or interpretation of these data is however not advisable, as individual samples from one small geographical area will have a different distribution as representatively sampled pools of the whole country and data on comparability of the applied quantification methods are not always available. Additionally, the 2011 dataset included a large number of left-censored data.
The remarkable difference between most WEOG and CEE samples might, like previously mentioned, be explained by differences in consumer behaviour, CP production industries, and availability of MCCP-containing products as result of global trade, but lack of data on both aspects does not allow for any conclusions in this regard.
There is no recent literature data on CP in human milk available for Latin American and African countries. Studies describing levels and SCCP/MCCP distributions in Chinese samples indicate a much higher level of contamination especially in China and more dominantly consisting of SCCP (Xia et al. 2017a; b). Other studies on Chinese, Korean and Japanese regional pooled samples unfortunately only determined SCCP levels, so no further information on other CP groups could be obtained (Cao et al. 2017; Yang et al. 2018). It seems that China as one of the predominant CP producing countries has a different contamination pattern and level than other Asian countries which most likely are primarily exposed through product use. Unfortunately, neither China nor India, which also produces copious quantities of CP, did participate in the studies presented here.
3.3 Relation to Other POPs 2015–2019
Considering concentrations of a broad spectrum of 28 recommended chlorinated and brominated analytes in the pooled human milk samples (Malisch et al. 2023c), ranking SCCP and MCCP among these other Stockholm Convention POPs as listed until 2019 becomes feasible. For ease of comparison, the following listed 27 POPs included in this ranking were sorted into two groups:
-
Pesticides—aldrin, chlordane, chlordecone, dichlorodiphenyltrichloroethane (DDT), dicofol; dieldrin, endosulfan, endrin, heptachlor, α-hexachlorocyclohexane (HCH), β-HCH; γ-HCH; mirex, pentachlorobenzene, pentachlorophenol (including pentachloroanisole) and toxaphene
-
(Other) Industrial chemicals and by-products—hexabromobiphenyl (HBB), hexabromocyclododecane (HBCDD), hexachlorobenzene (HCB), hexachlorobutadiene; polybrominated diphenyl ethers (PBDE: tetra- and pentabromodiphenyl ether; hexa- and heptabromodiphenyl ether; decabromobiphenylether), polychlorinated biphenyls (PCB), polychlorinated dibenzo-p-dioxins (PCDD), polychlorinated dibenzofurans (PCDF) and polychlorinated naphthalenes (PCN).
Apart from WEOG and CEE, SCCP and MCCP dominated the share of POPs grouped as industrial chemicals and by-products in most areas (Fig. 9). Especially in continental European countries, the well-documented legacy POP problem with PCB (Breivik et al. 2007) apparently led to a higher share of industrial chemicals in human milk samples (Costopoulou et al. 2006; Abballe et al. 2008; Fång et al. 2015; Antignac et al. 2016; Malisch et al. 2023d, e). However, no further conclusions as to the source(s) or time frame of the PCB exposure are possible, since the investigation of POPs sources was not the aim of the studies presented here. A more detailed description of POPs distribution and likely sources can be found in part one of this book (Fürst 2023) and, with more detail on regional distribution and trends, in the Stockholm Convention regional monitoring reports (Stockholm Convention 2022).
Median sum of all POPs except PFAS analysed in pooled human milk samples from 2015–2019, sorted by UN regions and broken down into Stockholm Convention POPs groups (bar charts) and further into components of the ‘industrial chemicals’ POPs group (pie charts). AFR, Africa; ASPAC,Asia-Pacific Group; CEE, Central and Eastern Europe; GRULAC, Group of Latin American and Caribbean Countries; WEOG, Western European and Others Group
Compared to all other POPs analysed in the samples and listed above, the concentrations of the sum of SCCP and MCCP were in most cases only surpassed by the pesticide DDT and its metabolites, excepting only continental European countries (part of WEOG and CEE) with high shares of PCB (Krätschmer et al. 2021a). Notably, DDT and its metabolites dominated the pesticide group throughout all regions with share of more than 90% (Krätschmer et al. 2021a; Malisch et al. 2023a, b).
Similarly elevated levels of DDT were also found in other human milk studies in Italy (Abballe et al. 2008), Jordan (Al Antary et al. 2017), France (Antignac et al. 2016), India (Bawa et al. 2018), Taiwan (Chen et al. 2018), Tanzania (Müller et al. 2017) and Siberia (Mamontova et al. 2017). The most likely reason for high DDT exposure in warmer climates is the use as insecticide to combat malaria (Conference of the Parties of the Stockholm Convention 2013). Additionally, a 2001 study with women of the Mohawk nation was also able to link higher levels of DDT and HCB in human milk to increased fish consumption of some of the participants (Fitzgerald et al. 2001), so it is likely that several factors have influenced DDT exposure (e.g., food chain, aim and time of restriction of use in countries/UN regions), similar as illustrated for main exposure pathways to CP in a previous section of this article.
As fish only plays a very minor role in dietary intake in most European countries (EFSA 2011), the elevated levels of CP and other POPs found in fish on the European market (Parera 2013; Fernandes et al. 2018; Krätschmer et al. 2019, 2021b; Labadie et al. 2019) are expected to only have a minor influence on CP level in human milk from that area (Albers et al. 1996).
4 Considerations on CP Homologue Patterns
4.1 UN Regional Group Characteristics
While concentrations of SCCP and MCCP are important from a risk assessment point of view, the homologue group patterns of samples can give indication on possible contamination sources or at least the severity of contamination with certain chlorination degrees or CP chain lengths. Homologue group patterns have previously been shown to be specific to geographical regions or species (Zhou et al. 2018; Krätschmer et al. 2019). When comparing these homologue group patterns, two aspects should be regarded:
-
(a)
Distribution of alkyl chain lengths (Fig. 10) and
-
(b)
Distribution of numbers of chlorine atoms (Fig. 11)
Relative contribution of alkyl chain lengths C10–C19 to the median CP homologue patterns for each UN Regional Group. Data derived using GC-ECNI-HRMS. AFR, Africa; ASPAC, Asia-Pacific Countries; CEE, Central and Eastern Europe; GRULAC, Group of Latin American and Caribbean Countries; WEOG, Western European and Others Group
Relative contribution of different chlorine atom numbers to median homologue patterns of the five UN Regional Groups. Data derived by GC-ECNI-HRMS with application of ECNI correction factors (Mézière et al. 2020). AFR, Africa; ASPAC, Asia-Pacific Countries; CEE, Central and Eastern Europe; GRULAC, Group of Latin American and Caribbean Countries; WEOG, Western European and Others Group
The median distribution of alkyl chain lengths in the five UN Regional Groups shows that SCCP (C10–C13) are still very present in all parts of the world, in agreement with the previously discussed SCCP/MCCP ratios. Interestingly, the amount of chlorinated decanes (C10-CPs) seems to have the highest variation among SCCP, with especially high shares in ASPAC sample pools. Such a distinct change in overall CP pattern might indicate a contamination source possibly typical for this area especially for the Pacific islands as they present most countries included in this group. Another interesting observation is the dominance of chlorinated tetradecanes (C14-CPs) among MCCP (C14–C17), but also among the whole CP pattern. This is in accordance with findings in environmental samples (Krätschmer et al. 2019). Even though they were not quantified during this study, some LCCP (C18–C20), here especially chlorinated octadecanes (C18-CPs), were detected in 8 European and 14 African sample pools.
Given the increasing reports of LCCP (C18–C20) and vLCCP (C21–C36) in food (e.g., Ding et al. 2021) and the environment (e.g., Yuan et al. 2021), a further increase of these CP groups can also be expected in human milk studies in the future. With CP being used in cable insulations and other parts of electronic equipment (Wang et al. 2018), mismanaged consumer product and electronic waste is a potential contamination source that is likely to show up in human surveys in addition to the primary use of the products themselves (Perkins et al. 2014). This might especially be of importance for countries with considerable amounts of e-waste recycling or large dumping sites outside government control, like it was reported in the past for Ghana and Nigeria (Schmidt 2006; UNEP 2018). Currently, e-waste management is regulated in eight African countries including Egypt, Ghana, Nigeria and Côte d’Ivoire, who are part of this human milk survey (Forti et al. 2020). In East Africa, a group of countries including the study participants Tanzania, Uganda and Kenya have published a common strategy paper on e-waste management 2017, though progress in implementation varies (EACO 2017). While this possible exposure pathway seems valid for a variety of compounds, present data on pooled human milk samples from the first (2008–2010) and second sampling periods (2015–2019) do not show a uniform increase or decrease of CP levels in the mentioned countries, likely surpassed by other predictors and natural variations between study subjects.
