Abstract
Throughout the world, hundreds of patients with advanced metastatic prostate cancer are currently being treated with 177Lu-PSMA radioligands on compassionate usage protocols in accord with published guidelines of the European Association of Nuclear Medicine (EANM). However, 7 years after the introduction of 68Ga/177Lu-PSMA theranostic management of metastatic castration-resistant prostate cancer (mCRPC), it remained unapproved by any national regulatory authority, and has yet to achieve oncologist/urologist acceptance into mainstream clinical practice. The reasons for the nonacceptance of 177Lu-PSMA-radioligand therapy (RLT) are explored in this review, which charts the evolution of this very promising treatment modality, pioneered in German, Austrian, and Australian academic hospitals, from which many retrospective reports of efficacy have been published. This efficacy has subsequently been demonstrated by completion of the Pharma randomized controlled trial, the VISION Study which led to formal regulatory approval. However, in order to promote worldwide availability, and to evaluate efficiency in respect of improved survival and quality of life, the proposed WARMTH NIGHTCAP (World Association for Radiopharmaceutical and Molecular Therapy National Investigators Global Harmonised Theranostics of Cancer of Prostate) Study was designed to prospectively audit 68Ga/177Lu-PSMA RLT in a large real-world population of mCRPC patients, in up to 50 countries, now being treated on compassionate access programs. The NIGHTCAP Study did not come to fruition due to the COVID pandemic but the design principles remain valid.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
What evidence is required to establish that 68Ga/177Lu-PSMA radioligand theranostic management of advanced metastatic prostate cancer provides meaningful clinical benefit in terms of prolonged overall survival (OS) and enhanced quality of life (QOL)?
How might it be unequivocally demonstrated that this radionuclide molecular-targeted approach represents a significant, affordable, available improvement in clinical outcome over that achievable with current standard of care, such that it becomes adopted into mainstream clinical oncology practice worldwide?
Where do we start?
If we were to choose 2018 as our point of departure, we would see a tabula rasa of oncologist ignorance, and even denial, of the existence of precision radionuclide targeted diagnosis and therapy of prostate cancer. The comprehensive, authoritative, state-of-the-art review on recent accomplishments and future challenges in management of metastatic prostate cancer, published in the New England Journal of Medicine in 2018 [1] failed to mention either 68Ga-PSMA PET-CT diagnosis or 177Lu-PSMA beta therapy, let alone 225Ac-PSMA alpha therapy. Viewed from a North American perspective, theranostic radionuclide precision oncology does not exist for the quarter million patients with metastatic prostate cancer diagnosed per annum in the USA by superseded CT methodology and 99mTc-MDP bone scans [2].
The first report of 68Ga-PSMA –PET localization of human tumors in prostate cancer patients was published in 2013 [3]. The potential for theranostics was quickly appreciated in Europe and Australia where, over the next 5 years, 68Ga-PSMA-PET replaced CT, and was shown to be superior to MR, in those centers offering this imaging modality [4]. A multicenter German study reported change in intended management in 39% of patients after 68Ga-PSMA-PET-CT [5]. A prospective multicenter Australian study of patients presenting with newly diagnosed, or recurrent, prostate cancer, demonstrated alteration of planned treatment in over half the patients [6]. Both these theranostic management studies were published in 2018, in the American scientific literature.
Most recently, a review of the German national experience showed even greater impact of 68Ga-PSMA-PET-CT on prostate cancer management, occasioning change of intended treatment in two-thirds of patients [7]. It was remarked that 68Ga-PSMA-PET-CT had been incorporated into the German guideline and, more significantly, into the prostate cancer management guideline of the European Association of Urology. Meanwhile, whilst an American-German coauthored paper reported the major impact of 68Ga-PSMA-PET-CT on salvage radiotherapy planning [8], the accompanying, more skeptical, editorial perspective, by a past president of the Society of Nuclear Medicine was entitled “Transformational Change in Prostate Cancer Management?” [9].
