Abstract
Prostate-specific membrane antigen (PSMA) has been firmly established as a clinically relevant biomarker in imaging and targeted radionuclide therapy (theranostics) of prostate cancer, particularly in the metastatic castrate-resistant state. Numerous investigations from around the world in both academic and pharma settings are focused on research and development of safe and effective PSMA-based theranostic agents. Encouraging results from retrospective studies using the β-particle emitting 177Lu-PSMA-617 radioligand therapy prompted prospective phase II and phase III randomized clinical trials with recently published favorable results of the VISION trial. While these pivotal investigations continue, there has also been major growing interest in the potential clinical utility of α-particle PSMA-targeted therapeutic agents. After a brief review of PSMA biology, imaging with positron emission tomography, and current experience with targeted β-particle (177Lu) therapy, this article summarizes the preclinical and early clinical studies that have evaluated the PSMA-targeted agents conjugated properly to α-particle radiolabels including 225Ac, 213Bi, 227Th, 212Pb, and 149Tb.
(Chapter in: Beyond Becquerel and Biology to Precision Radiomolecular Oncology: Festschrift in Honor of Richard P. Baum).
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Metastatic castrate-resistant prostate cancer (mCRPC) is incurable. Patients with oligometastatic disease (typically defined as fewer than 3–5 metastases that are evident on imaging) may be candidates for metastasis-directed therapies (e.g., metastatectomy, stereotactic body radiotherapy). There have been major recent strides in approved therapeutic armamentarium in patients with multiple sites of mCRPC including the next-generation microtubule inhibitor, cabazitaxel, agents that target androgen axis, such as abiraterone acetate (androgen synthesis inhibitor) and enzalutamide (androgen receptor antagonist), sipuleucel-T immunotherapy (cancer vaccine), and α-particle therapy of bone lesions with 223RaCl2.
15.2 Prostate-Specific Membrane Antigen as Biological Target
Prostate-specific membrane antigen (PSMA) is a promising target for diagnostics and therapy (theranostics) of prostate cancer. PSMA, also known as folate hydrolase I or glutamate carboxypeptidase II, is a type II, 750-amino-acid transmembrane protein (100–120 kDa), which is anchored in the secretory cells of prostate epithelium, small intestine, proximal renal tubule, salivary glands, and brain. In prostate cancer, PSMA is overexpressed in aggressive primary, recurrent and metastatic tumors and is correlated to androgen independence. PSMA is also overexpressed in neovasculature of many other tumors (e.g., kidney, bladder, pancreas, lung). There have been many designs for radiolabeled agents targeting the PSMA for PET imaging and targeted radionuclide therapy including 89Zr- and 64Cu-labeled anti-PSMA antibodies and antibody fragments, 64Cu-labeled aptamers, and 11C-, 18F-, 68Ga-, 64Cu-, 44Sc-, 86Y-, 177Lu-, 225Ac-, 213Bi-, 227Th-, and 203Pb/212Pb-labeled low molecular weight inhibitors of the external moiety of PSMA based on glutamate-urea-lysine dimers [1,2,3,4,5,6,7,8].
15.3 PSMA PET
The most reported small molecule PSMA inhibitor is 68Ga-PSMA-11 (also called HBED-CC) after its clinical introduction in 2012 by the group of investigators from Heidelberg, Germany [9]. Systematic review and meta-analysis studies have demonstrated the high diagnostic performance of 68Ga-PSMA-11 in both initial staging and restaging of prostate cancer leading to major impact on clinical management in about half of the patients [10,11,12,13,14]. Moreover, in the clinical space of biochemical recurrence, several investigations have demonstrated the competitive advantage of 68Ga-PSMA-11 over other relevant PET radiotracers (e.g., radiolabeled choline, 18F-fluciclovine, 18F-NaF) in detecting and localizing disease sites, particularly in the serum PSA levels below 1 ng/mL in which salvage therapy may be most clinically beneficial [15,16,17]. More recently, PSMA ligands radiolabeled with 18F have been examined primarily due to the advantage of longer half-life (110 min for 18F vs. 68 min for 68Ga) which facilitates regional distribution of the radiotracer without the local need for a gallium generator or a cyclotron, leading to potentially widespread adoption after regulatory and reimbursement approvals [18,19,20].