In general, discussions of homologue group patterns always necessitate knowledge of the applied instrumentation and correction measures to evaluate and compare results (Krätschmer and Schächtele 2019; EURL POPs 2021). As is clearly shown in Fig. 11, chlorine numbers above 10 play a negligible role, whereas the sum of six to nine chlorine atoms describes 43–82% of the detected CP homologue groups. McGrath et al. proposed a method only evaluating homologue groups with six and seven chlorine atoms and validated this for different food matrices (McGrath et al. 2020).
However, application of this method on the human milk samples in the present studies would lead to a significant underestimation of CP concentrations: Only the African pooled samples would be described to a degree of more than 50%, whereas all other median area patterns contain homologue groups with six and seven chlorine atoms only to 22–30%. It is therefore uncertain if the screening method of McGrath et al. (2020) would still adequately quantify the overall amount of CP present in those sample pools. ASPAC, WEOG and CEE samples seem to be similar in their overall chlorination degree with low amounts of five chlorine atoms and the increasing presence of decachlorinated alkyl chains.
Interestingly, similarities between African and European sample pools apparent in the comparison of alkyl chain length patterns are not reflected in the chlorination levels (Fig. 11): the median Cl pattern of African pool samples features a much larger share of pentachlorinated CP (>20%) than the European sample pools (<3).
The difference in number of chlorine atoms has different impact on SCCP, MCCP and LCCP due to their increasing carbon chain lengths (Fernandes et al. 2022). For example, the mean number of chlorine atoms in C10-CP in the WEOG area is 6.7 (Fig. 12a), translating into a mean chlorination degree of 63% (Fig. 12b). In comparison, the mean chlorine number for C14-CP in Africa is also 6.7, but it translates into only 55% Cl for this CP chain length. However, some variation between individual samples is to be expected, as the patterns discussed here are median values of several pooled samples which themselves consist of numerous individual samples.
Relative contribution of different SCCP and MCCP chain lengths to median homologue patterns of the five UN Regional Groups. Annotated are (a) the mean number of chlorine atoms and (b) mean relative chlorination degree per CP chain length for each area. Data derived by GC-ECNI-HRMS with application of ECNI correction factors (Mézière et al. 2020). AFR, Africa; ASPAC, Asia-Pacific Countries; CEE, Central and Eastern Europe; GRULAC, Group of Latin American and Caribbean Countries; WEOG, Western European and Others Group
4.2 Sub-groups within UN Regional Groups
The UN Regional Groups used throughout this article are a political and economic construct, often formed in the early 1960s (UN General Assembly 2000). As such, they do not necessarily reflect similar population characteristics or environmental influences, which might lead to observable changes in CP levels. While the African group and the GRULAC countries are defined by geographical boundaries, especially WEOG countries and the ASPAC group combine countries of vastly different characteristics and locations. These differences have been previously addressed at 25th plenary meeting of the 55th United Nations General Assembly, where the formation of an Oceania regional group including Australia and New Zealand was proposed by the representative of Nauru (UN General Assembly 2000). In the interest of a closer evaluation of the results in the ASPAC region, three sub-groups were made for the countries participating in these studies: AP-1 (Pacific Islands), AP-2 (South-East Asia) and AP-3 (Central Asia). The exact grouping is shown in Table 3. Between these sub-groups, strong differences in SCCP and MCCP levels, average chlorination degrees and median homologue group patterns could be observed only for AP-3 (Fig. 12).
Between these sub-groups, strong differences in SCCP and MCCP levels, average chlorination degrees and median homologue group patterns could be observed only for the Central Asia sub-group (Fig. 13). Although the South-East Asian countries present more MCCP than the Pacific Island countries evaluated here, the rest of the overall patterns both in terms of chain length distribution and chlorine distribution are very similar. This might indicate different CP sources on the Pacific coast and further inside continental Asia. As only pattern data is available for Mongolia in sub-group AP-3, no conclusions can be made on product streams or likely source countries.
Map of the sub-groups within the ASPAC region and relative contributions of carbon chains and chlorine numbers to median homologue group patterns for each of them. For conciseness, not all Pacific Island countries named in Table 3 are shown on this map
Without the participation of the USA or Canada in the surveys presented here, the WEOG region consists of Western European countries and Australia. Geographically, some of the European WEOG countries are directly neighbouring countries of the CEE group (Fig. 14). A comparison of the median carbon chain and chlorine distribution patterns confirms the alleged differences between European WEOG countries and Australia, as the latter has visible contributions of longer MCCP and even C18-LCCP in its median pattern. Interestingly, the median pattern of CEE countries does not match the other European countries, mostly due to a higher contribution of C14-MCCP and octachlorinated homologue groups to the overall pattern.
Map of Europe with countries participating in this survey highlighted in green (E-1, belonging to the Western Europe and Others Group WEOG) and blue (E-2, belonging to the Central and Eastern European countries group CEE). Column diagrams on the right indicate the median carbon chain patterns and median chlorination patterns of E-1, E-2, Australia (AUS) and Mongolia (AP-3)
However, this specific carbon chain pattern matches reasonably well the pattern of AP-3, the Central Asian sub-group including Mongolia already shown in Fig. 13. While the median chlorine distributions do not completely match, the pattern similarities are enough to suggest at least some overlap in CP sources between Central/Eastern Europe and Mongolia. Common economic pathways are likely to have played a part in the distribution of either CP mixtures or products containing CP in this area, at least in the past if not at present. Unfortunately, we do not have a Chinese pooled sample to compare patterns with, so it remains unclear if the present findings originate from the distribution of CP (formerly) produced in Eastern Europe (ECHA 2008; Euro Chlor 2021) or in China.
These two examples have shown that there are several different ways of grouping the countries participating in the UNEP human milk surveys. While the grouping by official UN Regional Groups as practised throughout this article is a valid option, some connections—or differences—can only be shown when a more geographically-based approach to grouping is chosen. As a variety of economic, political, geographical and social factors are likely to play a role in the distribution of and consequently exposure to CP, any kind of group approach will always limit the insight into sources for individual countries. This article is merely meant to give a first insight into available data and encourage competent authorities to investigate further.
4.3 Differences Depending on Sampling Region
Sampling guidelines for the UNEP human milk surveys describe the pooled samples of each country to be representative of the background contamination of the country’s citizens (UNEP 2017). However, even with careful screening and choosing of the participants, differences in consumer preferences, undisclosed or unknown contamination sources or even differences in food choices might influence the resulting CP homologue pattern of the pooled sample.
For example, in 2013, Brazil submitted three different samples, which varied considerably in the CP content, namely by a factor of ~3 for SCCP (17–60 ng/g lipid) and by a factor of ~8 for MCCP (5.5–40 ng/g lipid). These samples were collected in three different greater regions of Brazil: the barely populated North Region, additionally including the central-west federal states Mato Grosso and Mato Grosso do Sul (Northwest, BR-GR1); the Northeast Region (BR-GR2); and the densely populated South and Southeast Regions additionally including the Federal District and Goiás (BR-GR3). These differences in region and population are also visible in the homologue group patterns of these samples (Fig. 15).
