Abstract
Burrowing rodents have unusually disproportionate effects on rangeland ecosystems because they (1) engineer their environment through burrow construction and modification of vegetation structure, (2) influence ecosystem processes including aboveground plant production, nutrient cycling rates, and water infiltration patterns, (3) alter plant community composition, and (4) provide a prey base for a diverse array of predators. In some cases, engineering effects create habitat for certain faunal species that inhabit burrows or colonies of these rodents. We review the ecology and management of burrowing rodents that function as ecosystem engineers in western North America, which includes prairie dogs (five species in the genus Cynomys), ground squirrels (11 species in the genera Otospermophilus, Poliocitellus, and Urocitellus), pocket gophers (16 widespread species in the genera Cratogeomys, Geomys, and Thomomys), and kangaroo rats (eight widespread species in the genus Dipodomys). Effects of burrowing rodents on vegetation structure, species composition, and nutrient content vary with diet, degree of sociality, body size, and hibernation patterns, and potentially have significant effects on coexisting large grazers, including domestic livestock. Diets of prairie dogs overlap substantially with livestock. Impacts on ranching enterprises can vary with their abundance and seasonally, and may be greatest when burrowing rodents reduce dormant-season forage availability. Ground squirrel, pocket gopher, and kangaroo rat interactions with livestock vary among species in relation to their diet, degree of coloniality, and population density. All prairie dog and ground squirrel species are affected by outbreaks of plague caused by Yersinia pestis, a non-native disease. Plague and population control via rodenticides are the primary factors determining the distribution and abundance of these species. In contrast, pocket gophers and kangaroo rats are unaffected by plague. Management and conservation efforts that enable burrowing rodents to coexist with livestock across broad landscapes will likely be essential for the conservation of a unique suite of bird, mammal, herpetofaunal and arthropod species that depend on them as prey or on their engineering activities for habitat.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Rangelands around the world are inhabited and shaped by a diverse array of fossorial and semi-fossorial (burrowing), herbivorous mammals (Davidson et al. 2012). Many of these species function as ecosystem engineers (Jones et al. 1994) because they construct burrow systems and alter the structure of vegetation and soils (e.g., Huntly and Inouye 1988; Reichman and Seabloom 2002; Lenihan 2007; Davidson and Lightfoot 2008; Prugh and Brashares 2012; Baker et al. 2013). These engineering activities alter the composition of plant communities, and create habitat features upon which other fauna depend (e.g., Davidson et al. 2012; Augustine and Baker 2013). In addition, burrowing mammals often serve as the prey base for a diverse array of predators, including raptors and mammalian carnivores. Here, we provide a review of the burrowing rodent species that function as ecosystem engineers in rangelands of western North America. In this review, we examine a group of ground squirrels that are social and colonial, which often concentrates their effects on rangelands in a spatially heterogeneous manner. These colonial species can be divided in terms of taxonomy and body size into the prairie dogs (five species in the genus Cynomys, which tend to be larger than other colonial ground squirrels) versus the somewhat smaller ground squirrels in the genera Otospermophilus (one species), Poliocitellus (one species), and Urocitellus (nine species; Table 15.1). Non-colonial, burrowing rodents that exert important engineering effects on western rangelands consist of pocket gophers in the genera Cratogeomys, Geomys, and Thomomys (16 widespread species, plus several restricted-range endemics) and kangaroo rats in the genus Dipodomys (eight widespread species, plus several restricted-range endemics; Table 15.2). We first describe the general life history and distribution of representative species in each of these groups, followed by a description of their ecosystem associations and the ways in which they influence the structure and function of rangelands. We follow with a discussion of our current state of knowledge on how burrowing rodents influence livestock management and production, the degree to which they are regulated by diseases (especially plague, caused by the introduced bacterium Yersinia pestis), and how interactions with disease and livestock fundamentally shape the management and conservation of burrowing rodents.
2 Life History, Ecology, and Distribution
2.1 Prairie Dogs and Ground Squirrels
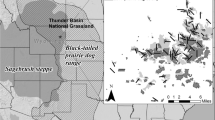
The black-tailed prairie dog (C. ludovicianus) is the most widespread species, occurring from the prairies of Saskatchewan to the northern Chihuahuan Desert of Mexico (Fig. 15.1). The closely-related Mexican prairie dog (C. mexicanus) occurs in a disjunct, southern portion of the Chihuahuan Desert, and is likely derived from a population of C. ludovicianus that became isolated from the main population following the Wisconsin glaciation (Ceballos and Wilson 1985). Their relatively long, black-tipped tail distinguishes these two species from the three white-tailed species (C. parvidens, C. gunnisoni, and C. leucurus). Of these, the white-tailed prairie dog (C. leucurus) predominantly inhabits sagebrush (Artemisia spp.) steppe in the Wyoming Basin of Wyoming, Colorado, and Utah as well as northern portions of the Colorado Plateau. Gunnison’s prairie dog (C. gunnisoni) occurs to the south, in sagebrush steppe and grasslands across the Colorado Plateau, Apache highlands, and Chihuahuan Desert, as well as high-elevation grasslands within the southern Rocky Mountains (Fig. 15.1). The Utah prairie dog (C. parvidens) inhabits grasslands and shrublands in a relatively restricted range in southwestern Utah (Fig. 15.1).
The extent to which prairie dogs affect ecosystem processes and interact with other taxa is largely influenced by degree of social organization (which influences animal density within a colony), body size, and hibernation patterns. All five species feed primarily on grasses and forbs, hence their diet overlaps substantially with that of livestock and native ungulates. C. ludovicianus additionally clip tall vegetation and girdle shrubs on their colonies to enhance visibility, whereas the three white-tailed species do not (Tileston and Lechleitner 1966). Body size of all species varies seasonally and is greater in males, with the range being ~ 700–1100 g for C. ludovicianus and mexicanus, ~ 650–1100 g for C. leucurus and C. parvidens, and ~ 400–800 g for C. gunnisoni (Hoogland 2003). Prairie dogs live in spatially discrete colonies composed of multiple family units (coteries or clans) and exhibit greater social complexity (as measured in terms of relative frequencies of different age and sex classes living within a single social group) than the other ground squirrels described below (Blumstein and Armitage 1997). C. ludovicianus and C. parvidens exhibit more complex social structure than C. leucurus and C. gunnisoni (Blumstein and Armitage 1997; Table 15.1), which is reflected to some extent in the density of individuals within colonies and degree of modification of vegetation structure. C. ludovicianus often occurs at densities on the order of 10–35 individuals per ha, and can even increase to > 100 individuals per ha in suburban landscapes where dispersal is curtailed (Magle et al. 2007; Table 15.1). Densities of the three white-tailed species can also vary widely (e.g., 1–60 individuals ha−1; Table 15.1), but typically occur at lower densities than C. ludovicianus, on the order of 2–32 ha−1 (Menkens and Anderson 1991; Nelson and Theimer 2012; USFWS 2012).
Although the pre-settlement distribution and abundance of prairie dogs are difficult to assess from journals of early explorers, comprehensive reviews of cumulative accounts of prairie dog control efforts in the late 1800s and early 1900s show that the distribution and abundance of all five prairie dog species declined dramatically after European settlement (Knowles et al. 2002). Effects of human control efforts were compounded by the introduction of plague in the early 1900s. Because of the cost of monitoring the vast areas often surveyed for C. ludovicianus, surveys typically measure colony area rather than animal numbers, using methods that include aerial photography or high spatial resolution satellite imagery to detect colonies (Brennan et al. 2020), aerial surveys of colony intercepts by observers in fixed-wing aircraft (White et al. 2005), and ground-based mapping of colony boundaries (Sidle et al. 2012). Thus far, ground-based surveys have been required to accurately distinguish colonies actively occupied by prairie dogs from former colony-sites recently extirpated by poisoning or plague (Sidle et al. 2012). For Utah prairie dogs, populations are monitored annually using direct counts of aboveground individuals on known colonies (USFWS 2012).
Twelve species of ground squirrels inhabit western North America all of which are smaller in body size and exhibit less complex social organization compared to prairie dogs (Table 15.1; Figs. 15.2 and 15.3). We focus primarily on five of these because of their relatively large ecological roles, widespread distribution, and existing research on impacts to western rangelands. The California ground squirrel (Otospermophilus beecheyi) primarily resides in open grasslands, oak (Quercus spp.) savannah, oak woodland, nearshore rocky outcrops, and on agricultural lands where the openness of the habitat permits individuals to detect predators. Abundant throughout its range in Pacific Coast states, O. beecheyi densities typically vary between 8 and 92 animals ha−1, mass of adults ranges from 280 to 738 g, and females usually produce a single litter each year of 4–11 young (Smith et al. 2016). Two species, the Richardson’s ground squirrel (Urocitellus richardsonii) and Franklin’s ground squirrel (Poliocitellus franklinii) occupy the northern Great Plains (Fig. 15.2). Adult mass of U. richardsonii varies greatly, with pre-hibernation masses for adult females of 350–435 g and males of 500–655 g (Michener and Koeppl 1985). P. franklinii is slightly smaller and is less social than the Urocitellus species, generally living alone or in pairs rather than in colonies (Ostroff and Finck 2003). The Wyoming ground squirrel (Urocitellus elegans) now includes three subspecies, each of which occurs in distinct geographic ranges (Thorington et al. 2012; Fig. 15.2). Here, we focus primarily on U. e. elegans due to their prevalence and impacts in sagebrush steppe (Zegers 1984; Thorington et al. 2012; Fig. 15.2). U. e. elegans select habitats with talus slopes or well-drained soils that facilitate burrow construction. The Uinta ground squirrel (Urocitellus armatus) is a large ground squirrel that resides mainly in the in or near Utah and Idaho in open meadows and sagebrush steppe (Fig. 15.3; Eshelman and Sonnemann 2000).
Seven other ground squirrel species have more restricted distribution in western North America (Figs. 15.2 and 15.3). These include the Belding’s (U. beldingii), Columbian (U. columbianus), Idaho (U. brunneus), Piute (U. mollis), Townsends (U. townsendii), and Washington (U. washingtonii) ground squirrels. Collectively, these seven species occupy a broad swath of the Intermountain Region extending from southern Alberta to southern Nevada (Figs. 15.2 and 15.3), and all of these species can be locally important in terms of effects on rangelands.
For most rangeland ground squirrels, population numbers are limited by food availability, as shown experimentally for U. columbianus (Dobson and Kjelgaard 1985). Population densities of O. beecheyi, for example, vary radically with typical densities of 1.2–11 adults/ha (Schitoskey and Woodmansee 1978). Moreover, U. u. elegans densities vary seasonally with hibernation but densities, including juveniles, reach up to 44 ha−1 in shortgrass prairie and vary from 14 to 48 ha−1 in montane meadows (Zegers 1984). P. franklinii typically occurs at lower densities (1.5–2.5 adults ha−1. Ostroff and Finck 2003).
2.2 Pocket Gophers and Kangaroo Rats
Pocket gophers of the family Geomyidae include 41 recognized species restricted to North and Central America. Pocket gophers are truly fossorial and possess multiple adaptations for a life spent mostly underground, including a stocky, fusiform body with stout forelimbs and enlarged claws, skin on the snout that grows behind the incisors to prevent soil from entering the mouth while digging, reduced eyes and ears, and a short, mostly naked tail (Baker et al. 2013). The pelage is short and fine, and dorsal coloration often matches the color of the specific soils in which they live, presumably as a mechanism for avoiding aerial predators (Krupa and Geluso 2000). They possess fur-lined, external cheek pouches that can be used to temporarily hold and transport food.
Three genera (Cratogeomys, Geomys, and Thomomys) inhabit arid, semiarid and montane rangelands of western North America (Fig. 15.4). Five widespread species (C. castanops, G. bursarius, T. bottae, T. talpoides, and T. townsendii) collectively occupy all of the rangeland ecoregions of western North America except the Nebraska sandhills (Fig. 15.4). Maximum adult body sizes vary from about 90 to 630 g; most species weigh less than 400 g (Reid 2006), and are similar morphologically and behaviorally. They are mostly active at night, and unlike prairie dogs and ground squirrels, are described as solitary, territorial, and asocial (Baker et al. 2013). In milder climates and irrigated farmlands, females can have multiple litters per year, whereas those in colder environments and shorter growing seasons tend to have a single litter of 4–6 pups. In highly seasonal environments, two peaks of burrowing activity correspond to the onsets of breeding and juvenile dispersal (Miller 1964). All species are strictly herbivores. In California, gophers move seasonally in response to drying of vegetation or flooding of burrows (Fitch and Bentley 1949).
Geographic ranges of eight species of pocket gophers that inhabit rangelands of western North America, overlaid with the distribution of rangeland ecoregions. The five most widespread species occupy all rangeland ecoregions with the exception of the Sand Hills of Nebraska. Three additional species with restricted ranges are also shown to illustrate their close association with specific ecoregions
Gopher species differ in soil affinities (Miller 1964), with larger species restricted to deeper, sandier soils, and widely distributed species such as T. bottae and T. talpoides inhabiting diverse soil types. Some species are specialized to specific ecoregions, such as T. clusius in the Wyoming Basins, G. texensis on the Edwards Plateau, and G. knoxjonesi in the southern shortgrass prairie (Fig. 15.4). These three species are shown as examples in Fig. 15.4, but nine additional pocket gopher species not displayed have more restricted ranges in western rangelands (Table 15.2). Soil moisture limits smaller Thomomys species. Typically, only one gopher occupies a burrow at a time and, in areas of high gopher activity, the distance between burrow systems is remarkably consistent, regardless of sex, age, or reproductive status (Reichman et al. 1982). Population densities of gophers are highly variable and biased by the size of the area studied; Smallwood and Morrison (1999) estimated an average density of 35 gophers ha−1 for six common western species, although higher densities are possible (49–83 ha−1; Hansen and Remmenga 1961). Population densities are usually estimated by kill- or live-trapping, although there have attempts to convert counts of mounds and burrows to densities (Smallwood and Morrison 1999).