15.4 PSMA β-Particle Radioligand Therapy
Apart from the apparent diagnostic advantage of small molecule PSMA inhibitor-based PET agents, these ligands may be radiolabeled to deliver targeted radionuclide therapy locally at the PSMA overexpressing disease sites, in accordance with the theranostic concept. There have been major recent strides in β-particle radioligand therapy with 177Lu-PSMA-617 in patients with mCRPC that have yielded promising results. These mostly retrospective studies have generally demonstrated that 177Lu-PSMA has low toxicity profile and is effective even in patients who have been pre-treated heavily and are refractory to standard therapies [21,22,23,24,25,26,27,28,29,30,31]. In a recent single-center prospective phase II clinical trial, 30 patients with high PSMA expression on 68Ga-PSMA-11 PET/CT (defined as a site of metastatic disease with intensity significantly greater than normal liver – standardized uptake value [SUV]max of tumor involvement at least 1.5 times mean SUV of liver –, and no FDG-positive disease without high PSMA expression) received intravenously a mean radioactivity of 7.5 GBq per cycle of 177Lu-PSMA-617 (up to 4 cycles at 6 weeks interval) [32]. The primary endpoint was PSA response according to the Prostate Cancer Clinical Trial Working Group criteria defined as a greater than 50% PSA decline from the pre-therapy baseline value. Seventeen of 30 patients (57%, 95% confidence interval [CI]; 37–75%) achieved a PSA decline of 50% or more. Objective response in nodal or visceral disease was noted in 82% of those patients with measurable disease. Grade 1 or 2 xerostomia, nausea, and fatigue were reported by 87%, 50%, and 50% of patients, respectively. Grade 3 or 4 thrombocytopenia was recorded in 13% of patients. These encouraging results received much publicity and led to development of procedure guidelines as an “unproven intervention in clinical practice in accordance with the best currently available knowledge” and paved the way for currently ongoing randomized clinical trials [33, 34].
TheraP trial (NCT03392428) is an open-label, 1:1 randomized, stratified, two-arm multicenter phase II trial (ANZUP 1603) designed to compare 177Lu-PSMA-617 radioligand therapy (8.5 GBq decreasing by 0.5 GBq per cycle i.v. every 6 weeks, for up to a maximum of six cycles) to cabazitaxel (20 mg/m2 i.v. every 3 weeks with prednisolone 10 mg daily orally, for a maximum of 10 cycles) in 200 patients [35]. The primary endpoint is PSA response with a number of other secondary endpoints including overall survival, progression-free survival (PFS), radiographic PFS, PSA PFS, etc. Eligibility criteria include prior docetaxel chemotherapy, rising PSA level, and no discordant FDG-avid PSMA-negative sites of disease. TheraP trial commenced in January 2018 and is currently ongoing with enrollment.
The VISION trial (NCT03511664) is an international, multicenter, 2:1 randomized, phase III trial comparing 177Lu-PSMA-617 radioligand therapy (7.4 GBq per cycle i.v. every 6 weeks for 4 to 6 cycles) plus best standard of care versus only best standard of care in patients with mCRPC who have PSMA-positive disease and have received at least one prior taxane and novel anti-androgen axis therapies [36]. The primary outcome measure is overall survival. The trial has concluded accrual of 814 patients. The favorable results of the trial have been published (Sartor O et al, N Eng J Med 2021; PMID: 34161051). Other notable trials include the PRINCE trial combining 177Lu-PSMA-617 radioligand therapy with immunotherapy (pembroluzimab), LuPARP trial combining 177Lu-PSMA-617 radioligand therapy with olaparib (DNA damage repair inhibitor), UpFrontPSMA trial that compares upfront 177Lu-PSMA-617 and anti-androgen therapy followed by docetaxel versus only anti-androgen therapy and docetaxel, and LuTectomy trial in patients with high risk localized prostate cancer and high PSMA expression who will receive 1–2 cycles of 177Lu-PSMA-617 followed in 6–8 weeks with radical prostatectomy and pelvic lymph node dissection.
15.5 PSMA α-Particle Radioligand Therapy
Alpha particle is a positively charged helium ion with typical kinetic energy of 5 MeV, a high linear energy transfer of about 80 keV/mm, and a short path travel (50–80 mm). α-particles can deposit large amount of energy locally that may lead to cellular apoptosis, independent of cellular oxygenation, through catastrophic double-strand DNA breaks in the nucleus [37,38,39].