The “Appropriate Use Criteria for Imaging Evaluation of Biochemical Recurrence of Prostate Cancer After Definitive Primary Treatment” published April 2020 in the Journal of Nuclear Medicine merely remarks that “the new class of PSMA-targeted PET radiotracers has generated considerable interest and are [sic] discussed briefly, although these agents are currently not approved for routine clinical use in the United States” [10]. The American Society of Clinical Oncology (ASCO) Guideline for optimum imaging strategies for advanced prostate cancer e-published January 2020 in Journal of Clinical Oncology stated “a number of studies have reported on the major impact of PSMA PET imaging on management of patients with prostate cancer, although the potential influence on outcome will need additional investigations” [11]. A 2020 UK review of management of de novo metastatic prostate cancer cited eight diverse definitive studies, none of which made reference to 68Ga-PSMA PET/CT [12]. Significantly, the reviewers remarked that currently no international consensus has been reached on the definition of oligometastatic disease. They did, however, acknowledge that the advent of improved imaging of metastatic disease, such as 68Ga-PSMA, is likely to positively affect the survival outcomes achieved with metastasis-directed therapy [12].
The definitive prospective randomized multicenter phase 3 study of 68Ga-PSMA PET/CT imaging commenced in 2017, in Australia [13]. The results in 302 men with newly diagnosed prostate cancer provide compelling evidence that PSMA PET/CT has better accuracy, with consequent change in management, fewer equivocal results, and lower radiation exposure compared with current standard-of-care imaging with CT and bone scanning [14]. Accuracy of 68Ga-PSMA PET/CT was 27% greater than for conventional imaging (92% vs. 65%). Comparison of sensitivity (85% vs. 38%) and specificity (98% vs. 91%) also demonstrated significant advantage of 68Ga-PSMA PET/CT over CT and bone scanning. The authors conclude that PSMA PET/CT is better than, and can replace, conventional imaging with CT and bone scan for staging men with high-risk prostate cancer before surgery or radiotherapy with curative intent, and they recommended that existing guidelines should be reviewed [14].
It is to be hoped that this well-designed Australian multicenter RCT will persuade oncologists, urologists, and radiologists worldwide to adopt the essential imaging component of the theranostic paradigm. 68Ga-PSMA-PET-CT is the mandated prerequisite for eligibility for 177Lu-PSMA, or 225Ac-PSMA therapy, as encapsulated in Professor Richard Baum’s maxim: “we see what we treat, and we treat what we see.” It is likely, however, that this truth will become self-evident only when the actual efficiency of 177Lu-PSMA radioligand therapy is irrefutably demonstrated.
So, how do we obtain such unassailable evidence?
Academic centers throughout Germany have been applying theranostic management of metastatic castrate-resistant prostate cancer (mCRPC) with 68Ga/177Lu-PSMA since 2013 [15, 16]. Hundreds of patients have been treated on compassionate patient usage protocols, and several retrospective reports of encouraging responses have been published [16,17,18]. In particular, OS was significantly longer in patients who were chemotherapy-naïve [19]. However, the protocols were diverse, patient populations were heterogeneous, and surrogate endpoints varied. The resulting “evidence” of efficacy was not deemed worthy of acceptance by the oncologist community, which demands rigorous prospective clinical trials on agreed protocols, with uniform patient eligibility criteria and predefined endpoints. Notwithstanding the absence of formal oncologist approbation, the manifestly favorable clinical outcomes of 177Lu-PSMA-RLTof mCRPC, achieved with minimal toxicity, have resulted in hundreds of patients requesting treatment with these theranostic agents, which are not currently approved in any regulatory jurisdiction in the world.