Kangaroo rats are solitary, bipedal, granivorous rodents (Genoways and Brown 1993). Collectively, the seven most widespread species occur in all of the rangeland ecoregions in western North America, except for the tall-structured grasslands and savannas of the eastern Great Plains (Fig. 15.5). They dig for seeds in the soil and fill their external fur-lined cheek pouches with seeds that they scatter-hoard in superficial subsurface caches often near their mounds and within their burrows (Brown et al. 1979). They complement their granivorous diet with green grass and insects when available, and they have highly efficient kidneys that enable them to extract water from their food. Body sizes range from 30 to 200 g, with many species weighing around 50 g, while the three largest species (D. deserti, D. ingens, and D. spectabilis) weigh ~ 150 g. Typical litter sizes are 2–4, with some species, e.g., D. merriami, capable of producing larger litters and breeding multiple times in a year if food resources and environmental conditions permit (Kenagy and Bartholomew 1985). Different-sized species of kangaroo rats and other rodents often coexist in the same environment, partitioning seed resources based on seed size (Brown et al. 2000).
Geographic ranges of seven species of kangaroo rats that inhabit rangelands of western North America, overlaid with the distribution of rangeland ecoregions. These seven species collectively occupy all rangeland ecoregions, with the exception of tall-structured grasslands in the Tallgrass Prairie, Prairie-Forest Border, and Aspen Parkland
Because of their ability to respond numerically to pulses of production of seed resources, population densities of kangaroo rats are highly variable, among species and populations, and across years. Their populations are typically monitored with Sherman live traps in trapping grids or webs. Lima et al. (2008) reported densities in southern Arizona ranging from approximately 2–16 ha−1 for D. ordii and 18–50 ha−1 for D. merriami; average densities for three species at the same site ranged from 2 to 12 ha−1 (Brown and Zongyong 1989), which is similar to estimates in other habitats (e.g., Orland and Kelt 2007; Stapp et al. 2008). Kangaroo rats also have been extensively studied as part of long-term experiments that have revealed much about their ecologies and species co-existence at the community level (Brown and Heske 1990; Kelt 2011).
3 Role of Burrowing Rodents as Ecosystem Engineers
Burrowing rodents have unusually disproportionate effects on rangelands because they (1) engineer their environment through burrow construction and modification of vegetation structure, (2) influence ecosystem processes including above ground plant production, nutrient cycling rates, and water infiltration patterns, (3) alter plant community composition, and (4) provide a prey base for a diverse array of predators (Coggan et al. 2018). In some cases, engineering effects create or enhance habitat for certain faunal species that uniquely inhabit burrows or colonies of these rodents. Modifications to vegetation structure, species composition, and nutrient content potentially have significant effects on coexisting large grazers, including domestic livestock (Krueger 1986; Derner et al. 2006; Augustine and Springer 2013). While all of the burrowing rodents we discuss here serve as ecosystem engineers in rangelands, the strength and specific nature of these effects varies among the different taxa, and among different types of rangelands (Stapp 1998; Cully et al. 2010a, b; Baker et al. 2013; Fig. 15.6). Furthermore, the strength and nature of effects can be contingent on rainfall patterns (Augustine and Springer 2013) and the spatial extent of areas occupied by different species (Derner et al. 2006).
Examples of burrow mounds created by prairie dogs and ground squirrels, and their effects on vegetation on and surrounding the mounds. Upper panels show the contrast between vegetation height and composition in the absence of prairie dogs a versus on an active black-tailed prairie dog (C. ludovicianus) colony b near the peak of the growing season in northern mixed-grass prairie on the Buffalo Gap National Grassland, South Dakota. Photos (a) and (b) were taken within 200 m of one another on the same day, and include a cow pat of approximately the same size in the lower right corner for scale. Panel c shows closely cropped vegetation on Gunnison’s prairie dog (C. gunnisoni) colony during the growing season at the Sevilleta National Wildlife Refuge, New Mexico, and d shows the extent of bare soil on black-tailed prairie dog colony during the dormant season in the Chihuahuan Desert, near Janos, Mexico. Insets in (c) and (d) show Gunnison’s and black-tailed prairie dogs respectively. Photo e illustrates the effect of a California ground squirrel (O. beecheyi) burrow mound on the plant community and bare soil exposure, and f illustrates the fan of soil left at the entrance of a recently excavated burrow. In (f), the burrow entrance is approximately 11 cm in diameter, and the inset illustrates a social group of California ground squirrels. Photo credits a, b David Augustine, c, d Ana Davidson, e, f Paul Stapp/Jennifer Smith
3.1 Prairie Dogs and Ground Squirrels
Large body size of C. ludovicianus relative to other rodents and high social complexity contribute to their ability to occur at high densities and exert dramatic effects on vegetation within their colonies. This includes a substantial increase in bare soil exposure, reduced vegetation height and biomass, and increased abundance of annual forbs, grazing-tolerant grasses, and some unpalatable subshrubs (e.g., Coppock et al. 1983; Cid et al. 1991; Hartley et al. 2009; Augustine et al. 2014; Fig. 15.6). Plant diversity is typically enhanced on versus off colonies, although diversity may decline with increasing years of occupancy as dominant mid-height grasses are lost (Archer et al. 1987; Fahnestock and Detling 2002). Mounds at burrow entrances are the most conspicuous aspect of prairie dog colonies, but typically only cover about 2% of the total colony area (Stapp et al. 2008). The unique habitats that colonies and mounds provide for plants and animals increases diversity across the landscape (Davidson et al. 2012). However, prairie dog grazing activity combined with mounds can increase total bare soil exposure to > 50% in some cases, even during the growing season (Augustine and Derner 2012). All prairie dog species construct extensive burrow systems that are typically 5–14 m in length and extend 1–2 m in depth below the ground’s surface. Burrow construction results in substantial mixing of soil horizons, estimated to affect 200–225 kg of soil per burrow system for C. ludovicianus (Whicker and Detling 1988), and burrow mounds have increased soil nutrient concentrations and water infiltration rates (Barth et al. 2014). Soil disturbance and intense grazing by prairie dogs accelerates nitrogen mineralization in the soil and uptake by plants, thereby improving forage quality for large herbivores (Holland et al. 1992; Fahnestock and Detling 2002; Augustine and Springer 2013).
The extent of bare soil exposure created on colonies provides key nesting habitat for mountain plovers (Charadrius montanus) throughout the western Great Plains (Dinsmore et al. 2005; Augustine and Derner 2012; Duchardt et al. 2019; Table 15.3), and enhances habitat for other birds such as horned larks (Eremophila alpestris), mourning doves (Zenaida macroura) and upland sandpipers (Bartramia longicauda; Augustine and Baker 2013; Geaumont et al. 2019) and some non-fossorial rodents (Stapp 1997; Cully et al. 2010a). C. ludovicianus burrows additionally provide essential nest sites for burrowing owls (Athene cunicularia; Desmond et al. 2000) and winter hibernacula for prairie rattlesnakes (Crotalus viridis; Shipley and Reading 2006). C. ludovicianus frequently girdle sagebrush, which creates uniquely herbaceous-dominated patches in some northern portions of their range (Baker et al. 2013) and suppress invasion by undesired shrubs such as mesquite (Prosopsis glandulosa) in the south (Weltzin et al. 1997; Ponce-Guevara et al. 2016; Hale et al. 2020).
The influence of the three white-tailed species of prairie dog on rangelands in some cases can be similar to black-tailed prairie dogs, such as creating open grassland and burrows that provide habitat for grassland fauna (Keeley et al. 2016; Davidson et al. 2018). However, in portions of their range, C. gunnisoni and C. lucurus have less effect on bare soil exposure and vegetation height, relative to C. ludovicianus, and do not clip or girdle shrubs (Baker et al. 2013). As a result, the white-tailed species have fewer cascading effects on ground-nesting birds, and domestic and native large herbivores. For example, because some rangelands inhabited by C. gunnisoni already have substantial bare soil exposure due to aridity, their grazing effect on vegetation is not necessary to create breeding habitat for mountain plovers (Pierce et al. 2017). C. gunnisoni can have substantial effects in some rangelands (e.g., Chihuahuan desert grassland; Davidson and Lightfoot 2008), but lesser effects in others (e.g., Stapp 1998, Baker et al. 2013).
Like prairie dogs, gregarious species of ground squirrels alter ecosystems through burrow construction and effects on rangeland vegetation. In particular, O. beecheyi imposes disproportional effects on rangelands through construction of burrows used for shelter and breeding, which results in soil mixing and deposition of large, fan-shaped mounds at burrow entrances. A typical tunnel is roughly 5 m in length (Van Vuren and Ordeñana 2012), but soil type and squirrel density influence length and complexity of tunnels, varying from 0.9 to 70 m (Grinnell 1923). Most burrows have interconnected tunnels with multiple (e.g., 6–20) openings to the surface, each with an average diameter of 11 cm (Grinnell 1923). In contrast, U. e. elegans’ burrow construction involves excavation of sticks, rocks, and sagebrush leaves to produce a pile of debris near each entrance (Andelt and Hopper 2016). Burrow construction and forage consumption by another ground squirrel, U. richardsonii, promotes overall plant community diversity and soil nitrate content on intensely grazed rangelands (Newediuk et al. 2015). Burrows of most ground squirrel species are used by a diversity of commensal species, including amphibians, reptiles and mammals (Lenihan 2007; McCullough Hennessy et al. 2016; Conway 2018; Table 15.3).
3.2 Pocket Gophers and Kangaroo Rats
Because pocket gophers are small and asocial, their engineering effects on aboveground vegetation appear less intensive than that of prairie dogs, and their effects primarily occur via soil disturbance. Pocket gophers create mounds by pushing soil through burrows to the surface in inclined tunnels, and mounds can be distinguished from those of other rodents and moles by their crescent shape and visible soil plug, which will be quickly replaced if removed. Individual mounds typically range in size from 20 to 50 cm; however, in areas of high activity, mounds exist as irregular clusters and disturbed soils overlying burrows. The density and coverage of mounds varies with soil type, texture, and topography, ranging up to about 20% (Laycock and Richardson 1975; Grant et al. 1980; Carlson and Crist 1999; Stapp et al. 2008), or in unusual cases up to 50% (Stromberg and Griffin 1996). Burrows tend to be 10–70 cm belowground (Wilkins and Roberts 2007). Burrow diameter, depth and length vary across and even within species, and may be more related to plant distribution and soil characteristics than to body size (Romañach et al. 2005; Wilkins and Roberts 2007). Due to the highly clustered nature of gopher mounds and burrows at low and intermediate densities (Hansen and Remmenga 1961), the effects of gophers are likely to be more spatially heterogeneous than those of prairie dogs, and more widespread on the landscape. The density and dispersion of gopher populations ultimately reflect the availability and spatial patterning of preferred food plants and the friability of the soil, which are determined by soil type, topography, and land-use practices (Huntly and Inouye 1988). Gopher species differ in their affinities for particular soil types and textures (Miller 1964), with larger species restricted to deeper, sandier soils, and widely distributed species such as T. talpoides and T. bottae inhabiting a broad range of soil conditions.
Gophers are capable of transporting large amounts of soil: synthesizing studies of five common western gopher species, Smallwood and Morrison (1999) estimated soil excavation rates of 12.6–21.7 m3 ha−1 yr−1 (mean 17.6 m3 ha−1 yr−1). A single gopher produces roughly 110 mounds per year (Romañach et al. 2007), mixing soil and nutrients between the surface and deeper soil layers (Huntly and Inouye 1988). Climate or land-use history of a given location can influence the degree to which mounds have more or less nutrients and moisture than off-mound sites. Regardless of location, mounds increase spatial heterogeneity and microtopographic variation, which affects primary productivity and plant communities (Reichman and Seabloom 2002). Mounds bury individual plants, creating small-scale openings where early seral species can establish. Increased availability of nitrogen adjacent to mounds enhances plant growth (Reichman 2007); in Colorado, this led to a 5.5% increase in primary productivity, which more than offset the loss of plants covered by mounds (Grant et al. 1980). By 3–4 years after appearance of a mound, cover of perennial and annual forbs increases (Foster and Stubbendieck 1980). In California, gopher mounds also favor establishment of exotic annual plants (Stromberg and Griffin 1996). As a consequence of the vertical mixing of the soils and increased spatial heterogeneity of resources and vegetative cover, gopher disturbances increase soil fertility, water flow, and plant species diversity (Reichman and Seabloom 2002), and in the some areas, alter soil development (Mielke 1977).
Vaughan (1961) reported at least 22 species of vertebrates using gopher mounds in Colorado and that the occurrence of tiger salamanders (Ambystoma tigrinum) was directly related to the presence of mounds. Connior (2011) documented 45 vertebrate species and numerous arthropods that were associated with G. bursarius habitat. The friable soils on mounds are used for dustbathing by grasshopper mice (Onychomys leucogaster) and the abundance of arthropods on mounds compared to other microhabitats may explain why foraging mice are oriented to gopher mounds, as they are with prairie dog burrows (Stapp 1997; Kraft and Stapp 2013). Gopher mounds enhance abundance of certain grasshopper species by providing favorable oviposition and sunning sites, in addition to a diversity of potential food plants (Huntly and Inouye 1988). Root foraging by gophers in Idaho resulted in higher damage to plants from mobile, chewing insects and decreased abundance of sedentary, sucking insects, such as aphids (Ostrow et al. 2002). Gophers also indirectly increase plant reproductive success by altering interactions between plants and their pollinators (Underwood and Inouye 2017).