15.5.1 Actinium-225
Actinium-225 (225Ac) is a useful α-emailer in targeted radionuclide therapy. It has a half-life of 9.9 days and decays to 209Bi (half-life of 1.9 × 1019 years) through net production of 4 α-particles with energies in the range of 5.8–8.4 MeV at tissue travel distance of 47–85 μm, 2 β-particles, and γ emissions at 218 KeV and 440 KeV. 225Ac may be sourced from 229Th with current worldwide production of approximately 68 GBq per year which is anticipated to grow [40,41,42].
There are several preclinical studies that have demonstrated the potential utility of 225Ac for targeted therapy in various malignancies including prostate cancer [43]. Kelly et al. showed that a single dose of 148 kBq 225Ac conjugated to albumin-binding prostate-specific membrane antigen (PSMA)-targeting RPS-074 (225Ac-RPS-074) in LNCaP xenograft mouse model of human prostate cancer induced a complete response in 6 of 7 tumors without major toxic effects [44]. More recently, researchers reported on a useful mouse model of human metastatic prostate cancer by injecting C4–2 cells into the left ventricle of immunodeficient male NSG mice which was then used to evaluate the effectiveness of 225Ac-PSMA-617 at various disease stages [45]. This preclinical study suggested that early 225Ac-PSMA-617 intervention may be efficacious in the setting of widespread metastatic prostate cancer. Delivery to tumor may also be accomplished by loading 225Ac into PEGylated liposomes targeted to mouse antihuman PSMA J591 antibody or A10 PSMA aptamer [46]. Liposomes when loaded with 225Ac or other α-emitters are attractive delivery systems since they can be decorated in different ways selectively to enhance therapeutic efficacy [47].
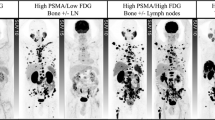
There has been much interest in the potential efficacy of 225Ac-labeled PSMA-targeted in the treatment of patients with mCRPC [48]. The results of these small case series have been encouraging with remarkably favorable responses in individual patients albeit at a potential cost of xerostomia [49] (Fig. 15.1). Kratochwil and colleagues reported on dosimetry estimates and empiric dose finding for targeted therapy of mCRPC with 225Ac-PSMA-617 [50]. They found that a treatment activity of 100 kBq/kg of 225Ac-PSMA-617 per cycle every 8 weeks was an apparent optimal trade-off between toxicity and biochemical efficacy. Swimmer-plot analysis has indicated favorable duration of tumor control even in the prognostically unfavorable clinical setting of mCRPC with xerostomia as the main cause for discontinuation of therapy [51]. Kratochwil et al. also recently reported on their observation that patients who were resistant to 225Ac-PSMA-617 often harbored mutations in DNA-damage repair and checkpoint genes suggesting that combination treatment with poly(ADP-ribose)-polymerase (PARP) inhibitors might be efficacious [52].
67-year-old male with a history of prostate cancer (Gleason score 4 + 5) who was initially treated with prostatectomy and androgen deprivation therapy now presenting with extensive castration-resistant skeletal metastases. The upper panel shows baseline 68Ga-PSMA-11 PET (left) and fused PET/CT (right) before treatment. The lower panel shows 68Ga-PSMA-11 PET (left) and fused PET/CT (right) after 3 cycles of therapy with 225Ac-PSMA-617. The post-therapy scans demonstrate remarkable response to radioligand therapy. The serum PSA declined from a baseline of 474.63 ng/mL to a post-treatment value of 0.14 ng/mL. There were no significant changes in hemoglobin and platelet counts and the glomerular filtration rate. (Courtesy of Dr. Mike Sathekge, University of Pretoria and Steve Biko Academic Hospital, South Africa)
Recently, Khreish and colleagues reported on a retrospective study of tandem therapy of mCRPC with 225Ac-PSMA-617/177Lu-PSMA-617 [53]. This limited study showed that a single course of tandem therapy with low activity 225Ac-PSMA-617 (range 1.5–7.9 MBq) following a full-activity 177Lu-PSMA-617 (range 5.0–1.6 GBq) may be therapeutically efficacious with minimal additional adverse events such as xerostomia. Rathke et al. suggested that 225Ac-PSMA-617 therapy may exert both an inflammatory and a direct radiation effect on the salivary glands that may lead to slavery gland dysfunction and xerostomia [54].
