Abstract
In the light of the current dissemination of robotic surgery, there will likely be fundamental robotic skills requirements to complete general surgery residency, and the need for a structured robotic training curriculum has been supported by several associations and program directors. Training a robotic colorectal surgeon has two different aspects to consider: learning how to use the platform, and learning procedural skills strictly related to colorectal surgery. Broad consensus exists on the fact that a structured robotic colorectal training program should have a modular approach including theoretical knowledge, case observation, simulation, and proctored training. Additionally, the ideal robotic colorectal training program should provide an objective assessment of acquired skills with well-established requirements to proceed from one step to the next, and non-operative robotic skills should also be implemented and evaluated. Another relevant feature of robotic colorectal training is the component-based approach, which consists in deconstructing the procedure in defined and measurable components that can be evaluated more objectively. Finally, we are not only training console surgeons, but also bedside assistants since correct trocar positioning and reliable feedback from the operating table are essential to the effective and safe completion of a robotic colorectal procedure.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Training
- Colorectal surgery
- Robotic training
- Simulation
- Structured training
- Surgical education
- Training program
1 Introduction
Robotic surgery represents the greatest revolution in general surgery of the last twenty years, and when a new technology is introduced in surgical practice standardized training becomes of utmost importance. Some of the challenges to young surgeons’ training in this field are represented by elevated costs, duty hours, and the presence of senior surgeons still going through their learning curve, which may limit teaching to residents and junior surgeons [1]. However, in the light of the current dissemination of robotic surgery, there will likely be fundamental robotic skills requirements to complete general surgery residency, and the need for a structured robotic training curriculum has been supported by several associations and program directors [2, 3].
Training a robotic colorectal surgeon has two different aspects to be considered: learning how to use the platform, and learning procedural skills strictly related to colorectal surgery. Considering the trainee’s previous practice is of paramount importance to differentiate educational pathways, but institutional experience with the platform and case volume should also be weighed, given that the absence of an expert surgeon and low program operative volumes could negatively impact on a robotics curriculum. Moreover, when assessing the overall costs of training, the acquisition of a virtual simulator and robotic dual console should be included. All these angles need to be considered prior to creating a structured training program, which should present realistic and achievable goals, in order to avoid frustration and loss of credibility towards hospital management [4]. Additionally, the ideal robotic colorectal surgery training program should provide an objective assessment of acquired skills with well-established requirements to proceed from one step to the next, and non-operative robotics skills should also be implemented and evaluated [5]. We are training not only console surgeons, but also bedside assistants since correct trocar positioning and reliable feedback from the operating table are essential to the effective and safe completion of a robotic colorectal procedure. Finally, training should not be limited to robotic novice surgeons, but also extended to trainers, as mentors need to adapt to new teaching technologies such as telementoring and the use of the robotic dual console [6]. Whether or not specific robotic colorectal training should be started during residency or reserved for post-residency fellowships is still debated. In our opinion, surgical residents should be familiar with the fundamentals of robotic surgery and should be able to act as table assistants by the end of residency, and during the last year of residency they should be able to perform low-complexity robotic procedures.
2 Learning Curves in Robotic Colorectal Surgery
Establishing learning curves for robotic colorectal procedures has implications on training planning and consequently on credentialing [7]. The most reported variables used to assess learning curves are time-related, but the learning process in robotic surgery involves multiple aspects, so a multidimensional analysis could be more reliable and should include evaluation of the trainee’s surgical background, surgery type, postoperative morbidity, oncological outcomes, and a risk score stratification of cases, since patient selection can heavily impact operative times [8].