Like pocket gophers, kangaroo rats are often described as ecosystem engineers, keystone species, or as a keystone guild (Davidson and Lightfoot 2008; Prugh and Brashares 2012). Experimental exclusion of multiple kangaroo rat species from grasslands has illuminated their effects on community structure and composition through selective harvesting of large seeds. For example, long-term removal of kangaroo rats from Chihuahuan Desert induced a transition from shrubland to a grassland dominated by an introduced, large-seeded grass (Brown and Heske 1990). Through a combination of selective foraging, burrowing, and other soil disturbances, D. ingens increased plant gamma diversity, biomass, and productivity, invertebrate biomass and diversity, and lizard and squirrel densities in the Carrizo Plain of California (Prugh and Brashares 2012). Similarly, removal of D. stephensi decreased overall plant species diversity and bare ground, causing dramatic increases in an introduced annual forb (Brock and Kelt 2004).
The larger species of kangaroo rats (D. deserti, D. ingens, D. spectabilis) create large mounds or precincts (e.g., 3–10 m in diameter) that dot rangeland landscapes (Davidson and Lightfoot 2008; Prugh and Brashares 2012; Fig. 15.7). Although mounds appear aggregated at landscape scales due to the spatial patterning of soils, plant communities, and livestock grazing, they are uniformly dispersed at finer scales, reflecting the intensity of intraspecific competition (Schooley and Wiens 2001). Mounds represent nutrient-rich patches with distinct soils and plant and animal assemblages compared to adjacent off-mound areas, increasing landscape heterogeneity and biodiversity (e.g. Davidson and Lightfoot 2008; Koontz and Simpson 2010; Fig. 15.7). In some cases, large kangaroo rat mounds can facilitate establishment of exotic weed species, because of the disturbed mound soil (Schiffman 1994). Grasshoppers that associate with bare soil and annual plants are abundant on the kangaroo rat mounds, as are insects that consume annual plant seeds (Davidson and Lightfoot 2007). Lizards use the mounds for basking and squirrels and prairie dogs use them as open, high points for viewing the landscape (Davidson et al. 2008; Prugh and Brashares 2012). Kangaroo rat mounds are honeycombed with shallow burrows, and provide homes and refugia for numerous species of arthropods, amphibians, lizards, snakes, other rodents, and rabbits (Hawkins and Nicoletto 1992; Hawkins 1996; Davidson and Lightfoot 2007; Prugh and Brashares 2012; Table 15.3). For example, Davidson and Lightfoot (2007) found that banner-tailed kangaroo rat (D. spectabilis) burrows enhanced abundance and species richness of multiple trophic and taxonomic groups of surface-active arthropods, as well as obligate arthropod burrow specialists. Burrows are also modified and used by larger vertebrates such as kit foxes (Vulpes macrotis), burrowing owls, American badgers (Taxidea taxus), and weasels (Hawkins and Nicoletto 1992; Conway 2018).
Examples of mounds and surface disturbance created by the burrowing activities of pocket gophers in California annual grasslands (a) with a Botta’s pocket gopher (T. bottae) shown in the inset, and by banner-tailed kangaroo rats (D. spectabilis) in the Janos grasslands of northern Chihuahua, Mexico, with a banner-tailed kangaroo rat in the inset. Photo credits a Paul Stapp, b Ana Davidson/David Lightfoot
4 Predators of Burrowing Rodents
Burrowing rodents in rangelands of western North America also serve as a key prey source supporting a diverse array of predators. The endangered black-footed ferret (Mustela nigripes) is well-known to rely exclusively on prairie dogs as a prey source, and long-term conservation of this species depends upon landscape-scale prairie dog conservation (Biggins and Eads 2018). In portions of their range, Mexican kit foxes (Vulpes macrotis zinseri) also rely on prairie dogs and their burrows as a critical habitat (Moehrenschlager et al. 2007). The abundance of ferruginous hawks (Buteo regalis) and American badgers (Taxidea taxus) are both closely tied to the abundance of prairie dogs, ground squirrels, and pocket gophers across much of their range (Goodrich and Buskirk 1998; Cook et al. 2003; Lomolino and Smith 2003; Lenihan 2007). Most ground squirrels contribute to biodiversity as major prey items for a diverse assemblage of snakes, raptors, and predatory mammals, including O. beecheyi (Lenihan 2007; McCullough Hennessy et al. 2016), U. richardsonii (Michener 1979), U. u. elegans (Andelt and Hopper 2016), U. columbianus (Macwhirter 1991), and U. armatus (Minta et al. 1992). Although pocket gophers spend relatively little time above ground, they are surprisingly common prey in the diet of many raptors and owls, presumably because soil movement reveals their presence, and their vision is poor (Cartron et al. 2004).
5 Interactions with Livestock
5.1 Prairie Dogs and Ground Squirrels
Diets of prairie dogs overlap substantially with livestock, and all five species have experienced widespread efforts to control their populations, typically via rodenticides (Detling 2006; Miller et al. 2007). Prairie dogs significantly increase bare soil exposure and reduce vegetation height and biomass on their colonies across a diverse suite of rangeland ecosystems (Baker et al. 2013). This creates substantial concern for livestock producers because forage limitations during dry periods are a major determinant of long-term stocking rates on western rangelands. Traditional models of rangeland dynamics (reviewed by Briske et al. 2005) also frequently associate vegetation conditions on prairie dog colonies with a degraded or overgrazed state (e.g., Augustine et al. 2014). However, prairie dogs are also well-known to enhance protein content and digestibility of forage on their colonies, and cattle sometimes graze preferentially on colonies (Sierra-Corona et al. 2015), raising questions about when, where, and to what extent prairie dogs negatively affect livestock. Early research found minimal differences in cattle weight gains in pasture with versus without black-tailed prairie dogs (O’Meilia et al. 1982), but more recent work found that weight gains of yearling steers declined by 15% as prairie dog abundance increased from 0 to 60% of a pasture in shortgrass steppe (Derner et al. 2006).
Prairie dogs influence forage quality and quantity in both shortgrass and mixed grass rangelands, with the largest effects in mixed grass (Augustine and Springer 2013). This can lead to prairie dogs enhancing cattle gains in wet years, but suppressing cattle gains in dry years (Augustine and Springer 2013; Connell et al. 2019). Because prairie dog populations now fluctuate dramatically throughout their range due to plague outbreaks (even in the absence of human control efforts), long-term studies are needed to understand how colony expansion and contraction interact with varying precipitation to influence livestock operations beyond a single growing season. Additionally, suppression of forage quantity by C. ludovicianus during the dormant season (as they are typically active in this time period, with only occasional facultative episodes of torpor; Lehmer and Biggins 2005), can clearly impact livestock operations. These dormant-season effects need to be incorporated into assessments of multi-year effects on livestock operations.
C. ludovicianus also have unique effects on Chihuahuan Desert grasslands, which have undergone widespread transitions from grassland to shrubland encroached by mesquite and other desert shrubs (Van Auken 2009). Prairie dogs clip and girdle desert shrubs to increase their ability to detect predators, and they consume the seedlings of shrubs (Weltzin et al. 1997; Ceballos et al. 2010; Baker et al. 2013; Ponce et al. 2016). By doing so they help maintain grasslands that ranchers depend on for their cattle. Cattle facilitate the presence of prairie dogs through their grazing, keeping vegetation height low, which helps promote the ecological role of prairie dogs in controlling mesquite expansion (Ponce et al. 2016, Sierra-Corona et al. 2015). For example, Ponce et al. (2016) found that mesquite abundance in the Chihuahuan Desert grasslands was up to five times greater in plots without versus with prairie dogs. Mesquite canopy cover increased 61% over a 23-year period following prairie dog eradication (Weltzin et al. 1997). Ceballos et al. (2010) found shrub cover expanded by 34% into a desert grassland over seven years following prairie dog poisoning.
We are unaware of studies directly examining effects of the three white-tailed prairie dog species on livestock. Of the three, C. parvidens may have had the greatest localized effect on livestock during the twentieth century because of their affinity for productive swales, which is likely linked to intensive control efforts across their range, followed by eventual listing as an Endangered Species in 1973 (USFWS 2012). Recovery efforts have aimed to relocate populations in conflict with agriculture on private lands to historically occupied public lands (USFWS 2012). All three white-tailed species hibernate during the dormant season, which reduces effects on dormant-season livestock forage. In addition, growing-season effects of C. gunnisoni and C. leucurus on forage growth are likely to be less than that of C. ludovicianus due to lower population densities and per-animal forage requirements (Grant-Hoffman and Detling 2006; Table 15.1).
Livestock grazing also has reciprocal effects on prairie dog populations. In portions of their range with tall vegetation, C. ludovicianus often depend on livestock or native large grazers to maintain sufficiently short vegetation for them to persist or increase in abundance (Davidson et al. 2010). In these areas, reductions in livestock grazing pressure can be an effective means of discouraging colony expansion or recolonization of former colonies (Cable and Timm 1987; Truett et al. 2001). In more arid rangelands, some prairie dog populations are strongly limited by forage availability during drought (e.g., C. gunnisoni; Davidson et al. 2018), and forage loss to simulated livestock grazing has been shown to suppress population growth (e.g. C. parvidens; Cheng and Ritchie 2006).
Ground squirrel interactions with livestock vary among species in relation to their diet, degree of coloniality, and population density. O. beecheyi often forage on seeds of grasses and oaks, which reduces direct competition with cattle for food (Linsdale 1946). However, during the growing season, O. beecheyi forage almost exclusively on herbaceous vegetation, giving rise to seasonal competition with livestock. In one direct test for cattle-ground squirrel competition, heifers gained more weight during the growing season where O. beecheyi were controlled with rodenticides compared to where they were not (Howard and Bentley 1959). A follow-up study showed that the overall energy requirements of O. beecheyi are minimal (e.g., 94 cal/g/d) and their dietary preferences generally differ from those of cattle (Schitoskey and Woodmansee 1978), but they may still reduce forage for livestock to a greater extent than indicated by energy requirements as a result of clipping and burrowing effects. O. beecheyi also benefits from foraging in open, grazed habitat alongside cattle (Ortiz et al. 2019; Hammond et al. 2019). O. beecheyi can also be highly opportunistic, frequently stealing feed grains, pellets, and molasses lick blocks from facilities with poultry and livestock (Baker 1984), making them a serious agricultural pest, responsible for at least 12–16 million dollars in annual losses in California (Marsh 1998).
Other ground squirrel species that focus heavily on green leaves and grasses, such as U. richardsonii (Michener and Koeppl 1985) and U. armatus (Eshelman and Sonnemann 2000), may overlap more with the foraging niche of grazing livestock. Competition between native rodents and cattle is particularly common when rangelands are heavily grazed by livestock. Drought can amplify the effects of poor management practices, favoring population irruptions such as those well documented for U. richardsonii in Canadian prairies (Proulx 2010). Much like prairie dogs, U. richardsonii population densities are favored in heavily grazed rangelands where squirrels benefit from foraging on leaves, flowers, and seeds in open areas with reduced predator risk (Michener and Koeppl 1985). In tallgrass prairie, potential impacts of P. franklinii on livestock are low compared to those of Urocitellus species because P. franklinii is omnivorous, burrows are dispersed at lower density, and the species declines in mowed or heavily grazed grasslands (Ostroff and Finck 2003).
Although ranchers often express concerns about livestock breaking legs in the holes of burrowing mammals (Minta and Marsh 1988), there is little empirical evidence that burrows are a significant source of injury, and none of the multiple long-term studies of livestock-prairie dog interactions have reported this as an issue. However, Weir et al. (2016) found that 16% of 131 ranchers surveyed from British Columbia, Canada, reported injuries to livestock from burrows during the previous five years. Although pastures were inhabited by ground squirrels (U. columbianus), pocket gophers, marmots (Marmota flaviventris), and badgers, most (79%) of the injuries were caused by rodent burrows, and were to horses (58%) rather than cattle or other livestock, with 25% of the injuries ultimately requiring euthanasia. Many (53%) ranchers also reported damage from burrows to agricultural machinery including swathers and balers, over that period.
5.2 Pocket Gophers and Kangaroo Rats
Interactions between gophers and livestock are not as well-studied as they are for prairie dogs and ground squirrels. Gophers affect livestock mostly through effects on the availability and nutritional quality of forage. Over the short term, the creation of mounds buries forage plants, reducing basal cover, and consumption of belowground parts increases mortality, although these impacts may be offset by higher plant growth of plants adjacent to mounds (Grant et al. 1980; Reichman and Seabloom 2002). Gopher mounds may facilitate the establishment of exotic species (Stromberg and Griffin 1996) and plant species that are unpalatable to livestock (Foster and Stubbendieck 1980). Over the longer term, gopher activity can improve deteriorated rangelands by breaking up compacted soils and redistributing belowground nutrients (Grinnell 1923). In turn, the activities of livestock and other large grazers may influence the abundance and dispersion of gophers via their effects on the productivity and spatial patterning of major food plants (Steuter et al 1995).