The European Association of Nuclear Medicine (EANM) has taken the unprecedented step of preparing a guideline for this unapproved radionuclide therapy, which they acknowledge can be offered individually on the basis of compassionate patient use and in accordance with the best actual knowledge [20]. The ethical basis of the EANM guideline is stated to be: “In line with the declaration of Helsinki, it is considered ethically justified (and a legally recognized necessity of excuse) to apply a well-reasoned but unapproved intervention compared with withholding such a promising treatment from patients due to formal regulatory or administrative issues.” The guideline is intended to provide a base for the harmonization of PSMA-radioligand therapy protocols, wherein the EANM “strongly advocates the development of PSMA-radioligand therapy within the context of adequately powered multicenter clinical trials with appropriate endpoints.”
What form should such clinical trials take, in order to establish efficiency in the global population of prostate cancer patients?
A 2020 review of five key studies of metastasis-directed therapy in men with oligometastatic prostate cancer failed to mention 177Lu/225Ac-PSMA radioligand therapy [12]. It was remarked that large-cohort randomized controlled trials (RCTs) exploring the effects of metastasis-directed therapies would help to establish the potential OS benefits of this approach and possibly overcome the prohibitive financial barrier currently preventing the use of such approaches beyond the clinical trial setting by facilitating insurance coverage and reimbursement [12].
RCTs have been the acknowledged gold standard for evaluation of the efficacy of novel anticancer agents over the past 50 years. However, with the advent of precision oncology, such as radionuclide molecular-targeted therapy of prostate cancer, major flaws have been exposed in RCT methodology [21]. The demonstration of efficacy, in terms of a statistically significant advantage in respect of arbitrary surrogate endpoints in a highly selected patient population, often does not translate to improved survival and QOL in the real world of clinical practice. In fact, most of the novel anticancer agents approved after RCT over the past decade failed to achieve a clinically meaningful benefit on the ASCO and the European Society of Medical Oncology (ESMO) scales which measure efficiency of drugs in terms of substantial improvement of OS and QOL, and which also show cost-benefit [22].
The magnitude of the increasing incidence and mortality of prostate cancer throughout the world render meeting the unmet need for a proven remedy an urgent imperative. RCTs, quite apart from their expense, and highly selected patient population, take years to come to fruition, during which time the affected population-at-large is denied access to the agent being tested.
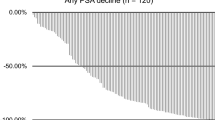
In addition, those patients allocated to the control arm of a RCT are also denied, what is postulated to be, the most effective treatment. For example, 250 of the mCRPC patients assigned to standard-of-care control arm in the VISION RCT (NCT03511664) of 177Lu-PSMA-617-RLT will not be able to receive the active treatment given to the 500 patients on the study arm [23]. The ethical rationale for this deprivation of 177Lu-PSMA-617 in the control cohort is said to be the existence of equipoise, as carefully explained to each RCT participant in the process of obtaining informed consent: “Investigators must impart a clear understanding that 177Lu-PSMA-617 has not, to date, shown any survival advantage or any other metric of clinical benefit over the standard of care.” However, in an earlier prospective study; the pre-VISION Study, using the same eligibility criteria, the same treatment protocol, and the same endpoints, the same authors reported favorable surrogate endpoints: best PSA response (>50% decline) in more than half the treated patients (53.7%), and median PFS 49.2 months, and conclude: “Therefore it seems reasonable to prefer the 7.5GBq [VISION] regimen in most patients” [24].
Nonetheless, the major ethical objection to any RCT is that the research subject is treated as a means to an end of demonstrating the statistically significant efficacy of the agent, rather than the clinical benefit of the patient on study. The patient treated on a research study has a moral right to be treated as an end in themselves, which is denied them in the design of RCTs.
How can we preserve the beneficent doctor-patient relationship and provide what is believed to be the best management of advanced prostate cancer, yet, at the same time, obtain the required evidence of efficiency which would be acceptable to oncologists, urologists, and regulatory authorities throughout the world?
