Abstract
Technological characteristics of the robotic platform may play a role in decreasing challenges of minimally invasive left colectomy for benign or malignant diseases. The literature regarding robotic left colectomy is scarce and focuses mainly on the robotic approach for complicated diverticular disease, showing good clinical results. Robotic left colectomy is performed with three robotic instruments (a monopolar hook or scissor; a bipolar forceps and a Cadiere forceps) and with one or two instruments for the assistant. The dissection of the left mesocolon is performed from medial to lateral and the splenic flexure is always fully mobilized. Section of the colon can be performed with a robotic stapler, and indocyanine green fluorescence can be employed to verify colon perfusion before anastomosis.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Although the robotic approach in colorectal surgery has been mainly described for rectal resection and right colectomy [1,2,3,4,5], technical advantages of the robotic platform over standard laparoscopy (high-definition three-dimensional vision of the surgical field, stable camera, ergonomics of the instruments) can also be gained for left colectomy.
Evidence in the literature regarding robotic left colectomy is scarce, and primarily concerns diverticular disease: in 2019 Al-Temini et al. [6] reported on 6776 patients undergoing laparoscopic left colectomy compared to 441 patients undergoing robotic left colectomy. Operative time was higher in the robotic group but conversions to open surgery were significantly lower. Similar results were observed by Bastawrous et al. [7]. Beltzer et al. [8] described similar clinical outcomes after robotic or laparoscopic left colectomy for diverticular disease, but reported more cases of complicated diverticulitis, with abscess or recurrent disease, in the robotic group. Together, this evidence may indicate a role for the robotic platform in decreasing the complexity of the minimally invasive approach in a complex surgical scenario such as diverticulitis.
In this chapter, we shall focus of the technical aspects of robotic left colectomy for cancer with the Xi da Vinci platform.
2 Patient Position and Robot Docking
The patient is in a supine position with arms alongside the trunk and legs abducted. A slight Trendelenburg position and right tilt are maintained to expose the operative field from the ileal loops (Fig. 9.1).
The procedure starts with induction of pneumoperitoneum through a Veress needle inserted in the Palmer point; once an intra-abdominal pressure of 12 mmHg is reached, the assistant port (a 12-mm optical trocar) is inserted in the right flank.
A preliminary exploration of the abdominal cavity is performed through this port with the robotic camera; if the site and resectability of the tumor are confirmed, the remaining ports are inserted under direct vision.
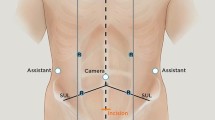
Four 8-mm robotic trocars are inserted along a line traced from the left subcostal margin to the right greater trochanter; the point where this line crosses the midline represents the insertion point of the camera trocar (R2); the remaining ports are inserted at 6–8 cm between them. Robotic trocar number one (R1) is on the left of R2, and robotic trocars number three (R3) and four (R4) are on the right (Fig. 9.2).
The robotic cart comes from the left side of the patient. R2 is connected to the arm, the camera is inserted and targeting is performed pointing on the sigmoid colon. After completing the targeting, arms one, three and four are connected to the trocars; a bipolar forceps is inserted in R1, a monopolar hook (or monopolar forceps) in R3, and a Cadiere grasper (or a tip-up grasper) in R4.
3 Dissection of the Left Mesocolon
The first step of the procedure is separation of the left mesocolon from the retroperitoneum: the bipolar forceps in R1 and the Cadiere forceps in R4 lift the mesocolon at the level of the inferior mesenteric vein (IMV), and the peritoneum is incised just below IMV, exposing the retrocolic plane.
The assistant forceps exerts countertraction on the retroperitoneum exposing the dissection plane (Fig. 9.3); the dissection is performed with the monopolar hook or scissors and proceeds from medial to lateral until reaching the colon.
4 Splenic Flexure Mobilization
The root of the transverse mesocolon is incised just anteriorly to the pancreas: the bipolar forceps lifts the transverse mesocolon cranially and the Cadiere forceps lifts the descending mesocolon; the assistant helps with a suction device or a grasper lowering the pancreatic body (Fig. 9.4). The root is incised two inches superiorly and two inches to the left of IMV, and the lesser sac in entered from below. Complete detachment of the root of transverse mesocolon from the pancreas is essential to fully mobilize the splenic flexure. IMV is isolated and sectioned between clips. The left coloparietal detachment is completed by reaching the previous medial-to-lateral dissection. Finally, a coloepiploic detachment is performed, and the splenic flexure is completely lowered.