To estimate forage loss to pocket gophers, California ground squirrels, and kangaroo rats in rangeland of central California, Fitch and Bentley (1949) stocked each rodent species in separate enclosures, all of which lacked cattle. They estimated that during the wet season, these rodents destroyed 25, 35, and 16% respectively of the potential forage yields in those enclosures. Losses included consumption, trampling, burying live plants, and clipping or caching belowground. Rodents removed comparatively little vegetation during the dry season. They concluded that competition between livestock and rodents is much more significant during the growing season, but the degree of competition depended on rodent population densities and annual variation in herbage production. Turner (1969) estimated that gophers may reduce standing crop biomass in Colorado mountain rangeland by as much as 20%. In western Nebraska, Foster and Stubbendieck (1980) reported that forage production, especially of perennial grasses, in gopher-disturbed pastures was 21–49% lower than in undisturbed pastures.
High densities of gopher mounds traditionally have been considered an indicator of overgrazing (Laycock and Richardson 1975), but mounds also tend to be more conspicuous in disturbed, heavily grazed pastures. Studies quantifying relationships between livestock grazing intensity and mound coverage have not identified consistent relationships (Grant et al. 1980; Stromberg and Griffin 1996; Carlson and Crist 1999).
Kangaroo rats are generally not considered major competitors with livestock in rangelands because they are primarily granivores, but they have been subject to past extermination efforts for rangeland management (Reynolds 1958). In some desert grasslands with heavy livestock grazing, their clipping of perennial grasses, consumption of large-seeded perennial grasses, and dispersal of mesquite seed pods has been suggested to further reduce perennial grass cover and therefore play a role in desertification (Reynolds 1958). However, long-term exclosure experiments (21 years) in the Chihuahuan desert grassland revealed significant increases in mesquite establishment when kangaroo rats were removed, suggesting they actually help prevent shrub invasion and therefore desertification of grasslands (Valone and Thornhill 2001).
6 Impacts of Disease
Prairie dogs and ground squirrels are affected by a wide array of bacterial, viral, parasitic and fungal diseases, some of which are zoonotic and hence of concern for human health (reviewed by Donnelly et al. 2015). Most notably, all of the prairie dog and ground squirrel species in western North America are affected to some extent by enzootic and/or epizootic outbreaks of plague. In contrast, pocket gophers and kangaroo rats are not known to be regulated by plague, nor are they known to be hosts of plague or other major zoonotic diseases. Plague was first documented in O. beecheyi in 1908, and then spread eastward, reaching C. ludovicianus populations in the Great Plains by the 1940s (Cully and Williams 2001; Biggins and Eads 2019). This disease is one of the most important factors currently driving population fluctuations in prairie dogs and ground squirrels. Plague can also be transmitted from prairie dogs and ground squirrels to humans and their pets through fleabites and direct contact with infected animals. As a result, it is important for people managing prairie dogs or ground squirrels to avoid movement of fleas from carcasses or burrows onto themselves.
Epizootic outbreaks of plague periodically decimate prairie dog populations throughout their range, often causing > 95% decline in the size of individual colonies or entire colony complexes (distributed across landscapes of up to > 100,000 ha) within a single year (Cully and Williams 2001; Stapp et al. 2004; Augustine et al. 2008; Cully et al. 2010b). Because plague transmission is density dependent and its spread depends on colony dispersion across the landscape, the introduction of plague to the Great Plains altered the historical metapopulation dynamics of prairie dogs by increasing the likelihood of extinction of large colonies, as well as the smaller, neighboring satellite colonies (Stapp et al. 2004, 2008). In prairie dogs, plague can be transmitted via multiple flea species, but the most important plague vectors are Oropsylla hirsuta and O. tuberculata cynomuris (Salkeld et al. 2016). Rapid plague spread across a large colony complex is related to colony size, low inter-colony distances, and proximity of colonies to dry creek drainages, which prairie dogs use for dispersal (Roach et al. 2001; Stapp et al. 2004; Johnson et al. 2011). Less rapid spread is related to large inter-colony distances, as well as the distribution of roads, lakes and streams that reduce dispersal and plague transmission (Collinge et al. 2005). In addition, overall population response to plague has been less severe for C. leucurus compared to C. gunnisoni and C. ludovicianus, presumably because C. leucurus has lower population densities (up to 10 times lower than that of C. gunnisoni and C. ludovicianus), is less social, and has smaller colony areas (Cully and Williams 2001). Recent studies suggest some populations of C. gunnisoni and C. ludovicianus that experienced plague outbreaks since the 1940s may be less susceptible than populations with no history of plague (Rocke et al. 2012; Busch et al. 2013). Nevertheless, inter and intra-species differences in vulnerability to plague remain. For example, C. gunnisoni and C. parvidens populations from high elevations remain highly susceptible, despite historical exposure (Russell et al. 2018).
How the disease is maintained between epizootics and factors driving epizootics are the subject of substantial ongoing research (Cully et al. 2010b; Salkeld et al. 2016; Biggins and Eads 2019). Uncertainty on these subjects and variability in colony size/connectivity across rangelands makes it difficult to predict the precise location and timing of epizootics, although observed spatial patterns suggest plague is spread by dispersing prairie dogs or carnivores that can move between colonies carrying infected fleas, rather than separate local foci (Stapp et al. 2004; Salkeld et al. 2016). Epizootics typically occur at intervals of 5–15 years, with slow, steady regrowth of the colonies in between (Stapp et al. 2004; Augustine et al. 2008; Hartley et al. 2009; Cully et al. 2010b). The likelihood of epizootics is also influenced by complex interactions between precipitation and temperature and the bacterium, fleas that transmit it, prairie dog health, the presence of amplifying alternate hosts, and the movements of other species capable of moving the pathogen between colonies (Stapp et al. 2004; Salkeld et al. 2016; Eads and Biggins 2017).
Prior to European settlement and the introduction of plague, prairie dog colonies in the western Great Plains were typically large, stable features on the landscape that did not undergo periodic, dramatic plague-induced declines (Knowles et al. 2002). Dramatic expansions of prairie dog colonies between epizootics are not necessarily population increases above historical levels, but rather are population recoveries following population collapse from a non-native disease (Cully et al. 2010b). Plague also kills and is a major impediment to recovery of the endangered black-footed ferret (Matchett et al. 2010). Epizootics can also prevent prairie dog colonies from occupying the same location continuously over long time periods, which reduces their impact on vegetation composition and productivity in the western Great Plains (Hartley et al. 2009; Augustine et al. 2014).
Like prairie dogs, ground squirrels in western rangelands host fleas that can transmit plague (Gage and Kosoy 2005). In California, O. beecheyi in combination with the fleas Orophsylla montana and Hoplopsyllus anomalous, form the principal complex for amplifying plague (Barnes 1982; Lang 2004), which are often detected because outbreaks can result in large numbers of dead squirrels. However, some populations of O. beecheyi show considerable resistance to plague mortality (e.g., Williams et al. 1979), so that they may not experience the extreme die-offs seen in prairie dog populations. Moreover, O. beecheyi hosts have consistent individual differences in their flea abundance/community stability (Smith et al. 2021) and degree of sociality (Smith et al. 2018) across years. Individuals vary considerably in these traits such that host heterogeneity is likely a strong determinant of plague transmission. As with prairie dogs, plague-related fluctuations in O. beecheyi numbers can influence prey availability for multiple predators (Lenihan 2007).
7 Threats
Three primary threats affect prairie dogs and ground squirrels: (1) periodic outbreaks of epizootic plague, (2) direct control by humans via rodenticide and shooting, and (3) loss and fragmentation of habitat. Plague and poisoning can rapidly reduce colony size and density, thereby removing the functional role of prairie dogs and ground squirrels in rangelands for several years until populations recover, with ramifications for prairie-dog/ground squirrel-associated species during these low points. The combination of plague and poisoning can influence metapopulation dynamics, whereby colonies extirpated by plague and/or control rely on recolonization from other colonies, critical to long-term prairie dog population viability. As a result, fragmented land ownership patterns can also threaten populations where lands upon which prairie dogs are controlled are closely interspersed with lands where they are not (Augustine et al. 2021).
Loss of habitat due to expanding human development is affecting some portions of the range of C. ludovicianus, and can even lead to small, isolated populations with unusually high density (Magle et al. 2007), but vast areas of their range remain unaffected. Because C. parvidens has a restricted range and preferentially colonizes productive swales in valleys where grasslands have been converted to housing, golf courses, and hay production, land development continues to affect recovery efforts (USFWS 2012).
Prairie dogs are additionally threatened by drought and climate change. In the southern and far northern portion of their range, drought can greatly limit recruitment, causing population declines and preventing recovery of reintroduced populations (Ceballos et al. 2010; Facka et al. 2010; Davidson et al. 2014; Hayes et al. 2016; Stephens et al 2018). One of the formerly largest remaining colonies of C. ludovicianus in Chihuahuan Desert grasslands (in Janos, Chihuahua, Mexico) has collapsed by 90% (Ceballos et al. 2010; Ponce pers. Comm.). It is uncertain if plague caused the population collapse, but the collapse occurred during multiple years of extreme drought and little offspring recruitment (Ceballos et al. 2010; Ponce pers. comm.). The population still has not recovered, presumably because of increasing aridity and desertification (Ceballos et al. 2010; Ponce pers. Comm.). Prairie dogs are also vulnerable in the northern portion of their range to harsh winters, which could be ameliorated by climate warming (Stephens et al. 2018).
Kangaroo rats that inhabit desert grasslands, such as D. spectabilis, have experienced major population declines due to desertification (Waser and Ayers 2003). Other species of kangaroo rats with small, endemic ranges throughout California’s grasslands and deserts have lost habitat and risk extinction due to expansive urban development, invasion of exotic annual grasses, and widespread agricultural conversion (Goldingay 1997; Longland and Dimitri 2020). Climate change is an increasing threat to kangaroo rats, with loss of habitat and range shifts predicted, as well as increased temperatures expected to exceed physiological tolerance of the desert-adapted rodents (Price et al. 2000; Widick and Bean 2019; Wilkening et al. 2019).
8 Management and Conservation Actions
8.1 Prairie Dogs and Ground Squirrels
As a result of widespread populations declines, four prairie dog species—C. ludovicianus, C. parvidens, C. gunnisoni, and C. mexicanus—have been proposed for listing under the U. S. Endangered Species Act. C. ludovicianus was proposed for listing in 1999. Following reviews, the species was removed as a Candidate for listing in 2004 on the basis that improved state agency surveys estimated 745,750 ha of occupied habitat in the United States (Federal Register Vol 69, No 159, 8/18/2004 pp 55,217–51,226). C. ludovicianus conservation in the United States is currently led by the 11 individual states where the species occurs, coordinated through a multi-state conservation plan (Luce 2003). In Canada, where C. ludovicianus inhabits a restricted portion of Saskatchewan that includes Grasslands National Park, the species is listed as threatened. C. ludovicianus also occupies a portion of the northern Chihuahuan Desert in Mexico, where it is listed as endangered and populations have contracted over the past three decades in response to severe droughts and shrub invasion (Ceballos et al. 2010). In 2009, the Mexican government established the Janos Biosphere Reserve to advance conservation of prairie dogs and associated species in Mexico.
C. gunnisoni was removed as a Candidate for listing in 2013 on the basis that occupancy surveys indicated that populations had stabilized, and dusting burrows with insecticide effectively controls plague (Federal Register Vol 78, No 220, 11/14/2013, ppl 68,660–68,665). C. parvidens was listed as an Endangered Species in the U.S. in 1973, and downlisted to threatened in 1984. Populations are limited to seven counties in southwest Utah, and have either increased or remained stable over the past 30 years (USFWS 2012). Management to recover populations of both C. gunnisoni and C. parvidens has focused primarily on translocations from agricultural fields and urban conflict areas onto public lands where they historically occurred (USFWS 2012; Nelson and Theimer 2012; Curtis et al. 2014). Attempts to translocate C. gunnisoni to historic sites in the southern portion of their range have been compromised by severe drought (Davidson et al. 2014).
C. mexicanus is listed as Endangered by the U.S. and Mexican governments, and occurs in six valleys within the south-central Chihuahuan Desert of Mexico, primarily on low-productivity rangelands with gypsum-derived soils (Yeaton and Flores-Flores 2006). Following decades of range contraction, populations may have stabilized where rangelands cannot be converted to cropland due to lack of irrigation water (Yeaton and Flores-Flores 2006); there is no formal conservation or recovery plan.
Public lands with a focus on C. ludovicianus conservation include Charles M. Russell and UL Bend National Wildlife Refuges (MT), Theodore Roosevelt, Badlands and Wind Cave National Parks (ND and SD), and Grasslands National Park (SK). Public lands with a multiple use focus that includes balancing prairie dog conservation and livestock production include 14 National Grasslands in 9 states managed by the US Forest Service, and areas administered by the Bureau of Land Management in New Mexico, Wyoming, and Montana. Because most of these public lands are closely intermingled with private lands, cross-boundary management of prairie dogs to both conserve populations and minimize impacts on livestock producers has emerged as a key management issue (e.g., Miller et al. 2007; Augustine et al. 2021). Tribal lands in the northern Great Plains also host extensive populations of C. ludovicianus, and in some cases are contiguous with public lands.
Management to enhance prairie dog populations primarily relies on plague mitigation and population translocations. The application of insecticides (e.g., deltamethrin) directly to burrows to control the flea vector is effective in preventing epizootic plague and maintaining prairie dog genetic diversity (Jones et al. 2012; Eads and Biggins 2019). Recent studies also show that application of flour-based baits containing fipronil that are consumed by prairie dogs can suppress fleas for up to a year (Eads et al. 2021). However, both external and internal insecticides still require labor-intensive application to colonies annually. A bait-based vaccine increased survivorship in field trials (Rocke et al. 2017), but it is still undergoing evaluation and refinement as a management tool. Best practices for translocation are discussed by Truett et al. (2001), Curtis et al. (2014) and Davidson et al. (2018).
