Abstract
The laparoscopic approach to rectal cancer is a technically difficult procedure associated with a long learning curve, leading to high rates of conversion to open surgery and to a high risk of involvement of the resection margins. The robotic-assisted approach could overcome some limitations of conventional laparoscopy in rectal surgery, thanks to its steady camera, wristed motion of instruments, and greater ergonomic comfort, especially in very small surgical fields and when a high level of precision is required. Although the results from recent randomized controlled trials comparing laparoscopic versus open surgery are still conflicting, data from population-based multicenter studies have shown the potential advantages of robotic surgery over conventional laparoscopy in terms of reduced conversion and complication rates and shorter length of stay. Otherwise, no differences have been found regarding involvement of the circumferential and distal resection margins and long-term oncological outcomes. One of the main drawbacks of robotic surgery is the higher costs related to the purchase and maintenance of robotic equipment. However, the costs should be carefully evaluated taking into account both the direct and indirect costs, the latter including the savings related to the lower complication rates and shorter hospital stays and therefore potentially counterbalancing the high direct costs.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Rectal cancer
- Colorectal surgery
- Minimally invasive surgery
- Robotic surgery
- Robotic rectal resections
- Total mesorectal excision
- Nerve-sparing surgery
- Da Vinci Xi
- Oncologic outcomes
- Comparative studies
1 Introduction
In the last three decades, laparoscopic colorectal surgery has become the standard of care for benign and malignant diseases thanks to its better postoperative outcomes (less pain and morbidity, shorter length of stay, earlier return to daily activities) and to its oncological results, if compared to conventional open surgery [1].
The laparoscopic approach to rectal cancer (total mesorectal excision, TME) is a technically demanding procedure because the limited range of motion of the straight laparoscopic devices and the narrow operative field may reduce the accuracy of movements, leading to high rates of conversion to open surgery and the risk of involvement of the circumferential resection margins [2].
The robotic-assisted approach may overcome the limitations of conventional laparoscopy in rectal surgery, thanks to wristed motion of instruments, steady camera, and ergonomic comfort [3,4,5], especially in narrow surgical fields and when high precision is required [6]. In the last two decades many studies have demonstrated that robotic rectal surgery (RRS) is feasible, effective and safe [7,8,9]. However, high quality of evidence regarding its superiority over open and laparoscopic rectal surgery (LRS) in postoperative outcomes is still lacking. Although the only RCT available to date failed to demonstrate the superiority of RRS in the conversion rate [9], two recent systematic reviews and meta-analysis concluded that RRS decreases the conversion rate when compared to LRS and is also associated with reduced blood loss [10, 11]. Moreover, long-term oncological outcomes remain to be demonstrated. Indeed, the costs of RRS are greater than those of LRS and this is a non-negligible aspect that impedes the wider spread of its use.
2 Robotic Surgical Techniques
Different surgical procedures have been described for RRS as a result of the technological evolution of the various devices, especially the da Vinci systems (Intuitive Surgical Inc., Sunnyvale, CA, USA).
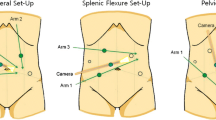
Initially, because of the difficult and time-consuming docking of the first da Vinci robotic cart, the hybrid approach (with previous laparoscopic splenic flexure mobilization) was described. More recently, full-robotic procedures (with double or single docking) have been reported with the use of the Si and Xi da Vinci devices (which allow faster docking, easier setup and multiquadrant access) (see Video 10.1).
2.1 Patient Positioning and Robotic Cart Docking
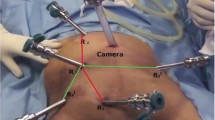
The patient is placed supine with abducted legs positioned on adjustable stirrups, secured on the table to prevent sliding when Trendelenburg and lateral tilt are used. The robotic cart is placed at the patient’s left side, docked according to the surgical step (splenic flexure or TME). After pneumoperitoneum induction, four 8-mm robotic ports are inserted along a straight line parallel and about 4 cm cranial to the costofemoral line, maintaining a distance of about 8 cm between ports. A 12-mm port is inserted in the right flank. The first assistant stands on the patient’s right side.
2.2 Surgical Procedure
The three main steps of the surgical procedure are: splenic flexure mobilization (SFM), vascular control, and TME (Fig. 10.1).