15.5.2 Bismuth-213
Bismuth-213 (213Bi) is an α-emitter that has received attention for potential clinical use. 213Bi emits both α (~92.7%) and β (~7.3%) particles with a relatively short half-life of 46 min. The 8.375 MeV α particle that is emitted by 213Po along the decay path of 213Bi comprises more than 98% of the α-particle energy from 213Bi disintegration [55]. The decay cascade also includes 26.1% probability of 440 KeV γ-ray emission that enable imaging.
An in-vitro and LNCaP xenograft animal model study of two PSMA targeted α-radioligands (213Bi-PSMA I&T and 213Bi-JVZ-008) confirmed α-particle induced double-strand DNA damage [56]. An investigation using a prostate cancer animal model compared 213Bi-DOTA-PESIN (DOTA-PEG(4)-bombesin) and 213Bi-AMBA (DO3A-CH(2)CO-8-aminooctanoyl-Q-W-A-V-G-H-L-M-NH(2)) with 177Lu-DOTA-PESIN reported that α-particle agent therapy was more efficacious than the counterpart β-particle agent therapy [57]. McDevitt and colleagues used 213Bi-J591 targeted to PSMA directed against androgen-sensitive LNCaP spheroids resulting in significantly improved median tumor-free survival [58].
Sathekge et al. from South Africa presented a case report of a patient with mCRPC who demonstrated exceptional response to 213Bi-PSMA-617 therapy [59]. Kratochwil et al. estimated the dosimetry with 213Bi-PSMA-617 based on extrapolation of 68Ga-PSMA-617 PET imaging data to half-life of 213Bi and compared the results to those for 225Ac-PSMA-617 [60]. These authors concluded that the higher perfusion-dependent off-target radiation and the shorter physical half-life of 213Bi in comparison to the longer biological half-life of PSMA-617 in dose-limiting organs renders 213Bi as a second-choice radiolabel for PSMA-targeted α-particle therapy.
15.5.3 Thorium-227
Thorium-227 (227Th) is another α-emitting radioisotope that is gaining traction in targeted radionuclide therapy of cancer. It has a half-life of 19 days decaying first to 223Ra and then follows the decay of 223Ra. Hammer et al. described the preclinical efficacy of 227Th-PSMA conjugates in PDX models of prostate cancer which prompted a currently ongoing phase I trial in patients with mCRPC (NCT03724747) [61]. This trial is being conducted by Bayer Inc. using the investigational drug 227Th-PSMA targeted conjugate, BAY 2315497.
15.5.4 Lead-212
Lead-212 (212Pb) is a β-emitter (half-life 10.64 h) and serves as an in-vivo nanogenerator of 212Bi (half-life 1.01 h) which decays to stable 208Pb via α-particle emission. Yong and Brechbiel have published comprehensive reviews of potential utility of 212Pb in targeted α-particle therapy of cancer [62, 63]. In a recently reported preclinical study, the small molecular PSMA ligand, 212Pb-NG001, was compared to the commonly used DOTA-based PSMA agent in mice bearing C4–2 human prostate cancer xenografts [64]. While both agents had similar binding, cellular internalization, and tumor uptake, the 212Pb-NG001 compound displayed 2.5-fold lower kidney uptake than the DOTA-conjugated compound. The researchers from the Johns Hopkins University reported recently on a proof-of-concept therapy study of dose-dependent inhibition of human prostate tumor growth in PSMA+ tumors implanted in animal hosts [65]. The tumor evolution could also be monitored with scintigraphy using the surrogate radionuclide, 203Pb (half-life 51.9 h, γ = 279 KeV) using single-photon computed tomography, supporting the notion of 203Pb/212Pb as a suitable theranostic radionuclide pair [66].