Conversely, management to reduce or eliminate prairie dog populations primarily occurs through direct poisoning with rodenticides consisting of toxicants or anticoagulants, and recreational shooting. The latter does not as dramatically affect numbers as poisoning efforts, but shooting can significantly alter behavior and suppress reproduction in local populations (Pauli and Buskirk 2007a) and is of concern due to lead fragment impacts on mammals and birds that scavenge carcasses (Pauli and Buskirk 2007b). Similarly, anticoagulants are of concern due to their impact on scavengers (Witmer et al. 2016).
One example of how all these management tools can be combined with cross-jurisdictional collaboration to enhance prairie dog conservation is in a portion of South Dakota encompassing Badlands National Park, Buffalo Gap National Grassland, the Pine Ridge Indian Reservation, and intermingled private lands. Here, management to enhance prairie dog populations by controlling plague (primarily via insecticides) in conservation zones is implemented alongside management to control prairie dogs via poisoning within boundary zones to prevent dispersal onto privately owned rangelands. Management of both plague and prairie dogs across jurisdictions at this landscape scale has been essential in sustaining a wild population of black-footed ferrets at this site (Phillips et al. 2020).
In contrast to prairie dogs, ground squirrel species described in this chapter are of least concern and their robust numbers are often the focus of integrated pest management programs; these include: (i) monitoring, (ii) preventative practices (e.g., educating farmers), and (iii) implementation of a variety of control methods (e.g., mechanical, physical, biological and chemical; Andelt and Hopper 2016). Over the past century, extensive efforts have been undertaken to control most rangeland ground squirrel species (e.g., Gilson and Salmon 1990; Marsh 1994; Proulx 2010). The application of toxicants, and to a lesser extent the use of shooting or fumigants, continue to be the most widely used control methods (e.g., Marsh 1994; Baldwin et al. 2014). The state of California now bans the use lead bullets because secondary ingestion of O. beecheyi carcasses can be lethal for wildlife at higher trophic levels (Smith et al. 2016). Historical effects include the killing of endangered species such as California condors (Gymnogyps californianus) and native predators (Marsh et al. 1987; Pattee et al. 1990). Toxic fumigants applied to O. beecheyi burrows may also kill commensal species, such as the California tiger salamander (Ambystoma californiense) and burrowing owl. Recreational shooting of other ground squirrel species also occurs throughout their ranges; carcasses of shot squirrels are known to pose a lead poisoning hazard for scavengers of U. richarsonii (Knopper et al. 2006) and U. beldingi (Herring et al. 2016).
While there has been some success in using targeted poisons applied at times that minimize death of nontarget species (Whisson 1999), nonlethal controls (e.g., habitat modification, translocations) can also be effective alternatives (Gilson and Salmon 1990; McCullough Hennessy et al. 2016; Swaisgood et al. 2019). Plague in ground squirrels can be suppressed effectively through application of insecticide at bait stations or burrow entrances (Barnes 1982). Despite this, lethal methods such as poisoning and gassing remain the most widely used method to reduce the size of the potential plague reservoir in rangelands (Wobeser 1994).
8.2 Pocket Gophers and Kangaroo Rats
For most species and throughout their ranges, pocket gophers are either considered innocuous or managed as pests. Control methods include flood irrigation, trapping, fumigants, or toxic baits (Baldwin et al. 2014), although most methods are effective only at small scales. There is increasing interest in biological control methods, such as erecting nest boxes for barn owls (Tyto alba; Browning et al. 2016), but degree of efficacy is not clear (Moore et al. 1998). In the past, kangaroo rats experienced some population control (Reynolds 1958); zinc and aluminum phosphide are approved to reduce kangaroo rat damage in some areas, but these animals are rarely considered pests and many species and populations are declining.
Some pocket gopher species (e.g., T. clusius) and subspecies (T. bottae curtatus) are of state or federal conservation concern. Six rangeland species of Dipodomys (elator, ingens, nitratoides exilis, nitratoides nitratoides, spectabilis, and stephensi) are considered Endangered, Vulnerable, or Near-Threatened by IUCN. Conservation efforts have primarily focused on establishing small parks and natural areas to prevent further habitat loss to urban and agricultural development, restoring habitat on these lands, and genetic studies to understand variation among disjunct populations (e.g., Price and Endo 1989; USFWS 2020).
9 Conclusion
Our review of the distribution and impact of ground squirrels, pocket gophers, and kangaroo rats in western North America highlights the strong and geographically widespread effects they have on rangeland ecosystems. Despite many differences among species and functional groups of burrowing rodents in terms of body size, diet, habitat ecology, and physiology, they all function as key agents of soil disturbance, engineers of belowground refugia, modifiers of vegetation structure and plant community composition, and a prey base for a suite of predators. Of these ecosystem functions, the extent of vegetation modification varies, with the more social, herbivorous, and larger-bodied prairie dogs having the strongest effects on vegetation structure and composition, and the non-social, granivorous kangaroo rats having comparatively more subtle effects. However, all four groups of burrowing rodents reviewed here can induce substantial movement and mixing of soils in rangelands, with important consequences for nutrient cycling, water infiltration, soil structure, the establishment of early-successional plants, and for associated species that use their burrows.
As the extent of rangelands contract in the face of human development and cropland conversion and as livestock production intensifies to meet the demands of our growing population, the management and conservation of burrowing rodents is likely to become both increasingly important and controversial on western rangelands. The conservation of burrowing rodents with relatively restricted ranges (e.g., C. parvidens, D. ingens) may be achieved through localized protection in parks and preserves, translocations to public lands where conflicts with livestock and human development are minimized, and the development of a plague vaccine. Yet, conservation of specific species at small scales on lands that do not support livestock grazing may not effectively conserve the role burrowing rodents play in creating habitat for associated species and as a prey base for predators across broad rangeland landscapes. Management and conservation efforts that enable burrowing rodents to coexist with livestock and native ungulates across broad landscapes will likely be essential for the conservation of black-footed ferrets, mountain plovers, burrowing owls, and ferruginous hawks, along with a diverse array of herpetofauna, other small mammals, and arthropods. Continued research is needed to develop creative management approaches that minimize impacts of burrowing rodents on livestock producers, while sustaining their ecologically important role as engineers. Such approaches may include both lethal control of burrowing rodent populations in some locations, while simultaneously mitigating plague and enhancing rodent populations in other locations at the spatial scales necessary to sustain associated species. As a result, cross-jurisdictional collaboration and coordination among managers of both public and private lands will be essential for achieving desired outcomes associated with burrowing rodents in rangelands.
References
Andelt WF, Hopper SN (2016) Managing Wyoming ground squirrels: fact sheet. Natural Resources Series: Wildlife, Colorado State University, Fort Collins, CO. https://extension.colostate.edu/topic-areas/natural-resources/managing-wyoming-ground-squirrels-6-505/
Archer S, Garrett MG, Detling JK (1987) Rates of vegetation change associated with prairie dog (Cynomys ludovicianus) grazing in North American mixed-grass prairie. Vegetatio 72:159–166. https://doi.org/10.1007/BF00039837
Augustine DJ, Baker BW (2013) Associations of grassland bird communities with black-tailed prairie dogs in the North American Great Plains. Conserv Biol 27(2):324–334. https://doi.org/10.1111/cobi.12013
Augustine DJ, Derner JD (2012) Disturbance regimes and mountain plover habitat in shortgrass steppe: large herbivore grazing does not substitute for prairie dog grazing or fire. J Wildl Manage 76(4):721–728. https://doi.org/10.1002/jwmg.334
Augustine DJ, Springer TL (2013) Competition and facilitation between a native and a domestic herbivore: trade-offs between forage quantity and quality. Ecol Appl 23(4):850–863
Augustine DJ, Matchett MR, Toombs TP, Cully JF Jr, Johnson TL, Sidle JG (2008) Spatiotemporal dynamics of black-tailed prairie dog colonies affected by plague. Landsc Ecol 23(3):255–267. https://doi.org/10.1007/s10980-007-9175-6
Augustine DJ, Derner JD, Detling JK (2014) Testing for thresholds in a semiarid grassland: the influence of prairie dogs and plague. Rangeland Ecol Manage 67(6):701–709. https://doi.org/10.2111/REM-D-14-00032.1
Augustine DJ, Davidson A, Dickinson K, Van Pelt B (2021) Thinking like a Grassland: challenges and opportunities for biodiversity conservation in the great plains of North America. Rangel Ecol Manag 78:281–295. https://doi.org/10.1016/j.rama.2019.09.001
Bak JM, Boykin KG, Thompson BC, Daniel D (2001) Distribution of wintering ferruginous hawks (Buteo regalis) in relation to black-tailed prairie dog (Cynomys ludovicianus) colonies in southern New Mexico and northern Chihuahua. J Raptor Res 35:124–129
Baker RO (1984) Comingling of Norway and roof rats with native rodents. In: Clark DO (ed) Proceedings of the 10th vertebrate pest conference. University of California, Davis, CA, USA, pp 103–111
Baker B, Augustine D, Sedgwick J, Lubow B (2013) Ecosystem engineering varies spatially: a test of the vegetation modification paradigm for prairie dogs. Ecography 36:230–239. https://doi.org/10.1111/j.1600-0587.2012.07614.x
Barnes A (1982) Surveillance and control of bubonic plague in the United States. In: Edwards M, McDonnell U (eds) Animal disease in relation to animal conservation. Academic Press, Zoological Society of London
Barnes AM (1982) Surveillance and control of plague in the United States. Animal disease in relation to animal conservation. Edwards MA, McDonnell U (eds) Symposia of the Zoological Society of London 50. Academic Press, New York, pp 237–270
Baldwin RA, Salmon TP, Schmidt RH, Timm RM (2014) Perceived damage and areas of needed research for wildlife pests of California agriculture. Integr Zool 9(3):265–279. https://doi.org/10.1111/1749-4877.12067
Bangert RK, Slobodchikoff CN (2006) Conservation of prairie dog ecosystem engineering may support arthropod beta and gamma diversity. J Arid Environ 67(1):100–115. https://doi.org/10.1016/j.jaridenv.2006.01.015
Barth CJ, Liebig MA, Hendrickson JR, Sedivec KK, Halvorson G (2014) Soil change induced by prairie dogs across three ecological sites. Soil Sci Soc Am J 78:2054–2060. https://doi.org/10.2136/sssaj2014.06.0263
Biggins DE, Eads DA (2018) Evolution, natural history, and conservation of black-footed ferrets. In: Biology and conservation of musteloids. pp 340–356. https://doi.org/10.1093/oso/9780198759805.003.0015
Biggins DE, Eads DA (2019) Prairie dogs, persistent plague, flocking fleas, and pernicious positive feedback. Front Vet Sci 6. https://doi.org/10.3389/fvets.2019.00075
Blumstein DT, Armitage KB (1997) Does sociality drive the evolution of communicative complexity? A comparative test with ground-dwelling sciurid alarm calls. Am Nat 150(2):179–200. https://doi.org/10.1086/286062
Brennan JR, Johnson PS, Hanan NP (2020) Comparing stability in random forest models to map Northern Great Plains plant communities in pastures occupied by prairie dogs using Pleiades imagery. Biogeosciences 17(5):1281–1291. https://doi.org/10.5194/bg-17-1281-2020