There are three phases to the common robotics learning curve: an initial learning stage with a rapid decrease in operative time, a second phase with stabilization of operative time (plateau or competence phase) and a third phase of mastery, with a decrease in operative time [4]. However, some studies reported an increase in console time during the mastery phase, which was attributed to the fact that the surgeons performed more complex cases as they progressed through the learning curve [9, 10]. The number of cases required to achieve competence in colorectal surgery is extremely variable in the literature. Recently, Nasseri et al. evaluated the learning curve of an expert laparoscopic colorectal surgeon by reviewing 111 consecutive colorectal procedures and found that the surgeon gained competence after 13 surgeries and mastery after 70 [7]. Park et al., in their multidimensional analysis of the learning curve for robotic low anterior rectal resection found that competence was achieved after 44 cases and mastery after 78 [11]. De Angelis et al. described a 16-case learning curve for robotic right colectomy for a surgical fellow with little experience in laparoscopic colorectal surgery. The learning curve for laparoscopic right colectomy was reported to be 25 cases [12]. Interestingly, a recently published systematic review questions the common perception of a shorter learning curve for robotic colorectal surgery compared to laparoscopy, claiming that the advantages of the robotic platform may result in a better baseline performance in early practice rather than a shorter learning curve. The authors found that conversion rates are significantly reduced in the early robotic learning curve when they are more common in laparoscopy. Moreover, all the studies taken under consideration in the review showed at some point a shorter robotic operating time, with a greater time advantage in complex tasks such as knot tying in simulation environments or total mesorectal excision in clinical practice [13]. When evaluating a trainee’s acquisition of a specific surgical technique, experience gained in other types of surgery is often neglected, as is operating room staff experience, despite the fact that these aspects can also impact learning. Guend et al. analyzed both individual and institutional learning curves and reported that the first surgeon who started practice achieved competence after 74 cases, but once the program was established other surgeons required only 25 to 30 cases to reach proficiency [14].
Another controversial topic is whether previous laparoscopic experience impacts the learning curve in a significant way. While many authors agree that limited laparoscopic experience should not discourage from approaching robotic colorectal surgery, especially in high volume centers [15, 16], others support the fact that experienced laparoscopic colorectal surgeons may have advantages in terms of learning curve. Wong et al., in their analysis of the learning curve of an experienced colorectal surgeon (1500 colorectal cases) during his transitioning to robotics, found that performance of complex cases early in the learning curve did not impact negatively postoperative outcomes. The authors suggested the adoption of audits on patient outcomes to assess the progression of the learning curve. The first audit was held with the hospital direction after the first 10 cases, and full accreditation was provided only after full review of the results [17].
As can be easily inferred from this quick overview, the published data are often difficult to replicate and to compare. Patient selection remains essential, and the training pathway should start with less complex cases to optimize outcomes. Operative volume, the application of a structured training curriculum, and the presence of an experienced mentor surgeon inside the institution are all factors with the power of shortening the learning curve, along with the choice of a fixed dedicated operating room team to improve workflow and communication.
3 Current Colorectal Training Programs, Educational Tools, and Assessment of Outcomes
A recent systematic review reported broad consensus on the fact that a structured robotic colorectal training program should have a modular approach including theoretical knowledge, case observation, simulation, and proctored training. All training programs reported in the study were designed for the da Vinci platform (Intuitive Surgical, Sunnyvale, CA) [18]. Several generic curricula have been developed by single institutions and residency programs. The Fundamentals of Robotic Surgery (FRS) is a proficiency-based curriculum created by surgery experts from multiple specialties that uses basic technical skills to train and assess robotic surgeons [19]. If we consider more specifically robotic colorectal training, four structured training programs can be identified in the literature: the European Academy of Robotic and Colorectal Surgery (EARCS) program, the National Colon and Rectal Surgery Robotic training program (CRSRTP) sponsored by the association of Program Directors for Colon and Rectal Surgery, the da Vinci Robotic System Intuitive Surgical program, and the Colorectal Robotic Surgery Training curriculum established by the European Society of Coloproctology (ESCP) [18, 20]. Moreover, a recent survey administered to American colorectal surgery program directors revealed that most programs have a robotic curriculum [21].