ASCO has released a policy statement asserting the importance of phase 1 clinical trials as a treatment modality with potential clinical benefit for patients with advanced stage malignancies [25]. Similarly, the US FDA also acknowledges that a primary aim of phase 1 trials is to gain early evidence of effectiveness [26]. This official recognition of early phase trials offering potential individual clinical benefit to all participants raises another ethical problem for subsequent RCTs. If drug access in phase 1 studies is considered therapeutic, how can investigators downstream of successful phase 1 trials ethically deprive half their human research subjects of study product in the RCT? Furthermore, drug regulator policies may restrict drug access, or limit commercial claims of efficacy based upon phase 1/2 trials, until validated by later restrictive RCT which confines availability to a highly select few.
The first prospective proof-of-concept phase 2 clinical trial of 177Lu-PSMA-617 in mCRPC was a single center Australian study (ANZ CTR 12615000912583), which demonstrated efficacy in 30 patients with advanced disease progressing after chemotherapy [27]. The treatment protocol was individualized within the parameters later enumerated in the EANM procedure guideline [20]. This seminal study demonstrates the practicality of personalizing 177Lu-PSMA treatment cycles to address the individual needs of the patient at the discretion of their treating physician. This real-world applicable study achieved rapid and substantial improvement in QOL and surrogate markers of response, without any significant toxicity. The authors concluded that this evidence supports the need for RCTs to further assess efficacy compared with current standard of care. However, whilst RCT may establish efficacy in a selected cohort of patients, it cannot provide the critical evaluation of efficiency in the global population of patients with advanced prostate cancer, nor can it address the practical problems of availability, affordability, and accessibility throughout the world [21].
The real issue of timely access to novel cancer therapies is not one of regulatory delay, but rather of archaic, overly restrictive, non-pragmatic RCT designs with limited distribution of investigation sites. RWE can help hasten the approval process and provide both access and strong evidence of meaningful gains in QOL and OS in large representative patient populations [28].
The important concept proven by the pathfinding prospective phase 2 study of 177Lu-PSMA-617 in mCRPC [27] is the capacity to individualize patient treatment within a harmonized protocol to obtain clinically meaningful scientific data which are credible and generalizable. Thus we now have a template for the translation of 177Lu-PSMA-RLT to real-world management of mCRPC on a harmonized protocol standardized to the EANM guideline.
Appropriate logistics exist in at least 50 countries where 68Ga/177Lu-PSMA theranostics is currently practiced on compassionate usage programs. The World Association for Radiopharmaceutical and Molecular Therapy (WARMTH) is coordinating an international prospective audit of patients receiving 177Lu-PSMA treatment under locally authorized individual patient access programs throughout the world and will collect, collate, and analyze real-world data (RWD) from patients treated on a harmonized protocol standardized on the EANM guideline. This multicenter international study: National Investigators Global Harmonization Theranostics CAncer of Prostate (NIGHTCAP) Study has very simple endpoints, comprising OS and QOL [29]. Assessment of QOL is by patient-reported outcome (PRO), which is language-independent and based upon patient selection of images on a standard 5-point emoji scale app on their smart phone [30].
Clinical access to 68Ga/177Lu-PSMA is provided through local compassionate patient usage programs, under existing national regulatory agency approvals, and all therapy and follow-up is at the discretion of the treating physician. This individualized molecular targeted theranostic management, within the harmonized EANM protocol guideline, does not require serial imaging or laboratory investigations to define surrogate response, given that the NIGHTCAP Study endpoints are limited to those which are of fundamental concern to the mCRPC patient: QOL and OS [29]. The COVID pandemic precluded performance of the NIGHTCAP Study but the design principles remain valid for real-world evidence of effectiveness.
Inevitably, novel evolving modifications will improve future outcomes of treatment of mCRPC. These potential developments may include incorporation of combination chemotherapy, such as cabazitaxel with 177Lu-PSMA in the ongoing TheraP Study [27], or sequential beta and alpha radionuclide therapy with 177Lu-PSMA and 225Ac-PSMA in tandem approaches [31, 32]. As soon as these novel combination therapies, which might also include chemo-immunotherapies, are shown to be safe and efficacious, they can be seamlessly incorporated into a modified harmonized adaptive NIGHTCAP Study protocol in real time as they become available. This rapid response and real-time flexibility contrasts with the rigid, locked-in protocol design, and inherent obsolescence of RCTs.