Briske DD, Fuhlendorf SD, Smeins FE (2005) State-and-transition models, thresholds, and rangeland health: a synthesis of ecological concepts and perspectives. Rangeland Ecol Manage 58:1–10. https://doi.org/10.2111/1551-5028(2005)58%3c1:SMTARH%3e2.0.CO;2
Brock RE, Kelt DA (2004) Keystone effects of the endangered Stephens' kangaroo rat (Dipodomys stephensi). Biol Conserv 116(1):131–139. https://doi.org/10.1016/S0006-3207(03)00184-8
Brown JH, Heske EJ (1990) Control of a desert-grassland transition by a keystone rodent guild. Science 250(4988):1705–1707. https://doi.org/10.1126/science.250.4988.1705
Brown JH, Zongyong Z (1989) Comparative population ecology of eleven species of rodents in the Chihuahuan Desert. Ecology 70(5):1507–1525. https://doi.org/10.2307/1938209
Brown JH, Reichman OJ, Davidson DW (1979) Granivory in desert ecosystems. Annu Rev Ecol Evol Syst 10(1):201–227. https://doi.org/10.1146/annurev.es.10.110179.001221
Brown JH, Fox BJ, Kelt DA (2000) Assembly rules: desert rodent communities are structured at scales from local to continental. Am Nat 156:314–321. https://doi.org/10.1086/303385
Browning M, Cleckler J, Knott K, Johnson M (2016) Prey consumption by a large aggregation of Barn Owls in an agricultural setting. In: Baldwin RA (ed) Proceedings of the 27th vertebrate pest conference. University of California, Davis, CA, USA, pp 337–344
Busch JD, Van Andel R, Stone NE, Cobble KR, Nottingham R, Lee J et al. (2013) J Wildl Dis 49:920–931. https://doi.org/10.7589/2012/08/209
Cable KA, Timm RM (1987) Efficacy of deferred grazing in reducing prairie dog reinfestation rates. In: Eighth Great Plains wildlife damage control workshop proceedings. Rapid City, South Dakota, USA
Carlson JM, Crist TO (1999) Plant responses to pocket-gopher disturbances across pastures and topography. J Range Manage 52(6):637–645. https://doi.org/10.2307/4003635
Cartron JLE, Polechla PJ, Cook RR (2004) Prey of nesting Ferruginous Hawks in New Mexico. Southwestern Nat 49:270–276
Ceballos G, Wilson DE (1985) Cynomys Mexicanus. Mamm Species 248:1–3. https://doi.org/10.2307/3503981
Ceballos G, Davidson A, List R, Pacheco J, Manzano-Fischer P, Santos-Barrera G, Cruzado J (2010) Rapid decline of a grassland system and its ecological and conservation implications. PLoS ONE 5(1):e8562. https://doi.org/10.1371/journal.pone.0008562
Cheng E, Ritchie ME (2006) Impacts of simulated livestock grazing on Utah prairie dogs (Cynomys parvidens) in a low productivity ecosystem. Oecologia 147(3):546–555. https://doi.org/10.1007/s00442-005-0286-y
Cid MS, Detling JK, Whicker AD, Brizuela MA (1991) Vegetational responses of a mixed-grass prairie site following exclusion of prairie dogs and bison. J Range Manage 44(2):100–105. https://doi.org/10.2307/4002305
Coggan NV, Hayward MW, Gibb H (2018) A global database and “state of the field” review of research into ecosystem engineering by land animals. J Anim Ecol 87(4):974–994. https://doi.org/10.1111/1365-2656.12819
Collinge SK, Johnson WC, Ray C, Matchett R, Grensten J, Cully Jr. JF, Gage KL, Kosoy MY, Loye JE, Martin AP (2005) Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the Western USA. Landsc Ecol 20(8):941–955. https://doi.org/10.1007/s10980-005-4617-5
Connell LC, Porensky LM, Scasta JD (2019) Prairie dog (Cynomys ludovicianus) influence on forage quantity and quality in a grazed grassland-shrubland ecotone. Rangeland Ecol Manage 72:360–373. https://doi.org/10.1016/j.rama.2018.10.004
Connior MB (2011) Geomys bursarius (Rodentia: Geomyiae). Mamm Species 43(879). https://doi.org/10.1644/879.1
Conway CJ (2018) Spatial and temporal patterns in population trends and burrow usage of burrowing owls in North America. J Raptor Res 52(2):129–142. https://doi.org/10.3356/JRR-16-109.1
Cook RR, Cartron JLE, Polechla PJ Jr (2003) The importance of prairie dogs to nesting ferruginous hawks in grassland ecosystems. Wildl Soc Bull 31(4):1073–1082
Coppock DL, Detling JK, Ellis JE, Dyer MI (1983) Plant-herbivore interactions in a North American mixed-grass prairie—I. Effects of black-tailed prairie dogs on intraseasonal aboveground plant biomass and nutrient dynamics and plant species diversity. Oecologia 56(1):1–9. https://doi.org/10.1007/BF00378210
Cully JF Jr, Williams ES (2001) Interspecific comparisons of sylvatic plague in prairie dogs. J Mammal 82(4):894–905. https://doi.org/10.1644/1545-1542(2001)082%3c0894:ICOSPI%3e2.0.CO;2
Cully JF, Collinge SK, Van Nimwegen RE, Ray C, Johnson WC, Thiagarajan B, Conlin DB, Holmes BE (2010a) Spatial variation in keystone effects: small mammal diversity associated with black-tailed prairie dog colonies. Ecography 33(4):667–677. https://doi.org/10.1111/j.1600-0587.2009.05746.x
Cully JF Jr, Johnson TL, Collinge SK, Ray C (2010b) Disease limits populations: plague and black-tailed prairie dogs. Vector-Borne Zoonotic Dis 10(1):7–15. https://doi.org/10.1089/vbz.2009.0045
Curtis R, Frey SN, Brown NL (2014) The effect of coterie relocation on release-site retention and behavior of Utah prairie dogs. J Wildl Manage 78(6):1069–1077. https://doi.org/10.1002/jwmg.755
Davidson AD, Lightfoot DC (2007) Interactive effects of keystone rodents on the structure of desert grassland arthropod communities. Ecography 30(4):515–525. https://doi.org/10.1111/j.2007.0906-7590.05032.x
Davidson AD, Lightfoot DC (2008) Burrowing rodents increase landscape heterogeneity in a desert grassland. J Arid Environ 72(7):1133–1145. https://doi.org/10.1016/j.jaridenv.2007.12.015
Davidson AD, Lightfoot DC, McIntyre JL (2008) Engineering rodents create key habitat for lizards. J Arid Environ 72:2142–2149. https://doi.org/10.1016/j.jaridenv.2008.07.006
Davidson AD, Ponce E, Lightfoot DC, Fredrickson EL, Brown JH, Cruzado J, Brantley SL, Sierra-Corona R, List R, Toledo D, Ceballos G (2010) Rapid response of a grassland ecosystem to an experimental manipulation of a keystone rodent and domestic livestock. Ecology 91(11):3189–3200. https://doi.org/10.1890/09-1277.1
Davidson AD, Detling JK, Brown JH (2012) Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Front Ecol Environ 10(9):477–486. https://doi.org/10.1890/110054
Davidson AD, Friggens MT, Shoemaker KT, Hayes CL, Erz J, Duran R (2014) Population dynamics of reintroduced Gunnison’s Prairie dogs in the southern portion of their range. J Wildl Manage 78(3):429–439. https://doi.org/10.1002/jwmg.755
Davidson AD, Shoemaker KT, Weinstein B, Costa G, Radeloff V, Rondinini C, Ceballos G, Graham C (2017) Geography of global mammal extinction risk. PLoS ONE 12(11):1–18. https://doi.org/10.1371/journal.pone.0186934
Davidson AD, Hunter EA, Erz J, Lightfoot DC, McCarthy AM, Mueller JK, Shoemaker KT (2018) Reintroducing a keystone burrowing rodent to restore an arid North American grassland: challenges and successes. Restor Ecol 26(5):909–920. https://doi.org/10.1890/110054
Derner JD, Detling JK, Antolin MF (2006) Are livestock weight gains affected by black-tailed prairie dogs? Front Ecol Environ 4(9):459–464. https://doi.org/10.1890/1540-9295(2006)4[459:ALWGAB]2.0.CO;2
Desmond MJ, Savidge JA, Eskridge KM (2000) Correlations between burrowing owl and black-tailed prairie dog declines: A 7-year analysis. J Wildl Manage 64(4):1067–1075. https://doi.org/10.2307/3803217
Detling JK (2006) Do prairie dogs compete with livestock? In: Hoogland JL (ed) Conservation of the black-tailed prairie dog. Island Press, Washington D.C., USA
Dinsmore SJ, White GC, Knopf FL (2005) Mountain plover population responses to black-tailed prairie dogs in Montana. J Wildl Manage 69(4):1546–1553. https://doi.org/10.2193/0022-541X(2005)69[1546:MPPRTB]2.0.CO;2
Dobson FS, Kjelgaard JD (1985) The influence of food resources on population dynamics in Columbian ground squirrels. Can J Zool 63(9):2095–2104. https://doi.org/10.1139/z85-308
Donnelly TM, Bergin I, Ihrig M (2015) Chapter 7—biology and diseases of other rodents. Laboratory animal medicine: 3rd edn. Academic Press, London, UK, pp 284–350
Duchardt CJ, Beck JL, Augustine DJ (2019) Mountain Plover habitat selection and nest survival in relation to weather variability and spatial attributes of black-tailed prairie dog disturbance. Condor 122(1):duz059. https://doi.org/10.1093/condor/duz059
Eads DA, Biggins DE (2017) Paltry past-precipitation: predisposing prairie dogs to plague? J Wildl Manage 81(6):990–998. https://doi.org/10.1002/jwmg.21281
Eads DA, Biggins DE (2019) Plague management of prairie dog colonies: degree and duration of deltamethrin flea control. J Vect Ecol 44(1):40–47. https://doi.org/10.1111/jvec.12327
Eads DA, Livieri TM, Dobesh P, Childers E, Noble LE, Vasquez MC, Biggins DE (2021) Fipronil pellets reduce flea abundance on black-tailed prairie dogs: potential tool for plague management and black-footed ferret conservation. J Wildl Dis 57:434–438. https://doi.org/10.7589/JWD-D-20-00161
Eshelman BD, Sonnemann CS (2000) Spermophilus Armatus. Mamm Species 637:1–6. https://doi.org/10.1644/1545-1410
Facka AN, Roemer GW, Mathis VL, Kam M, Geffen E (2010) Drought leads to collapse of black-tailed prairie dog populations reintroduced to the Chihuahuan Desert. J Wildl Manage 74(8):1752–1762. https://doi.org/10.2193/2009-208
Fahnestock J, Detling J (2002) Bison-prairie dog-plant interactions in a North American mixed-grass prairie. Oecologia 132:86–95. https://doi.org/10.1007/s00442-002-0930-8
Fitch HS, Bentley JJ (1949) Use of California annual-plant forage by rodents. Ecology 30:306–321. https://doi.org/10.2307/1932612
Foster MA, Stubbendieck J (1980) Effects of the plains pocket gopher (Geomys bursarius) on rangeland. J Range Manage 33:74–78. https://doi.org/10.2307/3898233
Gage KL, Kosoy MY (2005) Natural history of plague: perspectives from more than a century of research. Ann Rev Entomol 50:505–528. https://doi.org/10.1146/annurev.ento.50.071803.130337
Geaumont BA, Hovick TJ, Limb RF, Mack WM, Lipinski AR, Sedivec KK (2019) Plant and Bird community dynamics in mixed-grass prairie grazed by native and domestic herbivores. Rangeland Ecol Manage 72:374–384. https://doi.org/10.1016/j.rama.2018.10.002
Genoways HH, Brown JH (1993) Biology of the Heteromyidae, vol 10. American Society of Mammalogists, Provo, UT, USA
Gilson A, Salmon TP (1990) Ground squirrel burrow destruction: control implications. In: Davis LR, Marsh RE (eds) Proceedings of the 14th vertebrate pest conference. University of California, Davis, CA, USA, pp 97–98
Goldingay RL (1997) The kangaroo rats of California: endemism and conservation of keystone species. Pac Conserv Biol 3(1):47–60. https://doi.org/10.1071/pc970047
Goodrich JM, Buskirk SW (1998) Spacing and ecology of North American badgers (Taxidea taxus) in a prairie-dog (Cynomys leucurus) complex. J Mammal 79(1):171–179. https://doi.org/10.2307/1382852
Grant W, French N, Folse L (1980) Effects of pocket gopher mounds on plant production in shortgrass prairie ecosystems. Southwest Nat 25:215–224
Grant-Hoffman MN, Detling JK (2006) Vegetation on Gunnison’s prairie dog colonies in southwestern Colorado. Rangeland Ecol Manage 59:73–79. https://doi.org/10.2111/1551-5028(2006)59[073:VOGPDC]2.0.CO;2
Grinnell J (1923) The burrowing rodents of California as agents in soil formation. J Mammal 4:137–149
Hale SL, Koprowski JL, Archer SR (2020) Black-tailed prairie dog (Cynomys ludovicianus) reintroduction can limit woody plant proliferation in grasslands. Front Ecol Evol 8:233. https://doi.org/10.3389/fevo.2020.00233
Hammond TT, Vo M, Burton CT, Surber LL, Lacey EA, Smith JE (2019) Physiological and behavioral responses to anthropogenic stressors in a human-tolerant mammal. J Mammal 100(6):1928–1940. https://doi.org/10.1093/jmammal/gyz134
Hansen R, Remmenga E (1961) Nearest neighbor concept applied to pocket gopher populations. Ecology 42:812–814. https://doi.org/10.2307/1933511
Hartley LM, Detling JK, Savage LT (2009) Introduced plague lessens the effects of an herbivorous rodent on grassland vegetation. J Appl Ecol 46:861–869. https://doi.org/10.1111/j.1365-2664.2009.01660.x
Hawkins LK (1996) Burrows of kangaroo rats are hotspots for desert soil fungi. J Arid Environ 32(3):239–249. https://doi.org/10.1006/jare.1996.0020
Hawkins LK, Nicoletto PF (1992) Kangaroo rat burrows structure the spatial organisation of ground-dwelling animals in a semiarid grassland. J Arid Environ 23(2):199–208. https://doi.org/10.1016/s0140-1963(18)30531-7