CP homologue group patterns for human milk pooled samples collected in three greater regions of Brazil 2012 with indicated sum concentrations of SCCP and MCCP. Patterns derived from GC-ECNI-HRMS data with correction factors for lower chlorinated homologue groups. BR-GR1: North Region, with Mato Grosso and Mato Grosso do Sul; BR-GR2: Northeast Region; BR-GR3: South Region and Southeast Region, with additional states Federal District and Goiás
Most strikingly, the homologue group pattern of the sample pool from the southern federal states including the Federal District (BR-GR3) showed a completely different CP pattern than the other two, emphasizing MCCP and LCCP with lower chlorination degrees much more than the other patterns. It is at the same time also the pool with the highest amount of SCCP and MCCP quantified among the three. While the BR-GR1 and BR-GR2 pools share some similarities on the first look, the sample pool from the Northeast Region (BR-GR2) has an overlying higher chlorinated SCCP pattern (C10–C13, Cl9–Cl11) similar to known technical SCCP mixtures (Schinkel et al. 2018; Sprengel and Vetter 2019; Fernandes et al. 2022, example in Fig. 16).
In this case, further follow-up investigations might be advisable, as such a Gaussian distribution over several alkyl chain lengths is highly unusual in a metabolized sample. Therefore, in different regions of a country, various patterns can be observed. Such findings underline the need for robust, representative sampling in all areas of a participating country for the pooled sample. A follow-up with analyses of individual samples could reveal the ranges of CP concentrations and variation of patterns within Brazil or any other country with unusual homologue pattern distributions in the pooled samples.
While the correlation between age of the mothers and level of different (non-CP) chlorinated contaminants in human milk could be proven as early as 1996 (Albers et al. 1996), a newer study on CP in human milk from 2021 revealed no correlation between CP levels and age, height, weight, fish consumption or place of residence (urban/rural) of the participating mothers (Krätschmer et al. 2021a). With next to no data available on metabolization of CP and correlated changes in homologue group patterns, this might be an avenue worth investigating in the future, also to better understand such regional differences as shown here for Brazil.
5 Conclusions
While not being the primary or only tissue to bioaccumulate lipophilic contaminants, the levels of POPs found in human milk of primiparous mothers still represent their accumulated exposure since birth, partly modified by metabolism and further losses through excretion (Albers et al. 1996). Therefore, comparing background contamination levels in human milk samples with data on environmental (Fridén et al. 2011; Hilger et al. 2013; Gao et al. 2016; Brits et al. 2019) or domestic (Schinkel et al. 2019; Wang et al. 2019) samples can help identifying additional contamination sources not indicated in dietary intake studies. While such information is important for better understanding CP contamination across the globe, especially for breastfed infants, the amounts of CP found in human milk as their primary source of sustenance are decisive for intake.
The human milk studies of the United Nations Environment Programme (UNEP) were not designed to point out specific contamination hotspots or to accurately determine exposure levels. On the contrary, the focus on pooled samples collected from participants that are expressly not living near known contamination hotspots is conducive to UNEPs goal of monitoring changes in the overall background contamination levels in humans as part of an effectiveness evaluation of POPs regulations.
In the WHO/UNEP sampling campaigns presented here, CP were found in all pooled samples from five UN Regional Groups with levels ranging from 9 to 700 ng/g lipid. Compared to other compounds classified as industrial chemicals by the Stockholm Convention on POPs, the sum of SCCP and MCCP dominated the findings in the pooled human milk samples from all UN regions. If the sample pools with human milk from donors without any known major contamination sources nearby already shown this consistent, and in some cases, high abundance of CP, individual samples from local population close to emission spots or as result of exposure to consumer products or in the domestic environment might be markedly higher.
The presence of MCCP, hereto mostly unregulated, could be established worldwide, often in equal or higher amounts compared to the now regulated SCCP. Indications of LCCP in samples from WEOG, CEE and GRULAC countries also give cause for concern and a need for further research in this area. Both findings show that merely regulating SCCP has led to a shift in production toward longer carbon chain lengths; a situation that should prompt evaluations of MCCP and LCCP and further regulatory efforts in this area. CP production already surpasses the total PCB production volume (1.0–1.5 million tons, Breivik et al. 2007) in a single year and the 20–25% of the PCB production volume that became ‘environmentally available’ still have a major impact in the human milk samples in some countries (Grimm et al. 2015; Malisch et al. 2023e). It is to be expected that the amount of CP released into the environment will continue to rise, likely surpassing also the cumulative amount of DDT (currently ~2.8 million tons, ATSDR 2022) soon. Consequently, levels of all types of CP can also be expected to keep rising and become a steady presence in human milk for decades to come.
While available data represent a pool of several individual samples, the determined average CP homologue group patterns of each country sample allow for regional grouping consistent with geographic or economic relations of the countries. Contrastingly, evaluation of CP homologue group patterns in samples collected in specific parts of the same country illustrate the large regional variations that disappear within the average country patterns. Efforts to lower CP exposure therefore need to evaluate sources at a more local level, taking into account regional economic and nutritional variations, consumer behaviour and possible re-use of legacy products. Nevertheless, all evaluated CP homologue group patterns presented some common markers, i.e. the leaning toward higher chlorinated homologues and a general dominance of chlorinated decanes, undecanes and tetradecanes (C10-, C11- and C14-CPs) within most patterns, although the chlorinated decanes vary markedly in some of the pooled samples. Further research is needed to establish whether these commonalities stem from similarities in the technical CP products or from metabolization/distribution within the human body.
References
Abballe A, Ballard TJ, Dellatte E, Di Domenico A, Ferri F, Fulgenzi AR et al (2008) Persistent environmental contaminants in human milk: Concentrations and time trends in Italy. Chemosphere 73(1 Suppl):S220–S227. https://doi.org/10.1016/j.chemosphere.2007.12.036
Agency for Toxic Substances and Disease Registry (ATSDR) (2022) Toxicological profile for DDT, DDE, DDD. U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=81&tid=20. Accessed 31 Oct 2022
Al Antary TM, Alawi MA, Estityah H, Haddad N (2017) Organochlorine pesticides residues in human breast milk from the middle governorates in Jordan in 2013/2014. Bull Environ Contam Toxicol 99(1):89–92. https://doi.org/10.1007/s00128-017-2117-3
Albers JM, Kreis IA, Liem AK, van Zoonen P (1996) Factors that influence the level of contamination of human milk with poly-chlorinated organic compounds. Arch Environ Contam Toxicol 30(2):285–291. https://doi.org/10.1007/BF00215810