2.2.1 Splenic Flexure Mobilization
Different approaches have been described for SFM, according to the surgeons’ preference. Commonly, it is performed using a medial-to-lateral approach with patient in reverse-Trendelenburg. Firstly, the origin of the inferior mesenteric vein (IMV) is identified and the Toldt-Gerota’s plane is dissected; the transverse colon is lifted up with a grasper and, through the incision of the transverse mesocolic root at the level of the anterior pancreatic border, access to the lesser sac is obtained. The splenic flexure is then retracted medially by the assistant and the 4th arm, and the coloepiploic detachment is performed.
Other approaches are supramesocolic (“top-to-bottom”, starting with the gastrocolic ligament transection to enter the lesser sac) or lateral (starting with the coloparietal detachment along the Toldt’s fascia). A “bottom-to-up” approach along the pancreatic border is also described. SFM can be the last step as it may be omitted or partially performed in order to achieve a tension-free anastomosis (see Video 10.1).
2.2.2 Vascular Control
The approach to the origin of the inferior mesenteric artery (IMA) may be performed with the same docking as used for SFM or after re-docking for TME. In the latter case, the sigmoid colon is lifted up and the IMA is approached in a bottom-to-up fashion, cutting the peritoneum at the level of the sacral promontory to access the avascular presacral mesorectal plane, with identification and preservation of the hypogastric nerves. The superior rectal artery is identified as a landmark. The IMA is identified and isolated with the surrounding lymphatic tissue, divided 1–2 cm away from its origin (with or without left colic artery preservation) commonly using hem-o-lok clips (Teleflex, Wayne, PA, USA).
The medial-to-lateral dissection is performed to identify the left ureter and gonadal vessels up to the IMV, and the Toldt-Gerota’s plane is identified. The IMV is isolated and transected. The dissection continues downward.
2.2.3 Total Mesorectal Excision
The patient is placed in a 20–25° Trendelenburg position with a slight right tilt. TME is carried out after redocking the robotic cart and with the bipolar forceps placed in the left flank, according to Heald’s principles, along the avascular plane in order to preserve the hypogastric nerve and sacral venous plexus.
The dissection starts posteriorly along the plane between the endopelvic visceral fascia and endopelvic parietal fascia. The mesorectal dissection in a TME is performed in a “cylindrical” fashion down to the level of the levator ani; the assistant maintains a cranial traction of the sigmoid colon, during dissection, the seminal vesicles or vagina are important landmarks. The left lateral pelvic fascia is then dissected until the pelvic nerve plexus is identified. During this phase a 0° camera or up-down vision may be helpful for a better visualization.
Rectal transection is performed with robotic or conventional laparoscopic staplers. Vascular perfusion of the rectal stump and proximal colon may be evaluated with the integrated fluorescence imaging system after intravenous administration of indocyanine green [12].
A stapled end-to-end low/ultralow colorectal anastomosis or a manual coloanal anastomosis are performed depending on the tumor distance from the anal verge.
3 Results
3.1 Intraoperative Outcomes
Data from a meta-analysis and RCTs reported a longer operative time for RRS compared to LRS and open surgery [7,8,9,10,11, 13,14,15,16]. This was mainly due to time-consuming double-docking procedures and to the need to change the robotic instruments. In the last few years, the use of the da Vinci Xi platform, with its technology improvements (endoscope connection in any arm, multiquadrant access, longer instruments), has led to a significant reduction in operative time, now comparable to laparoscopy [7, 17].
The ROLARR trial failed to demonstrate superiority in the conversion rate for RRS compared to LRS [9]. However, other studies showed significantly lower conversion rates in the robotic group, especially in low rectal surgery and in the subgroup of high-risk patients (male, neoadjuvant radiochemotherapy, T3N1, obese) [7, 13, 18,19,20,21].
3.2 Short-Term Postoperative Outcomes
To date, no significant statistical difference was shown in complication rates between robotic, laparoscopic and open groups in most published studies [7, 9]. However, some recent papers reported lower overall septic complication rates in RRS versus LRS (1.6% vs. 3.1%, p = 0.02), lower wound dehiscence rates (0.1% vs. 0.7%, p = 0.05), shorter length of stay (3.8–4.8 vs. 4.7–6.3 days, p < 0.001), and shorter time to first flatus [22,23,24,25]. That is probably related to the reduction in conversion and complication rates.
3.3 Functional Outcomes
Two recent meta-analyses reported better functional results after RRS for cancer when compared to LRS: both urinary and sexual function in men at 6 and 12 months after surgery were significantly better in the RRS group [24, 25]. Mixed urinary and sexual function outcomes were also reported for women, with no significant differences in meta-analysis results.