Hayes CL, Talbot WA, Wolf BO (2016) Abiotic limitation and the C3 hypothesis: isotopic evidence from Gunnison’s prairie dog during persistent drought. Ecosphere 7(12):e01626. https://doi.org/10.1002/ecs2.1626
Heaney LR (1984) Climatic influences of life-history tactics and behavior of North American tree squirrels. In: Murie JO, Michener GR (eds) The biology of ground-dwelling squirrels. University of Nebraska Press, Lincoln, NE, pp 43–70
Helgen KM, Cole FR, Helgen LE, Wilson DE (2009) Generic revision in the Holartic ground squirrel genus Spermophilus. J Mammal 90(2):270–305. https://doi.org/10.1644/07-MAMM-A-309.1
Herring G, Eagles-Smith CA, Wagner MT (2016) Ground squirrel shooting and potential lead exposure in breeding avian scavengers. PLoS ONE 11(12):e0167926. https://doi.org/10.1371/journal.pone.0167926
Holekamp KE (1984) Dispersal in ground-dwelling sciurids. In: Murie JO, Michener GR (eds) The biology of ground-dwelling squirrels. University of Nebraska Press, Lincoln, NE, USA, pp 297–320
Holland EA, Parton WJ, Detling JK, Coppock DL (1992) Physiological responses of plant populations to herbivory and their consequences for ecosystem nutrient flow. Am Nat 140(4):685–706. https://doi.org/10.1086/285435
Hoogland JL (2003) Sexual dimorphism of prairie dogs. J Mammal 84(4):1254–1266. https://doi.org/10.1644/BME-008
Howard WE, Bentley JR (1959) Competition between ground squirrels and cattle for range forage. J Range Manage 12:110–115
Huntly N, Inouye RS (1988) Pocket gophers in ecosystems: patterns and mechanisms. Bioscience 38:786–793. https://doi.org/10.2307/1310788
Johnson TL, Cully JF, Collinge SK, Ray C, Frey CM, Sandercock BK (2011) Spread of plague among black-tailed prairie dogs is associated with colony spatial characteristics. J Wildl Manage 75(2):357–368. https://doi.org/10.1002/jwmg.v75.2
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 69(3):373–386. https://doi.org/10.2307/3545850
Jones PH, Biggins DE, Eads DA, Eads SL, Britten HB (2012) Deltamethrin flea-control preserves genetic variability of black-tailed prairie dogs during a plague outbreak. Conserv Genet 13(1):183–195. https://doi.org/10.1007/s10592-011-0275-0
Keeley WH, Bechard MJ, Garber GL (2016) Prey use and productivity of ferruginous hawks in rural and exurban New Mexico. J Wildl Manage 80(8):1479–1487. https://doi.org/10.1002/jwmg.21130
Kelt DA (2011) Comparative ecology of desert small mammals: a selective review of the past 30 years. J Mammal 92(6):1158–1178. https://doi.org/10.1644/10-MAMM-S-238.1
Kenagy GJ, Bartholomew GA (1985) Seasonal reproductive patterns in five coexisting California desert rodent species. Ecol Monogr 55:371–396. https://doi.org/10.2307/2937128
Knopper LD, Mineau P, Scheuhammer AM, Bond DE, McKinnon DT (2006) Carcasses of shot Richardon’s ground squirrels may pose lead hazards to scavenging hawks. J Wildl Manage 70:295–299. https://doi.org/10.2193/0022-541X(2006)70[295:COSRGS]2.0.CO;2
Knowles CJ, Proctor JD, Forrest SC (2002) Black-tailed prairie dog abundance and distribution in the Great Plains based on historic and contemporary information. Great Plains Res 12:219–254
Koontz TL, Simpson HL (2010) The composition of seed banks on kangaroo rat (Dipodomys spectabilis) mounds in a Chihuahuan Desert grassland. J Arid Environ 74(10):1156–1161. https://doi.org/10.1016/j.jaridenv.2010.03.008
Kraft JP, Stapp P (2013) Movements and burrow use by northern grasshopper mice as a possible mechanism of plague spread in prairie dog colonies. J Mammal 94(5):1087–1093. https://doi.org/10.1644/12-MAMM-A-197.1
Kretzer JE, Cully JF Jr (2001) Effects of black-tailed prairie dogs on reptiles and amphibians in Kansas shortgrass prairie. Southwest Nat 46(2):171–177. https://doi.org/10.2307/3672525
Krueger K (1986) Feeding relationships among bison, pronghorn, and prairie dogs: an experimental analysis. Ecology 67(3):760–770. https://doi.org/10.2307/1937699
Krupa JJ, Geluso KN (2000) Matching the color of excavated soil: cryptic coloration in the plains pocket gopher (Geomys bursarius). J Mammal 81(1):86–96. https://doi.org/10.1644/1545-1542(2000)081%3c0086:MTCOES%3e2.0.CO;2
Lang JD (2004) Rodent-flea-plague relationships at the higher eleveations of San Diego County, California. J Vector Ecol 29(2):236–247
Laycock WA, Richardson BZ (1975) Long-term effects of pocket gopher control on vegetation and soils of a subslpine grassland. J Range Manage 28:458–462. https://doi.org/10.2307/3897222
Lehmer EM, Biggins DE (2005) Variation in torpor patterns of free-ranging black-tailed and Utah prairie dogs across gradients of elevation. J Mammal 86(1):15–21. https://doi.org/10.1644/1545-1542(2005)086%3c0015:VITPOF%3e2.0.CO;2
Lenihan CM (2007) The ecological role of the California ground squirrel (Spermophilus beecheyi). University of California Press, Davis, California, USA
Lima M, Ernest SKM, Brown JH, Belgrano A, Stenseth NC (2008) Chihuahuan desert kangaroo rats: nonlinear effects of population dynamics, competition, and rainfall. Ecology 89(9):2594–2603. https://doi.org/10.1890/07-1246.1
Linsdale JM (1946) The California ground squirrel: a record of observations made on the Hastings natural history reservation. University of California Press, Berkeley, California, USA
Lomolino M, Smith G (2003) Terrestrial vertebrate communities at black-tailed prairie dog (Cynomys ludovicianus) towns. Biol Conserv 115:89–100. https://doi.org/10.1016/S0006-3207(03)00097-1
Longland WS, Dimitri LA (2020) Kangaroo rats: ecosystem engineers on western rangelands. Rangelands 2:1–9. https://doi.org/10.1016/j.rala.2020.10.004
Luce B (2003) A multi-state conservation plan for the black-tailed prairie dog, Cynomys ludovicianus, in the United States addendum to the black-tailed prairie dog conservation assessment and strategy of 3 Nov 1999. Available at https://wafwa.org/wpdm-package/a-multi-state-conservation-plan-for-the-black-tailed-prairie-dog/. Accessed 29 June 2022
Macwhirter RB (1991) Effects of reproduction on activity and foraging behaviour of adult female Columbian ground squirrels. Can J Zool 69(8):2209–2216. https://doi.org/10.1139/z91-30
Magle SB, McClintock BT, Tripp DW, White GC, Antolin MF, Crooks KR (2007) Mark-resight methodology for estimating population densities for prairie dogs. J Wildl Manage 71(6):2067–2073. https://doi.org/10.2193/2006-138
Marsh RE (1994) Current ground squirrel control practices in California. In: Halverson WS, Crabb AC (eds) Proceedings of the 16th vertebrate pest conference. University of California, Davis, CA, USA, pp 61–65
Marsh RE (1998) Historical review of ground squirrel crop damage in California. Int Biodeterior Biodegradation 42:93–99
Marsh RE, Schmidt RH, Howard WE (1987) Secondary hazards to coyotes of ground squirrels poisoned with 1080 or strychnine. Wildl Soc Bull 15:380–385
Matchett MR, Biggins DE, Carlson V, Powell B, Rocke T (2010) Enzootic plague reduces black-footed ferret (Mustela nigripes) survival in montana. Vector-Borne Zoonotic Dis 10(1):27–35. https://doi.org/10.1089/vbz.2009.0053
McCullough Hennessy S, Deutschman DH, Shier DM, Nordstrom LA, Lenihan C, Jp M, Wisinski CL, Swaisgood RR, Sarah McCullough Hennessy C, Diego Zoo S (2016) Experimental habitat restoration for conserved species using ecosystem engineers and vegetation management. Anim Conserv 19:506–514. https://doi.org/10.1111/acv.12266
Menkens GE Jr, Anderson SH (1991) Population dynamics of white-tailed prairie dogs during an epizootic of sylvatic plague. J Mammal 72(2):328–331. https://doi.org/10.2307/1382103
Michener GR (1979) Yearly variations in the population dynamics of Richardson’s ground squirrels. Can Field-Nat 93:363–370
Michener GR, Koeppl JW (1985) Spermophilus Richardsonii. Mamm Species 243:1–8. https://doi.org/10.2307/3503990
Mielke HW (1977) Mound building by pocket gophers (Geomyidae): their impact on soils and vegetation in North America. J Biogeogr 4:171–180. https://doi.org/10.2307/3038161
Miller RS (1964) Ecology and distribution of pocket gophers (Geomyidae) in Colorado. Ecology 45:256–272. https://doi.org/10.2307/1933839
Miller B, Reading R, Biggins D, Detling J, Forrest S, Hoogland J, Javersak J, Miller S, Proctor J, Truett J, Urest D (2007) Prairie dogs: an ecological review and current biopolitics. J Wildl Manage 71:2801–2810. https://doi.org/10.2193/2007-041
Minta SC, Marsh RE (1988) Badgers (Taxidea taxus) as occasional pests in agriculture. In: Crabb CA, Marsh RE (eds) Proceedings of the 13th vertebrate pest conference. University of California, Davis, CA, USA, pp 199–208
Minta SC, Minta KA, Lott DF (1992) Hunting associations between badgers (Taxidea taxus) and coyotes (Canis latrans). J Mammal 73(4):814–820. https://doi.org/10.2307/1382201
Moehrenschlager A, List R, Macdonald DW (2007) Escaping intraguild predation: Mexican kit foxes survive while coyotes and golden eagles kill Canadian swift foxes. J Mammal 88(4):1029–1039. https://doi.org/10.1644/06-MAMM-A-159R.1
Moore T, Van Vuren D, Ingels C (1998) Are Barn Owls a biological control for gophers? Evaluating effectiveness in vineyards and orchards. In: Baker RO, Crabb AC (eds) Proceedings of the 18th vertebrate pest conference. University of California, Davis, CA, USA
Nelson EJ, Theimer TC (2012) Translocation of Gunnison’s prairie dogs from an urban and suburban colony to abandoned wildland colonies. J Wildl Manage 76(1):95–101. https://doi.org/10.1002/jwmg.281
Newediuk LJ, Waters I, Hare JF (2015) Aspen parkland pasture altered by Richardson’s ground squirrel (Urocitellus richardsonii Sabine) activity: the good, the bad, and the not so ugly? Can Field-Nat 129(4):331–341. https://doi.org/10.22621/cfn.v129i4.1755
O’Meilia ME, Knopf FL, Lewis JC (1982) Some consequences of competition between prairie dogs and beef cattle. J Range Manage 5:580–585
Orland MC, Kelt DA (2007) Responses of a heteromyid rodent community to large- and small-scale resource pulses: diversity, abundance, and home-range dynamics. J Mammal 88(5):1280–1287. https://doi.org/10.1644/06-MAMM-A-408.1
Ortiz CA, Pendleton EL, Newcomb KL, Smith JE (2019) Conspecific presence and microhabitat features influence foraging decisions across ontogeny in a facultatively social mammal. Behav Ecol Sociobiol 73(4):42–42. https://doi.org/10.1007/s00265-019-2651-6
Ostroff AC, Finck EJ (2003) Spermophilus Franklinii. Mamm Species 724:1–5. https://doi.org/10.1644/724
Ostrow DG, Huntly N, Inouye RS (2002) Plant-mediated interactions between the northern pocket gopher, Thomomys talpoides, and aboveground herbivorous insects. J Mammal 83(4):991–998. https://doi.org/10.1644/1545-1542(2002)083%3c0991:PMIBTN%3e2.0.CO;2
Pattee OH, Bloom PH, Scott JM, Smith MR (1990) Lead hazards within the range of the California condor. Condor 92:931–937. https://doi.org/10.2307/1368729
Pauli JN, Buskirk SW (2007a) Risk-disturbance overrides density dependence in a hunted colonial rodent, the black-tailed prairie dog Cynomys ludovicianus. J Appl Ecol 44(6):1219–1230. https://doi.org/10.1111/j.1365-2664.2007.01337.x
Pauli JN, Buskirk SW (2007b) Recreational shooting of prairie dogs: a portal for lead entering wildlife food chains. J Wildl Manage 71(1):103–108. https://doi.org/10.2193/2005-620
Phillips P, Livieri TM, Swanson BJ (2020) Genetic signature of disease epizootic and reintroduction history in an endangered carnivore. J Mammal 101(3):779–789. https://doi.org/10.1093/jmammal/gyaa043
Phuong MA, Lim MC, Wait DR, Rowe KC, Moritz C (2014) Delimiting species in the genus Otospermophilus (Rodentia: Sciuridae), using genetics, ecology, and morphology. Bological J Linnean Soc 113:1136–1151
Pierce AK, Dinsmore SJ, Jorgensen D, Wunder MB (2017) Migration routes and timing of Mountain Plovers revealed by geolocators. J Field Ornithol 88(1):30–38. https://doi.org/10.1111/jofo.12184
Plumpton DL, Anderson DE (1997) Habitat use and time budgeting by wintering Ferruginous Hawks. Condor 99:888–893. https://doi.org/10.2307/1370139
Ponce-Guevara E, Davidson A, Sierra-Corona R, Ceballos G (2016) Interactive effects of black-tailed prairie dogs and cattle on shrub encroachment in a desert grassland ecosystem. PLoS ONE 11(5):e0154748. https://doi.org/10.1371/journal.pone.0154748
Price MV, Endo PR (1989) Estimating the distribution and abundance of a cryptic species, Dipodomys stephensi (Rodentia: Heteromyidae), and implications for management. Conserv Biol 3(3):293–301. https://doi.org/10.1111/j.1523-1739.1989.tb00089.x