15.5.5 Terbium-149
Terbium-149 (149Tb) is an α-emitter (16.7%) and positron emitter (83.3%) with a half-life of 4.1 h, produced with commercial cyclotrons using a proton beam with energies up to 70 MeV, and can be useful clinically for targeted radionuclide therapy [67]. Umbricht et al. investigated 149Tb-PSMA-617 for targeted α-therapy of mice bearing PSMA-positive PC-3 xenograft tumors [68]. When compared to control untreated mice, the median lifetime of treated mice was almost twice longer than that in untreated mice. The positron emission also allowed imaging localization of tumor sites with PET/CT. Of note, Muller and colleagues have also reported on first-in-human study of PET imaging of prostate cancer with 152Tb-PSMA-617 and on targeted radionuclide therapy of mouse model of human prostate cancer with the β-particle emitting agent 161Tb-PSMA-617 [69, 70].
15.6 Summary
The research and development with α-particle emitting PSMA targeted agents will provide new pathways for safe and effective therapy of metastatic prostate cancer. These novel agents when used in sequence or in combination with β-particle PSMA-based therapy or other standard drug regimens are anticipated to significantly improve the outcome of patients not only in terms of overall survival but also in terms of quality-of-life measures.
References
Rahbar K, Afshar-Oromieh A, Jadvar H, Ahamadzadehfar H. PSMA theranostics: current status and future directions. Mol Imaging. 2018;17:153601211877606.
Arsenault F, Beauregard JM, Pouliot F. Prostate-specific membrane antigen for prostate cancer theranostics: from imaging to targeted therapy. Curr Opin Support Palliat Care. 2018;12:359–65.
Kulkarni HR, Singh A, Langbein T, et al. Theranostics of prostate cancer: from molecular imaging to precision molecular radiotherapy targeting the prostate specific membrane antigen. Br J Radiol. 2018;91:20180308.
Wustermann T, Haberkorn U, Babich J, Mier W. Targeting prostate cancer: prostate-specific membrane antigen based diagnosis and therapy. Med Res Rev. 2019;39:40–69.
Awang ZH, Essler M, Ahmadzadehfar H. Radioligand therapy of metastatic castration-resistant prostate cancer: current approaches. Radiat Oncol. 2018;13:98.
Ahamadzadehfar H, Wegen S, Yordanova A, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44:1448–54.
Farolfi A, Fendler W, Iravani A, et al. Theranostics for advanced prostate cancer: current indications and future developments. Eur Urol Oncol. 2019;2:152–62.
Lutje S, Heskamp S, Cornelissen AS, et al. PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status. Theranostics. 2015;5:1388–401.
Eder M, Schafer M, Bauder-Wust U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–97.
Hope TA, Goodman JZ, Allen IE, et al. Systematic review and metaanalysis of 68Ga-PSMA-11 PET accuracy for detection of prostate cancer validated by histopathology. J Nucl Med. 2019;60:786–93.
Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74:179–90.
Von Eyben FE, Picchio M, von Eyben R, et al. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2018;4:686–93.
Lenzo NP, Meyrick D, Turner JH. Review of Gallium-68 PET/CT imaging in the management of prostate cancer. Diagnostics (Basel). 2018;8(1):E16.
Eapen RS, Nzenza TC, Murphy DG, et al. PSMA PET applications in the prostate cancer journey: from diagnosis to theranostics. World J Urol. 37:1255–61.
Zhou J, Gou Z, Wu R, et al. Comparison of PSMA-PET/CT, choline PET/CT, NaF PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: systematic review and meta-analysis. Skeletal Radiol. 2019;48:1915–24.
Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-center, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–94.
Treglia G, Pereira Mestre R, Ferrari M, et al. Radiolabeled choline versus PSMA PET/CT in prostate cancer restaging: a meta-analysis. Am J Nucl Med Mol Imaging. 2019;9:127–39.
Giesel FL, Knorr K, Spohn F, et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med. 2019;60:362–8.
Eiber M, Kronke M, Wurzer A, et al. 18F-rhPSMA-7 positron emission tomography for the detection of biochemical recurrence of prostate cancer following radical prostatectomy. J Nucl Med. 2019;18:e684. [Epub ahead of print].
Chen Y, Pullamvhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–53.
Yadav MP, Ballal S, Shaoo RK, et al. Radioligand therapy with 177Lu-PSMA for metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. AJR Am J Roentgenol. 2019;213:275–85.
Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen Radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–13.
Kim YJ, Kim YI. Therapeutic responses and survival effects of 177Lu-PSMA-617 Radioligand therapy in metastatic castrate-resistant prostate cancer: a meta-analysis. Clin Nucl Med. 2018;43:728–73.