Antignac JP, Main KM, Virtanen HE, Boquien CY, Marchand P, Venisseau A et al (2016) Country-specific chemical signatures of persistent organic pollutants (POPs) in breast milk of French, Danish and Finnish women. Environ Pollut 218:728–738. https://doi.org/10.1016/j.envpol.2016.07.069
Bawa P, Bedi JS, Gill JPS, Aulakh RS, Kumar A, Arora K (2018) Persistent organic pollutants residues in human breast milk from Bathinda and Ludhiana districts of Punjab, India. Arch Environ Contam Toxicol 75(4):512–520. https://doi.org/10.1007/s00244-018-0512-3
Bayen S, Obbard JP, Thomas GO (2006) Chlorinated paraffins: a review of analysis and environmental occurrence. Environ Int 32(7):915–929. https://doi.org/10.1016/j.envint.2006.05.009
Bendig P, Hägele F, Vetter W (2013) Widespread occurrence of polyhalogenated compounds in fat from kitchen hoods. Anal Bioanal Chem 405(23):7485–7496. https://doi.org/10.1007/s00216-013-7194-5
Berg V, Charles D, Bergdahl IA, Nøst TH, Sandanger TM, Tornevi A et al (2021) Cases experienced different longitudinal changes in POPs compared to controls Pre- and post-diagnostic blood profiles of chlorinated persistent organic pollutants and metabolic markers in type 2 diabetes mellitus cases and controls; a pilot study. Environ Res 195:110846. https://doi.org/10.1016/j.envres.2021.110846
Brandsma SH, van Mourik L, O’Brien JW, Eaglesham G, Leonards PEG, de Boer J et al (2017) Medium-chain chlorinated paraffins (CPs) dominate in Australian sewage sludge. Environ Sci Technol 51(6):3364–3372. https://doi.org/10.1021/acs.est.6b05318
Breivik K, Sweetman A, Pacyna JM, Jones KC (2002) Towards a global historical emission inventory for selected PCB congeners—a mass balance approach 1. Global production and consumption. Sci Total Environ 290(1-3):181–198. https://doi.org/10.1016/S0048-9697(01)01075-0
Breivik K, Sweetman A, Pacyna JM, Jones KC (2007) Towards a global historical emission inventory for selected PCB congeners—a mass balance approach 3. An update. Sci Total Environ 377(2-3):296–307. https://doi.org/10.1016/j.scitotenv.2007.02.026
Brits M, de Boer J, Rohwer ER, de Vos J, Weiss JM, Brandsma SH (2019) Short-, medium-, and long-chain chlorinated paraffins in South African indoor dust and cat hair. Chemosphere 238:124643. https://doi.org/10.1016/j.chemosphere.2019.124643
Cao Y, Harada KH, Hitomi T, Niisoe T, Wang P, Shi Y et al (2017) Lactational exposure to short-chain chlorinated paraffins in China, Korea, and Japan. Chemosphere 173:43–48. https://doi.org/10.1016/j.chemosphere.2016.12.078
Cao D, Gao W, Wu J, Lv K, Xin S, Wang Y et al (2018) Occurrence and human exposure assessment of short- and medium-chain chlorinated paraffins in dusts from plastic sports courts and synthetic turf in Beijing, China. Environ Sci Technol 53(1):443–451. https://doi.org/10.1021/acs.est.8b04323
Chaemfa C, Xu Y, Li J, Chakraborty P, Hussain Syed J, Naseem Malik R et al (2014) Screening of atmospheric short- and medium-chain chlorinated paraffins in India and Pakistan using polyurethane foam based passive air sampler. Environ Sci Technol 48(9):4799–4808. https://doi.org/10.1021/es405186m
Chakraborty J, Das S (2016) Molecular perspectives and recent advances in microbial remediation of persistent organic pollutants. Environ Sci Pollut Res Int 23(17):16883–16903. https://doi.org/10.1007/s11356-016-6887-7
Chen M-W, Santos HM, Que DE, Gou Y-Y, Tayo LL, Hsu Y-C et al (2018) Association between organochlorine pesticide levels in breast milk and their effects on female reproduction in a Taiwanese population. Int J Environ Res Public Health 15(5). https://doi.org/10.3390/ijerph15050931
Commission Regulation (EU) No 519/2012 of 19 June 2012 amending Regulation (EC) No 850/2004 of the European Parliament and of the Council on persistent organic pollutants as regards Annex I. Official Journal of the European Union. Reg (EU) 519/2012. L 159, OJ L 159, 20.6.2012, pp 1–4 (June 19, 2012)
Conference of the Parties of the Stockholm Convention (2013) Decision SC-6/1: DDT: Decision adopted by the Conference of the Parties to the Stockholm Convention at its sixth meeting, Geneva. http://www.pops.int/TheConvention/ConferenceoftheParties/Meetings/COP6/tabid/3074/ctl/Download/mid/11193/Default.aspx?id=5&ObjID=17118. Accessed 2 Jun 2023
Conference of the Parties of the Stockholm Convention (2017) Decision SC-8/11 listing Short-chain chlorinated paraffins (SCCPs) in Annex A of the Convention: Decision adopted by the Conference of the Parties to the Stockholm Convention at its eighth meeting, Geneva. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.8-SC-8-11.English.pdf. Accessed 2022 Feb 24
Contracting Parties to the Convention for the Prevention of Marine Pollution from Land-Based Sources (PARCOM) (1995) PARCOM Decision 95/1 on the phasing out of short chained chlorinated paraffins. Brussels. Summary record of the Joint Meeting of the Oslo and Paris Commissions (OSPAR). https://www.ospar.org/documents?d=32479. Accessed 24 Feb 2020
Cooley HM, Fisk AT, Wiens SC, Tomy GT, Evans RE, Muir DCG (2001) Examination of the behavior and liver and thyroid histology of juvenile rainbow trout (Oncorhynchus mykiss) exposed to high dietary concentrations of C10-, C11-, C12- and C14-polychlorinated n-alkanes. Aquat Toxicol 54(1-2):81–99. https://doi.org/10.1016/S0166-445X(00)00172-7
Costopoulou D, Vassiliadou I, Papadopoulos A, Makropoulos V, Leondiadis L (2006) Levels of dioxins, furans and PCBs in human serum and milk of people living in Greece. Chemosphere 65(9):1462–1469. https://doi.org/10.1016/j.chemosphere.2006.04.034
Darnerud PO, Bergman A (2022) Critical review on disposition of chlorinated paraffins in animals and humans. Environ Int 163:107195. https://doi.org/10.1016/j.envint.2022.107195
Darnerud PO, Aune M, Glynn A, Borgen A (2012) Chlorinated paraffins in Swedish breast milk, PM 18/12. Sundbyberg. 22 p. https://www.kemi.se/download/18.6df1d3df171c243fb23960cd/1591097408157/pm-18-12.pdf. Accessed 19 July 2019
Decision of the Council on Protection of the Environment by Control of Polychlorinated Biphenyls (13 Feb 1973). https://legalinstruments.oecd.org/en/instruments/OECD-LEGAL-0108. Accessed 2 Jun 2023
Department for General Assembly and Conference Management (DGACM) (2019) United Nations Regional Groups of Member States. https://www.un.org/dgacm/content/regional-groups. Accessed 24 Feb 2022
Ding L, Zhang S, Zhu Y, Zhao N, Yan W, Li Y (2021) Overlooked long-chain chlorinated paraffin (LCCP) contamination in foodstuff from China. Sci Tot Environ 801:149775. https://doi.org/10.1016/j.scitotenv.2021.149775
Dong Z, Li T, Wan Y, Sun Y, Hu J (2019) Physiologically based pharmacokinetic modeling for chlorinated paraffins in rats and humans: importance of biliary excretion. Environ Sci Technol 54(2):938–946. https://doi.org/10.1021/acs.est.9b03991
Du X, Yuan B, Zhou Y, Zheng Z, Wu Y, Qiu Y et al (2019) Tissue-specific accumulation, sexual difference, and maternal transfer of chlorinated paraffins in black-spotted frogs. Environ Sci Technol 53(9):4739–4746. https://doi.org/10.1021/acs.est.8b06350
East African Communications organisation (EACO) (2017) Regional E-waste strategy. Edited by Waste Management Steering Committee under Working Group 10: Environment and E-Waste Management. https://www.eaco.int/admin/docs/publications/EACO_Constitution_as_amended_in_July_2017.pdf. Accessed 22 Aug 2022
EFSA CONTAM Panel, Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J et al (2020) Scientific opinion—Risk assessment of chlorinated paraffins in feed and food. EFSA J 18(3). https://doi.org/10.2903/j.efsa.2020.5991
El-Sayed Ali T, Legler J (2010) Overview of the mammalian and environmental toxicity of chlorinated paraffins. In: de Boer J, El-Sayed Ali T (eds) Chlorinated paraffins: Handbook of environmental chemistry, vol 10. Springer, Heidelberg, pp 135–154