Thus, every patient on the NIGHTCAP Study would have received cutting-edge optimized theranostic management which is deemed to be most appropriate for them by their own personal physician, according to the most up-to-date real-time RWD. This ethically and scientifically sound approach to clinical outcome research is encapsulated in the ASCO Presidential Address of 2019 “Caring for every patient, learning from every patient” [33]. In the NIGHTCAP Study design, nothing be lost in translation into real-world evidence of efficiency of 177Lu-PSMA radioligand therapy of metastatic prostate cancer in routine oncology clinical practice throughout the world.
References
Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378:645–57.
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN. Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;394:17–31. https://doi.org/10.3322/caac.21492.
Afshar-Oromich A, Malcher A, Eder M, et al. PET imaging with a [68Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95.
Lenzo NP, Meyrick D, Turner JH. Review of gallium-68 PSMA PET/CT imaging in the management of prostate cancer. Diagnostics. 2018;8:16. https://doi.org/10.3390/diagnostics8010016.
Afaq A, Alahmed S, Chen S-H, et al. Impact of 68Ga-prostate-specific membrane antigen PET/CT on prostate cancer management. J Nucl Med. 2018;59:89–92. https://doi.org/10.2967/jnumed.117.192625.
Roach PJ, Francis R, Emmett L, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicentre study. J Nucl Med. 2018;59:82–8. https://doi.org/10.2967/jnumed.117.197160.
Schmidt-Hegemann N-S, Eze C, Li M, et al. Impact of 68Ga-PSMA PET/CT on the radiotherapeutic approach to prostate cancer in comparison to CT: a retrospective analysis. J Nucl Med. 2019;60:963–70. https://doi.org/10.2967/jnumed.118.220855.
Calais J, Czernin J, Cao M, et al. 68Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0ng/mL: impact on salvage radiotherapy planning. J Nucl Med. 2018;59:230–7. https://doi.org/10.2967/jnumed.117.201749.
Jadvar H, Ballas LK. Transformational change in prostate cancer management? J Nucl Med. 2018;59:228–9.
Jadvar H, Ballas LK, Choyke PL, et al. Appropriate use criteria for imaging evaluation of biochemical recurrence of prostate cancer after definitive primary treatment. J Nucl Med. 2020;61:552–62. https://doi.org/10.2967/jnumed.119.240929.
Trabulsi EJ, Rumble RB, Jadvar H, et al. Optimum imaging strategies for advanced prostate cancer: ASCO guideline. J Clin Oncol. 2020;38:1963. https://doi.org/10.1200/jco.19.02757.
Connor MJ, Shah TT, Horan G, et al. Cytoreductive treatment strategies for de novo metastatic prostate cancer. Nat Rev Clin Oncol. 2020;17:168. https://doi.org/10.1038/s41571.019-0284-3[13].
Hofman MS, Murphy DG, Williams SG, et al. A prospective randomised multicentre study of the impact of gallium-68 prostate specific membrane antigen (PSMA) PET/CTimaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): clinical trial protocol. BJU Int. 2018;122:783–93.
Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–16. https://doi.org/10.1016/S0140-6736(20)30314-7.
Weineisen M, Schottelius M, Simecek J, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–79. https://doi.org/10.2967/jnumed.115.158550.
Kulkarni HR, Singh A, Schuchardt C, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the bad berka experience since 2013. J Nucl Med. 2016;57:97S–104S. https://doi.org/10.2967/jnumed.115.170167.
Kulkarni HR, Singh A, Langbein T, et al. Theranostics of prostate cancer: from molecular imaging to precision molecular radiotherapy targeting the prostate-specific membrane antigen. Br J Radiol. 2018;91:20180308. https://doi.org/10.1259/bjr.20180308.
Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicentre study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. https://doi.org/10.2967/jnumed.116.183194.
Ahmadzadehfar H, Rahbar K, Baum RP, et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617. A WARMTH multicentre study (the 617 trial). Eur J Nucl Med Mol Imaging. 2020;48:113. https://doi.org/10.1007/s00259-020-04797-9.
Kratochwil C, Fendler PW, Eiber M, et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2019;46:2536–44. https://doi.org/10.1007/s00259-019-04485-3.
Turner JH. Theranostic outcomes in clinical practice of oncology: what, so what, now what? What’s more. Cancer Biother Radiopharm. 2019;34:135–40. https://doi.org/10.1089/cbr.2019.29006.jht(2019).
Das M. Many FDA-approved cancer drugs might lack clinical benefit. Lancet Oncol. 2018;19:e82. https://doi.org/10.1016/S1470-2045(17)30954-3.
Rahbar K, Bodei L, Morris MJ. Is the vision of radioligand therapy for prostate cancer becoming a reality? An overview of the phase 3 VISION trial and its importance for the future of theranostics. J Nucl Med. 2019;60:1504–6.
Seifert R, Kessel K, Schlack K, et al. Radioligand therapy using [(177)Lu]Lu-PSMA-617 in mCRPC: a pre-VISION single-center analysis. Eur J Nucl Med Mol Imaging. 2020;47:2106. https://doi.org/10.1007/s00259-020-04703-3.
Weber JS, Levit LA, Adamson PC, et al. American Society of Clinical Oncology policy statement update: the critical role of phase 1 trials in cancer research and treatment. J Clin Oncol. 2015;33:278–84. https://doi.org/10.1200/jco.2014.58.2635.
Burris HA III. Correcting the ASCO position on phase 1 clinical trials in cancer. Nat Rev Clin Oncol. 2020;17:125. https://doi.org/10.1038/s41571-019-0311-4(2020).
Hofman MS, Violet J, Hicks RJ, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–3.
Raphael MJ, Gyawali B, Booth CM. Real-world evidence and regulatory drug approval. Nat Rev Clin Oncol. 2020;17:271–2.
Turner JH. Real-world evidence of clinical outcomes in precision radionuclide oncology: the NIGHTCAP study of 177Lu-PSMA in metastatic prostate cancer. Curr Pharm Des. 2020;26:1–5. https://doi.org/10.2174/1381612826666200312141347.
Thompson CA, Novotny PJ, Bartz A, et al. Development of a novel emoji scale to measure patient-reported outcomes in cancer patients. J Clin Oncol. 2018;36(7 suppl):174. https://doi.org/10.1200/jco.2018.36.7suppl.174.
Khreish F, Ebert N, Ries M, et al. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47:721–8. https://doi.org/10.1007/s00259-019-04612-0.
Kulkarni HR, Zhang J, Singh A, et al. Tandem PSMA radioligand therapy using ac-225 and Lu-177 in advanced prostate cancer: safety and efficacy. Eur J Nucl Med Mol Imaging. 2019;46:S236–S7.
Bertagnolli MM. 2019 ASCO presidential address: caring for every patient, learning from every patient. J Clin Oncol. 2019;2019(37):2301–5. https://doi.org/10.1200/jco.19.01584.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Turner, J.H. (2024). Evaluation of Real-World Efficiency of 177Lu-PSMA Radioligand Therapy of Metastatic Prostate Cancer. In: Prasad, V. (eds) Beyond Becquerel and Biology to Precision Radiomolecular Oncology: Festschrift in Honor of Richard P. Baum. Springer, Cham. https://doi.org/10.1007/978-3-031-33533-4_33
Download citation
DOI: https://doi.org/10.1007/978-3-031-33533-4_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33532-7
Online ISBN: 978-3-031-33533-4
eBook Packages: MedicineMedicine (R0)