Ahmadzadehfar H, Rahbar K, Essler M, Biersack HJ. PSMA-based theranostics: a step-by-step practical approach to diagnosis and therapy for mCRPC patients. Semin Nucl Med. 2020;50:98–109.
Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90.
Beheshti M, Heinzel A, von Mallek D, et al. Prostate-specific membrane antigen radioligand therapy of prostate cancer. Q J Nucl Med Mol Imaging. 2019;63:29–36.
Siva S, Udovicich C, Tran B, et al. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat Rev Urol. 2020;17:107–18.
Virgolini I, Decristoforo C, Haug A, et al. Current status of theranostics in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:471–95.
Ferdinandus J, Violet J, Sandhu S, Hofman MS. Prostate-specific membrane antigen theranostics: therapy with lutetium-177. Curr Opin Urol. 2018;28:197–204.
Emmett L, Willowson K, Violet J, et al. Lutetium-177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017;64:52–60.
Kulkarni HR, Singh A, Schuchardt C, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med. 2016;57(Suppl 3):97S–104S.
Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-center, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Kratochwil C, Fendler WP, Eiber M, et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2019;46:2536–44.
Iravani A, Violet J, Azad A, Hofman MS. Leutetium-177 prostate-specific membrane antigen (PSMA) theranostics: practical nuances and intricacies. Prostate Cancer Prostatic Dis. 2020;23:38–52.
Hofman MS, Emmett L, Violet J, et al. TheraP: a randomized phase 2 trial of 177 Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (clinical trial protocol ANZUP 1603). BJU Int. 2019;124(Suppl 1):5–13.
Rahbar K, Bodei L, Morris MJ. Is the vision of radioligand therapy for prostate cancer becoming a reality? An overview of the phase III VISION trial and its importance for the future of theranostics. J Nucl Med. 2019;60:1504–6.
De Vincentis G, Gerritsen W, Gschwend JE, et al. Advances in targeted alpha therapy of prostate cancer. Ann Oncol. 2019;30:1728–39.
Chakravarty R, Siamof CM, Dash A, Cai W. Targeted a-therapy of prostate cancer using radiolabeled PSMA inhibitors: a game changer in nuclear medicine. Am J Nucl Med Mol Imaging. 2018;20:247–67.
Jadvar H. Targeted a-therapy in cancer management: synopsis of preclinical and clinical studies. Cancer Biother Radiopharm. 2020;35:475. [Epub ahead of print].
Morgenstern A, Apostolidis C, Kratochwil C, et al. An overview of targeted alpha therapy with 225Actinium and 213Bismuth. Curr Radiopharm. 2018;11:200–8.
Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted α-particle therapy. J Nucl Med. 2005;46:198S–204S.
Harvey JT. NorthStar perspectives for actinium-225 production at commercial scale. Curr Radiopharm. 2018;11:180–91.
Scheinberg DA, McDevitt MR. Actinium-225 in targeted alpha-particle therapeutic applications. Curr Radiopharm. 2011;4:306–20.
Kelly JM, Amor-Coarasa A, Ponnala S, et al. A single dose of 225Ac-RPS-074 induces a complete tumor response in LNCaP xenograft model. J Nucl Med. 2018;60:649. [Epub ahead of print].
Stuparu AD, Meyer CAL, Evans-Axelsson SL, et al. Targeted alpha therapy in a systemic mouse model of prostate cancer—a feasibility study. Theranostics. 2020;10:2612. [Epub ahead of print].
Bandekar A, Zhu C, Jindal R, et al. Anti-prostate-specific membrane antigen liposomes loaded with 225Ac for potential targeted antivascular a-particle therapy of cancer. J Nucl Med. 2014;55:107–14.
Chang MY, Seiderman J, Sofou S. Enhanced loading efficiency and retention of 225Ac in rigid liposomes for potential targeted therapy of micrometastases. Bioconjug Chem. 2008;19:1274–982.
Kratochwil C, Haberkorn U, Giesel FL. 225Ac-PSMA-617 for therapy of prostate cancer. Semin Nucl Med. 2020;50:133–40.
Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration resistant prostate cancer. J Nucl Med. 2016;57:1941–4.
Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted a-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimates and empiric dose finding. J Nucl Med. 2017;58:1624–31.
Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted a-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmers-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802.
Kratochwil C, Giesel FL, Heussel CP, et al. Patients resistant against PSMA-targeting alpha-radiation therapy often harbor mutations in DNA-repair associated genes. J Nucl Med. 2020;61:683. [Epub ahead of print].
Khreish F, Ebert N, Ries M, et al. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: pilot experience. Eur J Nucl Med Mol Imaging. 2020;47:721–8.
Rathke H, Kratochwil C, Hohenberger R, et al. Initial experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur J Nucl Med Mol Imaging. 2019;46:139–47.
Morgenstern A, Bruchertseifer F, Apostolidis C. Targeted alpha therapy with 213Bi. Curr Radiopharm. 2011;4:295–305.
Nonnekens J, Chatalic KL, Molkenboer-Kuenen JD, et al. 213Bi-labeled prostate-specific membrane antigen targeting agents induce DNA double-strand breaks in prostate cancer xenografts. Cancer Biother Radiopharm. 2017;32:67–73.
Wild D, Frischknerdt M, Zhang H, et al. Alpha- and beta-particle radiopeptide therapy in a human prostate cancer model (213Bi-DOTA-PESIN and 213Bi-AMBA versus 177Lu-DOTA-PESIN). Cancer Res. 2011;71:1009–18.
McDevitt MR, Barendswaard E, Ma D, et al. An alpha-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res. 2000;60:6095–100.
Sathekge M, Knoesen O, Meckel M, et al. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1099–100.
Kratochwil C, Schmidt K, Afshar-Oromieh A, et al. Targeted alpha therapy of mCRPC: dosimetry estimates of 213Bismuth-PSMA-617. Eur J Nucl Med Mol Imaging. 2018;45:31–7.
Hammer S, Hagemann UB, Zitzmann-Kolbe S, et al. Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted alpha therapy for prostate cancer. Clin Cancer Res. 2019;26:1985. [Epub ahead of print].
Yong K, Brechbiel MW. Application of 212Pb for targeted a-particle therapy (TAT): pre-clinical and mechanistic understanding through to clinical translation. AIMS Med Sci. 2015;2:228–45.
Yong K, Brechbiel MW. Towards translation of 212Pb as a clinical therapeutic; getting the lead in! Dalton Trans. 2011;40:6068–76.
Stenberg VY, Juzeniene A, Chen Q, et al. Preparation of the alpha-emitting prostate-specific membrane antigen targeted radioligand [212]Pb-NG001 for prostate cancer. J Labelled Comp Radiopharm. 2020;63:129–43.
Banerjee SR, Minn I, Kumar V, et al. Preclinical evaluation of 203/212Pb-labeled low-molecular weight compounds for targeted radiopharmaceutical therapy of prostate cancer. J Nucl Med. 2020;61:80–8.
Dos Santos JC, Schafer M, Bauder-Wust U, et al. Development and dosimetry of 203Pb/212Pb-labeled PSMA ligands: bringing the lead into PSMA-targeted alpha therapy. Eur J Nucl Med Mol Imaging. 2019;46:1081–91.
Muller C, Singh A, Umbricht CA, et al. Preclinical investigations and first-in-human application of 152Tb-PSMA-617 for PET/CT imaging of prostate cancer. EJNMMI Res. 2019;9:68.
Umbricht CA, Koster U, Bernhardt P, et al. Alpha-PET for prostate cancer: preclinical investigation using 149Tb-PSMA-617. Sci Rep. 2019;9:17800.
Muller C, Umbricht CA, Gracheva N, et al. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1919–30.
Muller C, Vermeulen C, Koster U, et al. Alpha-PET with terbium-149: evidence and perspectives for radiotheragnostics. EJNMMI Radiopharm Chem. 2017;1:5. https://doi.org/10.1186/s41181-016-0008-2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Jadvar, H. (2024). Precision Oncology with PSMA-Targeted α-Particle Therapy of mCRPC. In: Prasad, V. (eds) Beyond Becquerel and Biology to Precision Radiomolecular Oncology: Festschrift in Honor of Richard P. Baum. Springer, Cham. https://doi.org/10.1007/978-3-031-33533-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-33533-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33532-7
Online ISBN: 978-3-031-33533-4
eBook Packages: MedicineMedicine (R0)