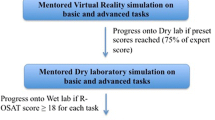
All programs include a theoretical phase along with a simulation phase. The CRSRTP mandates scores of >90% for key simulator exercises, and other programs have simulator time requirements ranging from 8 to 50 hours [20]. Recently, Intuitive is offering the possibility of practicing at the virtual simulator not only basic technical skills, but also steps of surgical procedures which include, amongst others, right colectomy [22].
Relevant features of colorectal training pathways are proctored cases and the component-based approach, which consists of deconstructing the procedure in defined and measurable components that could be evaluated more objectively. During proctored cases, the presence of the robotic dual console allows the proctor to take control of the robotic instruments when needed and to point resection planes without interrupting surgical workflow. The ESCP proposed a component-based approach for robotic low anterior resection, identifying for each step of the procedure errors and critical errors to help objective assessment. In this way the evaluation is not limited to a volume-outcome correlation, since performing a certain procedure an established number of times, does not always guarantee competency [23].
Objective assessment of outcomes outside the virtual simulation setting remains challenging. During the sixth Clinical Robotic Surgery Association (CRSA) congress an expert round table proposed a competence assessment scale for each specific colorectal procedure [2]; the EARCS too created a Global Assessment Score (GAS) form to objectify competence assessment [18, 24]. Lately, there is emerging interest in the use of automated performance metrics including kinematic and event data, such as instrument vibration, to evaluate robotics competency. The recently developed My Intuitive App (Intuitive Surgical, Sunnyvale, CA) gives the surgeon the possibility to see minute-by-minute use of instruments per arm, console time, operative and non-operative time, and compare the data with national trends; this could favorably impact competency evaluation [25]. Moreover, in the near future the development of the Internet of Surgical Things and the use of Artificial Intelligence could further improve objective assessment of robotic skills.
In 2020 a robotic surgery training curriculum was established in our institution. Junior surgeons experienced as table assistants and autonomous in the performance of robotic low-complexity procedures, but with limited experience in laparoscopic colorectal surgery started their robotic colorectal training from robotic right colectomy, leaving anterior resection as the final step. Senior surgeons, expert in laparoscopic colorectal surgery, started their transition to robotics from right colectomy as well, but rapidly proceeded to anterior resection. Since the introduction of the robotic colorectal program there has been a rapid shift in indications, and currently the majority of low anterior resections for rectal cancer in our institution are performed robotically. This – along with the growing evidence of the advantages of the robotic platform in rectal resection [26] – makes robotic training essential for colorectal surgeons. The future generation of colorectal surgeons might have learned how to perform rectal anterior resection directly with the robot, without going through laparoscopy, as already happened with prostatectomy.
References
Shaw RD, Eid MA, Bleicher J, et al. Current barriers in robotic surgery training for general surgery residents. J Surg Educ. 2022;79(3):606–13.
Petz W, Spinoglio G, Choi GS, et al. Structured training and competence assessment in colorectal robotic surgery. Results of a consensus experts round table. Int J Med Robot. 2016;12(4):634–41.
Waters PS, Flynn J, Larach JT, et al. Fellowship training in robotic colorectal surgery within the current hospital setting: an achievable goal? ANZ J Surg. 2021;91(11):2337–44.
Soliman MK, Tammany AJ. Teaching and training surgeons in robotic colorectal surgery. Clin Colon Rectal Surg. 2021;34(5):280–5.
AlJamal YN, Baloul MS, Mathis KL, et al. Evaluating non-operative robotic skills in colorectal surgical training. J Surg Res. 2021;260:391–8.
Eardley NJ, Matzel KE, Gómez Ruiz M, et al. European Society of Coloproctology Colorectal Robotic Surgery Training for the Trainers Course – the first pilot experience. Color Dis. 2020;22(11):1741–8.