Price MV, Waser NM, McDonald SA (2000) Elevational distributions of kangaroo rats (Genus Dipodomys): long-term trends at a Mojave Desert site. Am Midl Nat 144(2):352–361. https://doi.org/10.1674/0003-0031(2000)144[0352:EDOKRG]2.0.CO;2
Proulx G (2010) Factors contributing to the outbreak of Richardson’s ground squirrel populations in the Canadian prairies. In: Proceedings of the 24th vertebrate pest conference. University of California, Davis, CA, USA, pp 213–217
Prugh LR, Brashares JS (2012) Partitioning the effects of an ecosystem engineer: Kangaroo rats control community structure via multiple pathways. J Anim Ecol 81(3):667–678. https://doi.org/10.1111/j.1365-2656.2011.01930.x
Rayor LS (1988) Social organization and space-use in Gunnison’s prairie dog. Behav Ecol Sociobiol 22:69–78. https://doi.org/10.1007/BF00395699
Reichman OJ (2007) The influence of pocket gophers on the biotic and abiotic environment. In: Begall S, Burda H, Schleich CE (eds) Subterranean rodents: news from underground. Springer-Verlag, Berlin, pp 271–286
Reichman OJ, Seabloom EW (2002) The role of pocket gophers as subterranean ecosystem engineers. Trends Ecol Evol 17(1):44–49. https://doi.org/10.1016/S0169-5347(01)02329-1
Reichman OJ, Whitham TG, Ruffner GA (1982) Adaptive geometry of burrow spacing in two pocket gopher populations. Ecology 63(3):687–695. https://doi.org/10.2307/1936789
Reid F (2006) Peterson field guide to the Mammals of North America. Houghton Mifflin, New York, NY, USA
Reynolds HG (1958) The ecology of the Merriam kangaroo rat (Dipodomys merriami Mearns) on the grazing lands of southern Arizona. Ecol Monogr 28(2):111–127. https://doi.org/10.2307/1942205
Roach JL, Stapp P, Van Horne B, Antolin MF (2001) Genetic structure of a metapopulation of black-tailed prairie dogs. J Mammal 82(4): 946–959
Rocke TE, Williamson J, Cobble KR, Busch JD, Antolin MF, Wagner DM (2012) Resistance to plague among black-tailed prairie dog populations. Vector Borne Zoonot Dis 12(2):111–116. https://doi.org/10.1089/vbz.2011.0602
Rocke TE, Tripp DW, Russell RE, Abbott RC, Richgels KLD, Matchett MR, Biggins DE, Griebel R, Schroeder G, Grassel SM, Pipkin DR, Cordova J, Kavalunas A, Maxfield B, Boulerice J, Miller MW (2017) Sylvatic plague vaccine partially protects prairie dogs (Cynomys spp.) in field trials. EcoHealth 14(3):438–450. https://doi.org/10.1007/s10393-017-1253-x
Romañach SS, Seabloom EW, Reichman OJ, Rogers WE, Cameron GN (2005) Effects of species, sex, age, and habitat on geometry of pocket gopher foraging tunnels. J Mammal 86(4):750–756. https://doi.org/10.1644/1545-1542(2005)086[0750:EOSSAA]2.0.CO;2
Romañach SS, Seabloom EW, Reichman OJ (2007) Costs and benefits of pocket gopher foraging: linking behavior and physiology. Ecology 88(8):2047–2057. https://doi.org/10.1890/06-1461.1
Russell RE, Abbott RC, Tripp DW, Rocke TE (2018) Local factors associated with on-host flea distributions on prairie dog colonies. Ecol Evol 8(17):8951–8972. https://doi.org/10.1002/ece3.4390
Salkeld DJ, Stapp P, Tripp DW, Gage KL, Lowell J, Webb CT, Brinkerhoff RJ, Antolin MF (2016) Ecological traits driving the outbreaks and emergence of zoonotic pathogens. Bioscience 66(2):118–129. https://doi.org/10.1093/biosci/biv179
Schiffman PM (1994) Promotion of exotic weed establishment by endangered giant kangaroo rats (Dipodomys ingens) in a California grassland. Biodiv Conserv 3(6):524–537. https://doi.org/10.1007/BF00115158
Schitoskey F, Woodmansee SR (1978) Energy requirements and diet of the California ground squirrel. J Wildl Manage 42:373–382. https://doi.org/10.2307/3800273
Schooley RL, Wiens JA (2001) Dispersion of kangaroo rat mounds at multiple scales in New Mexico, USA. Landsc Ecol 16:267–277. https://doi.org/10.1023/A:1011122218548
Shipley BK, Reading RP (2006) A comparison of herpetofauna and small mammal diversity on black-tailed prairie dog (Cynomys ludovicianus) colonies and non-colonized grasslands in Colorado. J Arid Environ 66(1):27–41. https://doi.org/10.1016/j.jaridenv.2005.10.013
Sidle JG, Augustine DJ, Johnson DH, Miller SD, Cully JF Jr, Reading RP (2012) Aerial surveys adjusted by ground surveys to estimate area occupied by black-tailed prairie dog colonies. Wildl Soc Bull 36(2):248–256. https://doi.org/10.1002/wsb.146
Sierra-Corona R, Davidson A, Fredrickson EL, Luna-Soria H, Suzan-Azpiri H, Ponce-Guevara E, Ceballos G (2015) Black-tailed prairie dogs, cattle, and the conservation of North America’s Arid Grasslands. PLoS ONE 10(3):e0118602. https://doi.org/10.1371/journal.pone.0118602
Smallwood KS, Morrison ML (1999) Estimating burrow volume and excavation rate of pocket gophers (Geomyidae). Southwest Nat 44(2):173–183
Smith G, Lomolino M (2004) Black-tailed prairie dogs and the structure of avian communities on the shortgrass plains. Oecologia 138:592–602. https://doi.org/10.1007/s00442-003-1465-3
Smith JE, Long DJ, Russell ID, Newcomb KL, Muñoz VD (2016) Otospermophilus beecheyi (Rodentia: Sciuridae). Mammal Species 48(939):91–108. https://doi.org/10.1093/mspecies/sew010
Smith JE, Gamboa DA, Spencer JM, Travenick SJ, Ortiz CA, Hunter RD, Sih A (2018) Split between two worlds: automated sensing reveals links between above-and belowground social networks in a free-living mammal. Philos Trans R Soc Lond B: Biol Sci 373(1753):20170249. https://doi.org/10.1098/rstb.2017.0249
Smith JE, Smith IB, Working CL, Russell ID, Krout SA, Singh KS, Sih A (2021) Host traits, identity, and ecological conditions predict consistent flea abundance and prevalence on free-living California ground squirrels. Int J Parasitol 51:587–598. https://doi.org/10.1016/j.ijpara.2020.12.001
Stapp P (1997) Habitat selection by an insectivorous rodent: patterns and mechanisms across multiple scales. J Mammal 78(4):1128–1143. https://doi.org/10.2307/1383055
Stapp P (1998) A reevaluation of the role of prairie dogs in Great Plains grasslands. Conserv Biol 12(6):1253–1259. https://doi.org/10.1111/j.1523-1739.1998.97469.x
Stapp P, Antolin MF, Ball M (2004) Patterns of extinction in prairie dog metapopulations: plague outbreaks follow El Niño events. FrontEcol Environ 2(5):235–240. https://doi.org/10.1890/1540-9295(2004)002[0235:POEIPD]2.0.CO;2
Stapp P, Van Horne B, Lindquist M (2008) Ecology of mammals of the shortgrass steppe. In: Lauenroth WK, Burke IC (eds) Ecology of the shortgrass steppe: a long-term perspective. Oxford University Press, Oxford, UK, pp 132–180
Stephens T, Wilson SC, Cassidy F, Bender D, Gummer D, Smith DHV, Lloyd N, McPherson JM, Moehrenschlager A (2018) Climate change impacts on the conservation outlook of populations on the poleward periphery of species ranges: a case study of Canadian black-tailed prairie dogs (Cynomys ludovicianus). Glob Change Biol 24(2):836–847. https://doi.org/10.1111/gcb.13922
Steuter AA, Steinauer EM, Hill GL, Bowers PA, Tieszen LL (1995) Distribution and diet of bison and pocket gophers in a sandhills prairie. Ecol Appl 5(3):756–766. https://doi.org/10.2307/1941983
Stromberg MR, Griffin JR (1996) Long-term patterns in coastal California grasslands in relation to cultivation, gophers, and grazing. Ecol Appl 6(4):1189–1211. https://doi.org/10.2307/2269601
Swaisgood RR, Montagne JP, Lenihan CM, Wisinski CL, Nordstrom LA, Shier DM (2019) Capturing pests and releasing ecosystem engineers: translocation of common but diminished species to re-establish ecological roles. Anim Conserv 22(6):600–610. https://doi.org/10.1111/acv.12509
Thorington RW, Koprowski JL, Steele MA, Whatton JF (2012) Squirrels of the world. Johns Hopkins University Press Books, Baltimore, MD, USA
Tileston J, Lechleitner R (1966) Some comparisons of black-tailed and white-tailed prairie dogs in north-central Colorado. Am Midl Nat 75:292–316. https://doi.org/10.2307/2423393
Truett JC, Dullum JALD, Matchett MR, Owens E, Seery D (2001) Translocating prairie dogs: a review. Wildl Soc Bull 29(3):863–872
Turner GT (1969) Responses of mountain grassland vegetation to gopher control, reduced grazing, and herbicide. J Range Manage 22:377–383
Underwood N, Inouye BD (2017) Pathways for effects of small-scale disturbances on a rare plant: How Mimulus angustatus benefits from gopher mounds. Ecosphere 8(6):e01838. https://doi.org/10.1002/ecs2.1838
U.S. Fish and Wildlife Service (2012) Utah prairie dog (Cynomys parvidens) revised recovery plan. U.S. Fish and Wildlife Service, Denver, CO, USA
U.S. Fish and Wildlife Service (2020) Species status assessment report for the Giant Kangaroo Rat (Dipodomys ingens) U.S. Fish and Wildlife Service, Sacramento, CA plan. U.S. Fish and Wildlife Service, Denver, CO, USA
Valone TJ, Thornhill DJ (2001) Mesquite establishment in arid grasslands: an experimental investigation of the role of kangaroo rats. J Arid Environ 48(3):281–288. https://doi.org/10.1006/jare.2000.0757
Van Auken OW (2009) Causes and consequences of woody plant encroachment into western North American grasslands. J Environ Manage 90(10):2931–2941. https://doi.org/10.1016/j.jenvman.2009.04.023
Van Vuren DH, Ordeñana MA (2012) Factors influencing burrow length and depth of ground-dwelling squirrels. J Mammal 93:1240–1246. https://doi.org/10.1644/12-MAMM-A-049.1
Vaughan TA (1961) Vertebrates inhabiting pocket gopher burrows in Colorado. J Mammal 42:171–174. https://doi.org/10.2307/1376826
Waser PM, Ayers JM (2003) Microhabitat use and population decline in banner-tailed kangaroo rats. J Mammal 84(3):1031–1043. https://doi.org/10.1644/BBa-032
Weir RD, Davis H, Gayton DV, Lofroth EC (2016) Chapter 11. Fact or fantasy? Damage to livestock and agricultural machinery by American badgers and other burrowing mammals in British Columbia, Canada. In: Proulx G, Do Linh San E (eds) Badgers: systematics, biology, conservation and research techniques. Alpha Wildlife Publications, Sherwood Park, Alberta, Canada, pp 299–310
Weltzin J, Archer S, Heitschmidt R (1997) Small-mammal regulation of vegetation structure in a temperate savanna. Ecology 78(3):751–763. https://doi.org/10.1890/0012-9658(1997)078[0751:SMROVS]2.0.CO;2
Whicker A, Detling J (1988) Ecological consequences of prairie dog disturbances. Bioscience 38:778–785
Whisson DA (1999) Modified bait stations for California ground squirrel control in endangered kangaroo rat habitat. Wildl Soc Bull 27:172–177
White GC, Dennis JR, Pusateri FM (2005) Area of black-tailed prairie dog colonies in eastern Colorado. Wildl Soc Bull 33(1):265–272. https://doi.org/10.2193/0091-7648(2005)33[265:AOBPDC]2.0.CO;2
Widick IV, Bean WT (2019) Evaluating current and future range limits of an endangered, keystone rodent (Dipodomys ingens). Divers Distrib 25(7):1074–1087. https://doi.org/10.1111/ddi.12914
Wilkening J, Pearson-Prestera W, Mungi NA, Bhattacharyya S (2019) Endangered species management and climate change: when habitat conservation becomes a moving target. Wildl Soc Bull 43(1):11–20. https://doi.org/10.1002/wsb.944
Wilkins KT, Roberts HR (2007) Comparative analysis of burrow systems of seven species of pocket gophers (Rodentia: Geomyidae). Southwest Nat 52(1):83–88. https://doi.org/10.1894/0038-4909(2007)52[83:CAOBSO]2.0.CO;2
Williams JE, Moussa MA, Cavanaugh DC (1979) Experimental plague in the California ground squirrel. J Infec Dis 140:618–621.https://doi.org/10.1093/infdis/140.4.618
Witmer GW, Snow NP, Moulton RS (2016) Retention time of chlorophacinone in black-tailed prairie dogs informs secondary hazards from a prairie dog rodenticide bait. Pest Manage Sci 72(4):725–730. https://doi.org/10.1002/ps.4045
Wobeser GA (1994) Disease management through manipulation of the host population. In: Wobeser GA (ed) Disease in wild animals. Springer, NY, USA, pp 217–245. https://doi.org/10.1007/978-3-540-48978-8_12
Yeaton RI, Flores-Flores JL (2006) Patterns of occurrence and abundance in colony complexes of the Mexican prairie dog (Cynomys mexicanus) in productive and unproductive grasslands. Acta Zool Mexicana 22(3):107–130
Zegers DA (1984) Spermophilus Elegans. Mammal Species 214:1–7. https://doi.org/10.2307/3503955
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Augustine, D.J., Smith, J.E., Davidson, A.D., Stapp, P. (2023). Burrowing Rodents. In: McNew, L.B., Dahlgren, D.K., Beck, J.L. (eds) Rangeland Wildlife Ecology and Conservation . Springer, Cham. https://doi.org/10.1007/978-3-031-34037-6_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-34037-6_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34036-9
Online ISBN: 978-3-031-34037-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)