Environment and Climate Change Canada (2008) Follow-up report on a PSL1 assessment for which data were insufficient to conclude whether the substances were “toxic” to the environment and to the human health. Government of Canada. https://www.canada.ca/en/environment-climate-change/services/canadian-environmental-protection-act-registry/publications/chlorinated-paraffins.html. Accessed 2 Jun 2023
EURL for Halogenated POPs in Feed and Food (2021) Guidance document on the analysis of chlorinated paraffins: sample preparation and quantification in food and feed [version 1.9]. Freiburg im Breisgau. https://eurl-pops.eu/news/guidance-document-on-cp-analysis. Accessed 24 Feb 2022
Euro Chlor (2021) Chloroalkanes Product Group: Member companies of the CAPG. https://www.eurochlor.org/chloro-alkanes-product-group/. Accessed 24 Mar 2021
European Chemicals Agency (ECHA) (2008) Data on manufacture, import, export, uses and releases of alkanes, C10-13, chloro (SCCPs) as well as information on potential alternatives to its use: Technical report under contract ECHA/2008/02/SR2/ECA.225. https://echa.europa.eu/documents/10162/13640/tech_rep_alkanes_chloro_en.pdf. Accessed 24 Mar 2021
European Chemicals Agency (ECHA) (2014) Decision on substance evaluation pursuant to article 46(1) of regulation (EC) 1907/2006 for alkanes, C14-17, chloro (MCCP). UK REACH CA Health and Safety Executive, Helsinki. https://echa.europa.eu/documents/10162/b707a807-583e-62f2-c899-85ea2bbda9c2. Accessed 2 Jun 2023
European Chemicals Agency (ECHA) (2019) Harmonised classification—Annex VI of Regulation (EC) No 1272/2008 (CLP Regulation), Index-No. 602-095-00-X: alkanes, C14-17, chloro. https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/94445. Accessed 5 Aug 2019
European Chemicals Agency (ECHA) (ed) (2021a) Member State Committee Support Document for identification of Medium-Chain Chlorinated Paraffins (MCCP) as Substances of Very High Concern because of their PBT (Article 57d), vPvB (Article 57e) properties: SVHC Support Document. https://echa.europa.eu/documents/10162/cfbf3980-263c-7f53-f384-1bd1cf45c3c9. Accessed 21 July 2021
European Chemicals Agency (ECHA) (2021b) Candidate list updated with eight hazardous chemicals: ECHA/NR/21/20. https://echa.europa.eu/de/-/candidate-list-updated-with-eight-hazardous-chemicals. Accessed 21 July 2021
European Chemicals Bureau (ECB) (2005) Summary risk assessment report: alkanes, C14-17, chloro (MCCP): Part I—Environment. Institute for Health and Consumer Protection. https://echa.europa.eu/documents/10162/ad6eebf1-49b2-4a7b-9f73-a2c11109ff0c. Accessed 24 Feb 2022
European Chemicals Bureau (ECB) (2008) Risk assessment of Alkanes, C14-17, chloro (Medium-chained chlorinated paraffins): Draft of February 2008. 190 p., https://echa.europa.eu/documents/10162/13630/trd_rar_uk_mccp_en.pdf/b879f97d-9cea-49e1-9a84-4b3c6a4eb447. Accessed 12 June 2020
European Food Safety Authority (EFSA) (2011) Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA J 9(3):2097–2130. https://doi.org/10.2903/j.efsa.2011.2097
Fång J, Nyberg E, Winnberg U, Bignert A, Bergman Å (2015) Spatial and temporal trends of the Stockholm Convention POPs in mothers’ milk—a global review. Environ Sci Pollut Res Int 22(12):8989–9041. https://doi.org/10.1007/s11356-015-4080-z
Feo ML, Eljarrat E, Barceló D (2009) Occurrence, fate and analysis of polychlorinated n-alkanes in the environment. Trends Anal Chem 28(6):778–791. https://doi.org/10.1016/j.trac.2009.04.009
Fernandes AR, Mortimer D, Holmes M, Rose M, Zhihua L, Huang X et al (2018) Occurrence and spatial distribution of chemical contaminants in edible fish species collected from UK and proximate marine waters. Environ Int 114:219–230. https://doi.org/10.1016/j.envint.2018.02.047
Fernandes AR, Vetter W, Dirks C, van Mourik L, Cariou R, Sprengel J et al (2022) Determination of chlorinated paraffins (CPs): Analytical conundrums and the pressing need for reliable and relevant standards. Chemosphere 286(3):131878. https://doi.org/10.1016/j.chemosphere.2021.131878
Fiedler H (2010) Short-chain chlorinated paraffins: production, use and international regulations. In: de Boer J, El-Sayed Ali T (eds) Chlorinated paraffins: Handbook of environmental chemistry, vol 10. Springer, Heidelberg, London, pp 1–40
Fisk AT, Tomy GT, Cymbalisty CD, Muir DCG (2000) Dietary accumulation and quantitative structure-activity relationships for depuration and biotransformation of short (C10), medium (C14), and long (C18) carbon-chain polychlorinated alkanes by juvenile rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 19(6):1508–1516. https://doi.org/10.1002/etc.5620190606
Fitzgerald EF, Hwang SA, Deres DA, Bush B, Cook K, Worswick P (2001) The association between local fish consumption and DDE, mirex, and HCB concentrations in the breast milk of Mohawk women at Akwesasne. J Expo Anal Environ Epidemiol 11(5):381–388. https://doi.org/10.1038/sj.jea.7500180
Forti V, Baldé CP, Kuehr R, Bel G (2020) The global E-waste monitor 2020: quantities, flows and the circular economy potential. United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR)—co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), Bonn/Geneva/Rotterdam, https://ewastemonitor.info/wp-content/uploads/2020/11/GEM_2020_def_july1_low.pdf. Accessed 2 Jun 2023
Fridén U, McLachlan MS, Berger U (2011) Chlorinated paraffins in indoor air and dust: concentrations, congener patterns, and human exposure. Environ Int 37(7):1169–1174. https://doi.org/10.1016/j.envint.2011.04.002
Fürst P (2023) Human milk surveys on persistent organic pollutants from a historical perspective. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Gallistl C, Lok B, Schlienz A, Vetter W (2017) Polyhalogenated compounds (chlorinated paraffins, novel and classic flame retardants, POPs) in dishcloths after their regular use in households. Sci Total Environ 595:303–314. https://doi.org/10.1016/j.scitotenv.2017.03.217
Gallistl C, Sprengel J, Vetter W (2018) High levels of medium-chain chlorinated paraffins and polybrominated diphenyl ethers on the inside of several household baking oven doors. Sci Total Environ 615:1019–1027. https://doi.org/10.1016/j.scitotenv.2017.09.112
Gao W, Wu J, Wang Y, Jiang G (2016) Distribution and congener profiles of short-chain chlorinated paraffins in indoor/outdoor glass window surface films and their film-air partitioning in Beijing, China. Chemosphere 144:1327–1333. https://doi.org/10.1016/j.chemosphere.2015.09.075
Geng N, Zhang H, Xing L, Gao Y, Zhang B, Wang F et al (2016) Toxicokinetics of short-chain chlorinated paraffins in Sprague-Dawley rats following single oral administration. Chemosphere 145:106–111. https://doi.org/10.1016/j.chemosphere.2015.11.066
Gewurtz SB, Backus SM, Bhavsar SP, McGoldrick DJ, de Solla SR, Murphy EW (2011) Contaminant biomonitoring programs in the Great Lakes region: Review of approaches and critical factors. Environ Rev 19:162–184. https://doi.org/10.1139/a11-005
Global Monitoring Plan Data Warehouse (2020) Global monitoring plan data warehouse of the Stockholm Convention on Persistent Organic Pollutants: visualisation platform and on-line tool for the analysis of global levels of POPs in air, water, breast milk and blood: Masaryk University. https://www.pops-gmp.org
Glüge J, Wang Z, Bogdal C, Scheringer M, Hungerbühler K (2016) Global production, use, and emission volumes of short-chain chlorinated paraffins—A minimum scenario. Sci Total Environ 573:1132–1146. https://doi.org/10.1016/j.scitotenv.2016.08.105
Glüge J, Schinkel L, Hungerbühler K, Cariou R, Bogdal C (2018) Environmental risks of medium-chain chlorinated paraffins (MCCPs): a review. Environ Sci Technol 52(12):6743–6760. https://doi.org/10.1021/acs.est.7b06459
Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman Å, Robertson LW (2015) Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol 45(3):245–272. https://doi.org/10.3109/10408444.2014.999365
Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P (2018) An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 344:179–199. https://doi.org/10.1016/j.jhazmat.2017.10.014
Hilger B, Coelhan M, Fromme H, Völkel W (2011) Determination of chlorinated paraffins in human breast milk by HRGC-ECNI-LRMS. Organohalogen Comp 73:1611–1613
Hilger B, Fromme H, Völkel W, Coelhan M (2013) Occurrence of chlorinated paraffins in house dust samples from Bavaria, Germany. Environ Pollut 175:16–21. https://doi.org/10.1016/j.envpol.2012.12.011
Houde M, Muir DCG, Tomy GT, Whittle DM, Teixeira C, Moore S (2008) Bioaccumulation and trophic magnification of short- and medium-chain chlorinated paraffins in food webs from Lake Ontario and Lake Michigan. Environ Sci Technol 42(10):3893–3899. https://doi.org/10.1021/es703184s
Jansen A, Lyche JL, Polder A, Aaseth J, Skaug MA (2017) Increased blood levels of persistent organic pollutants (POP) in obese individuals after weight loss—A review. J Toxic Environ Health Part B 20(1):22–37. https://doi.org/10.1080/10937404.2016.1246391
Kemikalieinspektionen (2012) Chlorinated paraffins: statistics in brief. http://www3.kemi.se/en/Content/Statistics/Statistics-in-brief/Statistics-in-brief%2D%2D-Substances-and-substance-groups/Chlorinated-paraffins. Accessed 7 Oct 2016
Kemikalieinspektionen (2013) Flödesanalyser för kemiska ämnen: Klorparaffiner. http://webapps.kemi.se/flodesanalyser/FlodesanalyserSchema.aspx?SchemaID=1324. Accessed 7 Oct 2016
Komsta Ł (2013) Median-based linear models (mblm). R-Package, current version 0.12.1 (update 13 Oct 2022). https://cran.r-project.org/web/packages/mblm/mblm.pdf. Accessed 14 Dec 2022
Krätschmer KS (2022) Development, validation and application of a method for routine analysis of chlorinated paraffins in food and human milk. Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften (Dr. rer. nat.). University of Hohenheim, Institute of Food Chemistry (170b), Stuttgart. ISBN: 978-3-8439-4998-9
Krätschmer K, Schächtele A (2019) Interlaboratory studies on chlorinated paraffins: Evaluation of different methods for food matrices. Chemosphere 234:252–259. https://doi.org/10.1016/j.chemosphere.2019.06.022
Krätschmer K, Cojocariu C, Schächtele A, Malisch R, Vetter W (2018) Chlorinated paraffin analysis by gas chromatography Orbitrap high-resolution mass spectrometry: Method performance, investigation of possible interferences and analysis of fish samples. J Chromatogr A 1539:53–61. https://doi.org/10.1016/j.chroma.2018.01.034
Krätschmer K, Schächtele A, Malisch R, Vetter W (2019) Chlorinated paraffins (CPs) in salmon sold in southern Germany: Concentrations, homologue patterns and relation to other persistent organic pollutants. Chemosphere 227:630–637. https://doi.org/10.1016/j.chemosphere.2019.04.016
Krätschmer K, Malisch R, Vetter W (2021a) Chlorinated paraffin levels in relation to other persistent organic pollutants found in pooled human milk samples from primiparous mothers in 53 countries. Environ Health Perspect 129(8). https://doi.org/10.1289/EHP7696
Krätschmer K, Schächtele A, Vetter W (2021b) Short- and medium-chain chlorinated paraffin exposure in South Germany: a total diet, meal and market basket study. Environ Pollut 272. https://doi.org/10.1016/j.envpol.2020.116019
Labadie P, Blasi C, Le Menach K, Geneste E, Babut M, Perceval O et al (2019) Evidence for the widespread occurrence of short- and medium-chain chlorinated paraffins in fish collected from the Rhône River basin (France). Chemosphere 223:232–239. https://doi.org/10.1016/j.chemosphere.2019.02.069
Malisch R, Hardebusch B, Lippold R, van Leeuwen FXR, Moy G, Tritscher A, Šebková K, Klánová J, Kalina J (2023a) Time trends in human milk derived from WHO- and UNEP-coordinated exposure studies, Chapter 2: DDT, beta-HCH and HCB. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part IV)
Malisch R, Kypke K, Dambacher B, Hardebusch B, Lippold R, van Leeuwen FXR, Moy G, Tritscher A (2023b) WHO- and UNEP-coordinated exposure studies 2000-2019: Findings of selected organochlorine pesticides and industrial chemicals. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
Malisch R, Malisch K, van Leeuwen FXR, Moy G, Tritscher A, Witt A, Alvarez J (2023c) Overview of WHO- and UNEP-coordinated human milk studies and their link to the Stockholm Convention on persistent organic pollutants. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part I)
Malisch R, Schächtele A, van Leeuwen FXR, Moy G, Tritscher A, Šebková K, Klánová J, Kalina J (2023d) Time trends in human milk derived from WHO- and UNEP-coordinated exposure studies, Chapter 1: polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part IV)
Malisch R, Schächtele A, van Leeuwen FXR, Moy G, Tritscher A (2023e) WHO- and UNEP-coordinated exposure studies 2000-2019: Findings of polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part III)
Mamontova EA, Tarasova EN, Mamontov AA (2017) PCBs and OCPs in human milk in Eastern Siberia, Russia: Levels, temporal trends and infant exposure assessment. Chemosphere 178:239–248. https://doi.org/10.1016/j.chemosphere.2017.03.058
Matsukami H, Kajiwara N (2019) Destruction behavior of short- and medium-chain chlorinated paraffins in solid waste at a pilot-scale incinerator. Chemosphere 230:164–172. https://doi.org/10.1016/j.chemosphere.2019.05.048
Matsukami H, Takemori H, Takasuga T, Kuramochi H, Kajiwara N (2020) Liquid chromatography-electrospray ionization-tandem mass spectrometry for the determination of short-chain chlorinated paraffins in mixed plastic wastes. Chemosphere 244:125531. https://doi.org/10.1016/j.chemosphere.2019.125531
McGrath TJ, Poma G, Bombeke J, Covaci A (2020) A simplified screening method for short- and medium-chain chlorinated paraffins in food by gas chromatography-low resolution mass spectrometry. J Chromatogr A 1631:461574. https://doi.org/10.1016/j.chroma.2020.461574
McGrath TJ, Poma G, Matsukami H, Malarvannan G, Kajiwara N, Covaci A (2021) Short- and medium-chain chlorinated paraffins in polyvinylchloride and rubber consumer products and toys purchased on the Belgian market. Int J Environ Res Public Health 18(3). https://doi.org/10.3390/ijerph18031069
Mézière M, Krätschmer K, Perkons I, Zacs D, Marchand P, Dervilly G et al (2020) Addressing main challenges regarding short- and medium-chain chlorinated paraffin analysis using GC-ECNI-MS and LC-ESI-MS methods. J Am Soc Mass Spectrom 31(9):1885–1895. https://doi.org/10.1021/jasms.0c00155
Milbrath MO, Wenger Y, Chang C-W, Emond C, Garabrant D, Gillespie BW et al (2009) Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ Health Perspect 117(3):417–425. https://doi.org/10.1289/ehp.11781
Muir DCG, Bennie D, Teixeira C, Fisk AT, Tomy GT, Stern GA et al (2001) Short chain chlorinated paraffins: are they persistent and bioaccumulative? In: Lipnick RL (ed) Persistent, bioaccumulative, and toxic chemicals, vol 773. American Chemical Society, Washington, DC, pp 184–202
Müller MHB, Polder A, Brynildsrud OB, Karimi M, Lie E, Manyilizu WB et al (2017) Organochlorine pesticides (OCPs) and polychlorinated biphenyls (PCBs) in human breast milk and associated health risks to nursing infants in Northern Tanzania. Environ Res 154:425–434. https://doi.org/10.1016/j.envres.2017.01.031
Nilsson M-L, Waldebäck M, Liljegren G, Kylin H, Markides KE (2001) Pressurized-fluid extraction (PFE) of chlorinated paraffins from the biodegradable fraction of source-separated household waste. Fresenius J Anal Chem 370(7):913–918. https://doi.org/10.1007/s002160100833