Nasseri Y, Stettler I, Shen W, et al. Learning curve in robotic colorectal surgery. J Robot Surg. 2021;15(3):489–95.
Wong SW, Crowe P. Factors affecting the learning curve in robotic colorectal surgery. J Robot Surg. 2022;16(6):1249–56.
Bokhari MB, Patel CB, Ramos-Valadez DI, et al. Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc. 2011;25(3):855–60.
Shaw DD, Wright M, Taylor L, et al. Robotic colorectal surgery learning curve and case complexity. J Laparoendosc Adv Surg Tech A. 2018;28(10):1163–8.
Park EJ, Kim CW, Cho MS, et al. Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc. 2014;28(10):2821–31.
de’Angelis N, Lizzi V, Azoulay D, Brunetti F. Robotic versus laparoscopic right colectomy for colon cancer: analysis of the initial simultaneous learning curve of a surgical fellow. J Laparoendosc Adv Surg Tech A. 2016;26(11):882–92.
Flynn J, Larach JT, Kong JCH, et al. The learning curve in robotic colorectal surgery compared with laparoscopic colorectal surgery: a systematic review. Color Dis. 2021;23(11):2806–20.
Guend H, Widmar M, Patel S, et al. Developing a robotic colorectal cancer surgery program: understanding institutional and individual learning curves. Surg Endosc. 2017;31(7):2820–8.
Formisano G, Esposito S, Coratti F, et al. Structured training program in colorectal surgery: the robotic surgeon as a new paradigm. Minerva Chir. 2019;74(2):170–5.
Ferraro L, Formisano G, Salaj A, et al. Robotic right colectomy with complete mesocolic excision: senior versus junior surgeon, a case-matched retrospective analysis. Int J Med Robot. 2022;18(3):e2383.
Wong SW, Ang ZH, Crowe P. The learning curve to attain surgical competency in robotic colorectal surgery. ANZ J Surg. 2022;92(5):1117–24.
Harji D, Houston F, Burke J, et al. The current status of robotic colorectal surgery training programmes. J Robot Surg. 2023;17(2):251–63.
Institute for Surgical Excellence. Fundamentals of robotic surgery (FRS). https://www.surgicalexcellence.org/fundamentals-of-robotic-surgery-frs. Accessed 3 Feb 2023.
Gomez Ruiz M, Tou S, Matzel KE. Setting a benchmark in surgical training – robotic training under the European School of Coloproctology, ESCP. Colorectal Dis. 2019;21(4):489–90.
Shellito AD, Kapadia S, Kaji AH, et al. Current status of robotic surgery in colorectal residency training programs. Surg Endosc. 2022;36(1):307–13.
Intuitive Surgical. da Vinci SimNow Library. https://www.intuitive.com/en-us/products-and-services/da-vinci/education/simnow/library. Accessed 3 Feb 2023.
Tou S, Gómez Ruiz M, Gallagher AG, et al. European expert consensus on a structured approach to training robotic-assisted low anterior resection using performance metrics. Color Dis. 2020;22(12):2232–42.
Panteleimonitis S, Popeskou S, Aradaib M, et al. Implementation of robotic rectal surgery training programme: importance of standardisation and structured training. Langenbeck's Arch Surg. 2018;403(6):749–60.
Intuitive Surgical. My intuitive. https://www.intuitive.com/en-us/products-and-services/my-intuitive. Accessed 3 Feb 2023.
Corrigan N, Marshall H, Croft J, et al. Exploring and adjusting for potential learning effects in ROLARR: a randomised controlled trial comparing robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection. Trials. 2018;19(1):339.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Esposito, S., Francescato, A., Piccoli, M. (2024). Training in Robotic Colorectal Surgery. In: Ceccarelli, G., Coratti, A. (eds) Robotic Surgery of Colon and Rectum. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-33020-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-33020-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33019-3
Online ISBN: 978-3-031-33020-9
eBook Packages: MedicineMedicine (R0)