Incision of the root of the transverse mesocolon. With the robotic graspers in R1 lifting the transverse mesocolon and the grasper in R4 lifting the descending mesocolon, the assistant forceps lowers the body of the pancreas and the root of the transverse mesocolon is completely detached from pancreas, to enter the lesser sac from below
5 Inferior Mesenteric Artery Isolation and Lymphadenectomy
The peritoneum is incised at the level of sacral promontory and the mesorectal space is entered; the Cadiere forceps in R4 lifts the proximal rectum cranially to expose the root of inferior mesenteric artery (IMA), while the assistant forceps tractions the descending colon. The bipolar forceps in R2 lifts the left mesocolon and IMA is dissected at its origin, performing a central lymphadenectomy [9]; then, IMA is sectioned between clips (Fig. 9.5).
6 Section and Anastomosis
The upper mesorectum in dissected and the upper rectum is sectioned with a robotic linear stapler introduced in R3 after having removed the 8-mm trocar and having replaced it with a 12-mm robotic trocar able to harbor the stapler. Usually, a blue 45- or 60-mm cartridge is used for the transection; before extracting the colon, the mesocolon between the IMV and the IMA stumps is incised intracorporeally.
A suprapubic transverse incision is performed, and the colon is extracted; before the proximal transection, 3 mL of a reconstituted solution of indocyanine green with sodium chloride is injected intravenously, and the correct perfusion of the proximal colonic stump is assessed by switching the vision modality of the robotic camera from normal to infrared light (Fig. 9.6). This vision modality can be used extracorporeally, provided that all the operating room lights are switched off and a sterile drape is positioned over the robotic camera to lower the ambient light as much as possible.
After colon transection, the anvil of a circular stapler is introduced in the proximal colonic stump and fixed by a purse-string suture. During the extracorporeal phase of the surgery, the robot remains docked. The colon is then reinserted in the abdominal cavity and the minilaparotomy is closed; the pneumoperitoneum is reinduced and a Knight-Griffen mechanical terminoterminal anastomosis is performed under robotic guidance.
References
Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. 2020;3(1):e1918911.
Konstantinidis IT, Ituarte P, Woo Y, et al. Trends and outcomes of robotic surgery for gastrointestinal (GI) cancers in the USA: maintaining perioperative and oncologic safety. Surg Endosc. 2020;34(11):4932–42.
Liu R, Liu Q, Wang Z. Worldwide diffusion of robotic approach in general surgery. Updates Surg. 2021;73(3):795–7.
Jayne D, Pigazzi A, Marshall H, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: The ROLARR randomized clinical trial. JAMA. 2017;318(16):1569–80.
Park JS, Choi GS, Park SY, et al. Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg. 2012;99(9):1219–26.
Al-Temimi MH, Chandrasekaran B, Agapian J, et al. Robotic versus laparoscopic elective colectomy for left side diverticulitis: a propensity score–matched analysis of the NSQIP database. Int J Colorectal Dis. 2019;34(8):1385–92.
Bastawrous AL, Landmann RG, Liu Y, et al. Incidence, associated risk factors, and impact of conversion to laparotomy in elective minimally invasive sigmoidectomy for diverticular disease. Surg Endosc. 2020;34(2):598–609.
Beltzer C, Knoerzer L, Bachmann R, et al. Robotic versus laparoscopic sigmoid resection for diverticular disease: a single-center experience of 106 cases. J Laparoendosc Adv Surg Tech -A. 2019;29(11):1451–5.
Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23(1):1–34.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Robotic resection of splenic flexure (MOV 642129 kb)
Robotic rectal resection (MOV 356843 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Petz, W.L. (2024). Robotic Left Colectomy. In: Ceccarelli, G., Coratti, A. (eds) Robotic Surgery of Colon and Rectum. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-33020-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-33020-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33019-3
Online ISBN: 978-3-031-33020-9
eBook Packages: MedicineMedicine (R0)