Niu S, Chen R, Hageman KJ, Zou Y, Dong L, Zheng R et al (2021) Disentangling the contributions of urban and production sources in short- and medium-chain chlorinated paraffin concentrations in a complex source region. J Hazard Mater 405:124117. https://doi.org/10.1016/j.jhazmat.2020.124117
Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL et al (2007) Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305. https://doi.org/10.1289/ehp.10009
Parera J (2013) Polychlorinated dibenzo-p-dioxins, dibenzofurans, biphenyls, paraffins and polybrominated diphenyl ethers in marine fish species from Ebro River Delta (Spain). Chemosphere 93(3):499–505. https://doi.org/10.1016/j.chemosphere.2013.06.022
Perkins DN, Brune Drisse M-N, Nxele T, Sly PD (2014) E-waste: a global hazard. Ann Glob Health 80(4):286–295. https://doi.org/10.1016/j.aogh.2014.10.001
Perkons I, Pasecnaja E, Zacs D (2019) The impact of baking on chlorinated paraffins: Characterization of C10–C17 chlorinated paraffins in oven-baked pastry products and unprocessed pastry dough by HPLC–ESI–Q–TOF–MS. Food Chem 298:125100. https://doi.org/10.1016/j.foodchem.2019.125100
Persistence Market Research (2021) Chlorinated paraffins market: Chlorinated paraffins market: Global industry trend analysis 2012 to 2017 and forecast 2017-2025. https://www.persistencemarketresearch.com/market-research/chlorinated-paraffins-market.asp. Accessed 10 Mar 2021
Persistent Organic Pollutants Review Committee (POPRC) (2010) Supporting document for the draft risk profile on short-chained chlorinated paraffins: UNEP/POPS/POPRC.6/INF/15. Geneva. http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-POPRC.6-INF-15.English.pdf. Accessed 21 Apr 2021
Persistent Organic Pollutants Review Committee (POPRC) (2015) Short-chained chlorinated paraffins: Risk profile: Document UNEP/POP/POPRC.11/10/Add.2. Geneva
Polanco Rodríguez ÁG, Inmaculada Riba López M, Angel DelValls Casillas T, León JAA, Anjan Kumar Prusty B, Álvarez Cervera FJ (2017) Levels of persistent organic pollutants in breast milk of Maya women in Yucatan, Mexico. Environ Monit Assess 189(2):59. https://doi.org/10.1007/s10661-017-5768-y
Qiao L, Gao L-R, Zheng M-H, Xia D, Li J-G, Zhang L et al (2018) Mass fractions, congener group patterns, and placental transfer of short- and medium-chain chlorinated paraffins in paired maternal and cord serum. Environ Sci Technol 52(17):10097–10103. https://doi.org/10.1021/acs.est.8b02839
Schächtele A, Hardebusch B, Krätschmer K, Tschiggfrei K, Zwickel T, Malisch R (2023) Analysis and quality control of WHO- and UNEP-coordinated human milk studies 2000-2019—polybrominated diphenylethers, hexabromocyclododecanes, chlorinated paraffins and polychlorinated naphthalenes. In: Malisch R, Fürst P, Šebková K (eds) Persistent organic pollutants in human milk. Springer, Cham (in this volume, Part II)
Schinkel L, Bogdal C, Canonica E, Cariou R, Bleiner D, McNeill K et al (2018) Analysis of medium-chain and long-chain chlorinated paraffins: the urgent need for more specific analytical standards. Environ Sci Technol Lett 5(12):708–717. https://doi.org/10.1021/acs.estlett.8b00537
Schinkel L, Canonica E, Lienemann P, Bleiner D, Heeb N (2019) Mass spectrometric analysis of short-chain chlorinated paraffins in plastic consumer products. Chimia (Aarau) 73(6):504. https://doi.org/10.2533/chimia.2019.504
Schmidt CW (2006) Unfair trade: e-waste in Africa. Environ Health Perspect 114(4):a232–a235. https://doi.org/10.1289/ehp.114-a232
Sen PK (1968) Estimates of the regression coefficient based on Kendall’s Tau. J Am Stat Assoc 63:1379–1389. https://doi.org/10.1080/01621459.1968.10480934
Sharma BM, Kalina J, Whaley P, Scheringer M (2021) Towards guidelines for time-trend reviews examining temporal variability in human biomonitoring data of pollutants. Environ Int 151:106437. https://doi.org/10.1016/j.envint.2021.106437
Sprengel J, Vetter W (2019) Synthesis and characterization of eight single chain length chlorinated paraffin standards and their use for quantification. Rapid Commun Mass Spectrom 33(1):49–56. https://doi.org/10.1002/rcm.8310
Sprengel J, Vetter W (2021) Chlorinated paraffins in hinges of kitchen appliances. Environ Monit Assess 193(5):250. https://doi.org/10.1007/s10661-021-09023-z
Stockholm Convention (ed) (2013) Results of the global survey on concentrations in human milk of persistent organic pollutants by the United Nations Environment Programme and the World Health Organization: UNEP/POPS/COP.6/INF/33
Stockholm Convention (2019) Report on progress towards the elimination of polychlorinated biphenyls: UNEP/POPS/COP.9/INF/10
Stockholm Convention (2021) Proposal to list chlorinated paraffins with carbon chain lengths in the range C14-17 and chlorination levels at or exceeding 45 per cent chlorine by weight in Annexes A, B and/or C to the Stockholm Convention on Persistent Organic Pollutants: UNEP/POPS/POPRC.17/6
Stockholm Convention (2022) Monitoring reports of the Global Monitoring Plan. http://chm.pops.int/implementation/globalmonitoringplan/monitoringreports/tabid/525/default.aspx. Accessed 31 Oct 2022
Summary of War Regulations (1943 July) Chlorinated paraffin—no longer under allocation: Order M-189 revoked. Chemical industries. Chem Bus Mag 53(1):116. https://delibra.bg.polsl.pl/dlibra/publication/49027/edition/44823/content. Accessed 21 Apr 2021
Swedish Chemicals Agency (2018) ROHS Annex II Dossier MCCP: Proposal for a restriction of a substance in electrical and electronic equipment under RoHS. https://ec.europa.eu/environment/pdf/waste/rohs_eee/SE%20ROHS%20Annex%20II%20Dossier%20MCCP.pdf. Accessed 2 Jun 2023
Theil H (1992) A rank-invariant method of linear and polynomial regression analysis. In: Raj B, Koerts J (eds) Henri Theil’s contributions to economics and econometrics. Advanced studies in theoretical and applied econometrics, vol 23. Springer, Dordrecht, pp 345–381. https://doi.org/10.1007/978-94-011-2546-8_20
Thomas GO, Farrar D, Braekevelt E, Stern GA, Kalantzi OI, Martin FL et al (2006) Short and medium chain length chlorinated paraffins in UK human milk fat. Environ Int 32(1):34–40. https://doi.org/10.1016/j.envint.2005.04.006
Thompson R, Vaughan M (2014) Medium-chain chlorinated paraffins (MCCPs): A review of bioaccumulation potential in the aquatic environment. Integr Environ Assess Manag 10(1):78–86. https://doi.org/10.1002/ieam.1452
Thompson J, Lorber M, Toms L-ML, Kato K, Calafat AM, Mueller JF (2010) Use of simple pharmacokinetic modeling to characterize exposure of Australians to perfluorooctanoic acid and perfluorooctane sulfonic acid. Environ Int 36(4):390–397. https://doi.org/10.1016/j.envint.2010.02.008
Tomy GT (2010) Analysis of chlorinated paraffins in environmental matrices: the ultimate challenge for the analytical chemist. In: de Boer J, El-Sayed Ali T (eds) Chlorinated paraffins: Handbook of environmental chemistry, vol 10. Springer, Heidelberg, London, pp 83–106
Tsunemi K (2010) Risk assessment of short-chain chlorinated paraffins in Japan. In: de Boer J, El-Sayed Ali T (eds) Chlorinated paraffins: Handbook of environmental chemistry, vol 10. Springer, Heidelberg, London, pp 155–194
Ueberschär K-H, Dänicke S, Matthes S (2007) Dose-response feeding study of short chain chlorinated paraffins (SCCPs) in laying hens: Effects on laying performance and tissue distribution, accumulation and elimination kinetics. Mol Nutr Food Res 51(2):248–254. https://doi.org/10.1002/mnfr.200600168
United Nations (UN) General Assembly 55. New York 2000. Plenary session, PV 25: A/55/PV.25_EN. https://digitallibrary.un.org/record/422798/files/A_55_PV.25-EN.pdf?ln=en. Accessed 29 Sept 2022
United Nations Environmental Programme (UNEP) (2017) UNEP-coordinated survey of human milk for persistent organic pollutants: guidelines for organization, sampling and analysis. https://wedocs.unep.org/bitstream/handle/20.500.11822/21024/UNEP_Guidance_HumanMilk_2017_En.pdf?sequence=1&isAllowed=y. Accessed 24 Feb 2022
United Nations Environmental Programme (UNEP) (2018) Turning e-waste into gold: the untapped potential of African landfills. https://www.unep.org/news-and-stories/story/turning-e-waste-gold-untapped-potential-african-landfills. Accessed 25 Oct 2021
United Nations Environmental Programme (UNEP) (2020) All POPs listed in the Stockholm Convention. http://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx. Accessed 24 Feb 2022
U.S. Environmental Protection Agency (USEPA) (2009) Short-chain chlorinated paraffins (SCCPs) and other chlorinated paraffins action plan. U.S. Environmental Protection Agency. https://www.epa.gov/sites/production/files/2015-09/documents/sccps_ap_2009_1230_final.pdf. Accessed 4 June 2020
U.S. Environmental Protection Agency (USEPA) (2015) TSCA new chemicals review program standard review risk assessment on medium-chain chlorinated paraffins (PMN P-12-0453) and long-chain chlorinated paraffins (PMN P-12-0433). Washington, DC. https://www.epa.gov/sites/production/files/2015-12/documents/standard_review_risk_assessment_ineos_p-12-0433-0453_docket.pdf. Accessed 10 June 2021
U.S. Environmental Protection Agency (USEPA) (2016) Significant new use rule on certain chemical substances. Washington, DC. 81 FR 7455. https://www.federalregister.gov/documents/2016/02/12/2016-02952/significant-new-use-rule-on-certain-chemical-substances. Accessed 26 Mar 2021
van Mourik L, Leonards PEG, Gaus C, de Boer J (2015) Recent developments in capabilities for analysing chlorinated paraffins in environmental matrices: A review. Chemosphere 136:259–272. https://doi.org/10.1016/j.chemosphere.2015.05.045
van Mourik L, Gaus C, Leonards PEG, de Boer J (2016) Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015. Chemosphere 155:415–428. https://doi.org/10.1016/j.chemosphere.2016.04.037
Vetter W, Sprengel J, Krätschmer K (2022) Chlorinated paraffins—a historical consideration including remarks on their complexity. Chemosphere 287(1):132032. https://doi.org/10.1016/j.chemosphere.2021.132032
Wang C, Gao W, Liang Y, Jiang Y, Wang Y, Zhang Q et al (2019) Migration of chlorinated paraffins from plastic food packaging into food simulants: Concentrations and differences in congener profiles. Chemosphere 225:557–564. https://doi.org/10.1016/j.chemosphere.2019.03.039
Wei G-L, Liang X-L, Li D-Q, Zhuo M-N, Zhang S-Y, Huang Q-X et al (2016) Occurrence, fate and ecological risk of chlorinated paraffins in Asia: A review. Environ Int 92-93:373–387. https://doi.org/10.1016/j.envint.2016.04.002
World Health Organisation (WHO), United Nations Environmental Programme (UNEP), International Labour Organisation (1996) Chlorinated paraffins. Environmental Health Criteria 181. International Programme on Chemical Safety, Geneva. http://www.inchem.org/documents/ehc/ehc/ehc181.htm. Accessed 20 Feb 2019
Xia D, Gao L-R, Zheng M-H, Li J-G, Zhang L, Wu Y-N et al (2017a) Health risks posed to infants in rural China by exposure to short- and medium-chain chlorinated paraffins in breast milk. Environ Int 103:1–7. https://doi.org/10.1016/j.envint.2017.03.013
Xia D, Gao L-R, Zheng M-H, Li J-G, Zhang L, Wu Y-N et al (2017b) Human exposure to short- and medium-chain chlorinated paraffins via mothers’ milk in Chinese urban population. Environ Sci Technol 51(1):608–615. https://doi.org/10.1021/acs.est.6b04246
Yang L, Wang Z, Li J, Ma Y, Kong L, Yang H et al (2018) Evaluation of short chain chlorinated paraffins in human milk and their intake by infants in Hebei Province, China. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 35(10):2011–2021. https://doi.org/10.1080/19440049.2018.1492155
Yuan B, Strid A, Darnerud PO, de Wit CA, Nyström J, Bergman Å (2017) Chlorinated paraffins leaking from hand blenders can lead to significant human exposures. Environ Int 109:73–80. https://doi.org/10.1016/j.envint.2017.09.014
Yuan B, Vorkamp K, Roos AM, Faxneld S, Sonne C, Garbus SE et al (2019) Accumulation of short-, medium-, and long-chain chlorinated paraffins in marine and terrestrial animals from Scandinavia. Environ Sci Technol 53(7):3526–3537. https://doi.org/10.1021/acs.est.8b06518
Yuan B, McLachlan MS, Roos AM, Simon M, Strid A, de Wit CA (2021) Long-chain chlorinated paraffins have reached the arctic. Environ Sci Technol Lett 8(9):753–759. https://doi.org/10.1021/acs.estlett.1c00470
Zellmer S, Heiserich L, Kappenstein O, Merkel S, Schulte A, Luch A (2020) MCCP: Are medium-chain chlorinated paraffins of concern for humans? Arch Toxicol 405(23):7485. https://doi.org/10.1007/s00204-020-02681-x
Zhang B, Zhao B, Xu C, Zhang J (2017) Emission inventory and provincial distribution of short-chain chlorinated paraffins in China. Sci Total Environ 581–582:582–588. https://doi.org/10.1016/j.scitotenv.2016.12.167
Zhou Y, Yin G, Du X, Xu M, Qiu Y, Ahlqvist P et al (2018) Short-chain chlorinated paraffins (SCCPs) in a freshwater food web from Dianshan Lake: Occurrence level, congener pattern and trophic transfer. Sci Total Environ 615:1010–1018. https://doi.org/10.1016/j.scitotenv.2017.10.026
Zhou Y, Yuan B, Nyberg E, Yin G, Bignert A, Glynn A et al (2020) Chlorinated paraffins in human milk from Urban Sites in China, Sweden, and Norway. Environ Sci Technol 54(7):4356–4366. https://doi.org/10.1021/acs.est.9b06089
Acknowledgements
This publication was developed in the framework of the projects titled ‘Implementation of the POPs Monitoring Plan in the Asian Region’ and ‘Continuing regional Support for the POPs Global Monitoring Plan under the Stockholm Convention in the Africa, Pacific and Latin-American and Caribbean Region’, funded by the Global Environment Facility and in close collaboration with and support of CVUA Freiburg.
The worldwide implementation of the Global Monitoring Plan for POPs, including that of the UNEP/WHO global human milk survey, is made possible thanks to the generous contributions to the Stockholm Convention Voluntary Trust Fund by the Governments of Japan, Norway and Sweden and through the European Union’s Global Public Goods and Challenges Programme (GPGC). Further, the substantial contributions made by the Global Environment Facility to support POPs monitoring activities in regions implemented by UNEP, in close collaboration with WHO, particularly for the global human milk surveys, are greatly appreciated.
The authors would like to thank all the participating countries, the national and regional coordinators for their commitment and hard work in these human milk studies. The team of Chemical and Veterinary Institute (CVUA) Freiburg is gratefully acknowledged for analysing the samples discussed here in their role as UNEP Reference Laboratory for POPs in human milk. Data analysis for determination of time trends was supported by the RECETOX Research Infrastructure (No. LM2018121) and the CETOCOEN EXCELLENCE Teaming Phase 2 project financed by the European Union’s Horizon 2020 (No 857560) and the European Structural and Investment Funds (No. CZ.02.1.01/0.0/0.0/17_043/0009632). The two anonymous reviewers are also acknowledged for their valuable insights and commentary that allowed further improvement of this manuscript and allowed to develop additional perspectives for data evaluation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Krätschmer, K., Vetter, W., Kalina, J., Malisch, R. (2023). WHO- and UNEP-Coordinated Human Milk Studies 2000–2019: Findings of Chlorinated Paraffins. In: Malisch, R., Fürst, P., Šebková, K. (eds) Persistent Organic Pollutants in Human Milk. Springer, Cham. https://doi.org/10.1007/978-3-031-34087-1_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-34087-1_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34085-7
Online ISBN: 978-3-031-34087-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)