3.4 Oncological Outcomes
The ROLARR trial reported no statistically significant differences in positivity of the circumferential resection margin (5.1% vs. 6.3% in RRS and LRS groups, respectively, p = 0.56), involvement of the distal resection margin, and the pathological assessment of the quality of the plane of surgery [9]. Another RCT from Korea reported the same results [26]. A recent meta-analysis showed that RRS is the better way to achieve a complete TME [25]. Therefore, to date it cannot be concluded that RRS is superior to LRS.
Reports of long-term oncologic outcomes for RRS are still limited. Park et al. and Cho et al. found no differences in the 5-year overall survival, disease-free survival and local recurrence rates [27, 28]. Kim et al. showed that RRS was a significant positive prognostic factor for overall survival in a multivariate analysis [29]. However, Park et al. found RRS to be advantageous in the subgroup of patients who received preoperative chemoradiation and had ypT3–4 tumors after neoadjuvant treatment. The 5-year distant and local recurrence rates were 44.8% and 5.0% in the LRS group and 9.8% and 9.8% in the RRS group, respectively, reaching statistical significance. These data suggest that RRS may be advantageous in most complex cases with high-risk features of recurrence [7].
3.5 Cost Analysis
One of the most debated questions of robotic surgery is the costs of acquisition and maintenance. To date, most of the available studies in different surgical specialties show higher costs related to robotic surgery compared to laparoscopy [30,31,32]. However, most of these studies focused only on the direct costs related to the purchase and maintenance of the robot and to the purchase of the robotic devices. The indirect costs related to the higher conversion rate (with consequent prolonged length of stay and postoperative complications) of laparoscopy and open surgery are rarely taken into account, but they could be carefully evaluated because they have a significant negative impact on the overall costs for each institution and may counterbalance the higher expenditure related to the robotic equipment.
References
Di B, Li Y, Wei K, et al. Laparoscopic versus open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol. 2013;22(3):e39–43.
Tekkis PP, Senagore AJ, Delaney CP, Fazio VW. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. 2005;242(1):83–9.
Patriti A, Ceccarelli G, Bartoli A, Casciola L. Perspective of robotic rectal surgery. Minerva Chir. 2010;65(2):153–9.
Pigazzi A, Luca F, Patriti A, et al. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol. 2010;17(6):1614–20.
Patriti A, Ceccarelli G, Bartoli A, et al. Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS. 2009;13(2):176–83.
Pigazzi A, Ellenhorn JDI, Ballantyne GH, Paz IB. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc. 2006;20(10):1521–5.
Giuratrabocchetta S, Formisano G, Salaj A, et al. Update on robotic total mesorectal excision for rectal cancer. J Pers Med. 2021;11(9):900.
Bhama AR, Obias V, Welch KB, et al. A comparison of laparoscopic and robotic colorectal surgery outcomes using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Surg Endosc. 2015;30(4):1576–84.
Jayne D, Pigazzi A, Marshall H, et al. Effect of robotic-assisted vs conventional laparoscopic surgery on risk of conversion to open laparotomy among patients undergoing resection for rectal cancer: the ROLARR randomized clinical trial. JAMA. 2017;318(16):1569–80.
Memon S, Heriot AG, Murphy DG, et al. Robotic versus laparoscopic proctectomy for rectal cancer: a meta-analysis. Ann Surg Oncol. 2012;19(7):2095–101.
Yang Y, Wang F, Zhang P, et al. Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol. 2012;19(12):3727–36.
Baiocchi GL, Guercioni G, Vettoretto N, et al. ICG fluorescence imaging in colorectal surgery: a snapshot from the ICRAL study group. BMC Surg. 2021;21(1):190.
Sun Z, Kim J, Adam MA, et al. Minimally invasive versus open low anterior resection: equivalent survival in a national analysis of 14,033 patients with rectal cancer. Ann Surg. 2016;263(6):1152–8.
Tam MS, Kaoutzanis C, Mullard AJ, et al. A population-based study comparing laparoscopic and robotic outcomes in colorectal surgery. Surg Endosc. 2016;30(2):455–63.
Lee SH, Kim DH, Lim SW. Robotic versus laparoscopic intersphincteric resection for low rectal cancer: a systematic review and meta-analysis. Int J Color Dis. 2018;33(12):1741–53.
Ohtani H, Maeda K, Nomura S, et al. Meta-analysis of robot-assisted versus laparoscopic surgery for rectal cancer. In Vivo. 2018;32(3):611–23.
Morelli L, Guadagni S, Di Franco G, et al. Use of the new da Vinci Xi during robotic rectal resection for cancer: a pilot matched-case comparison with the da Vinci Si. Int J Med Robot. 2017;13(1):e1728.
Al-Temimi MH, Chandrasekaran B, Agapian J, et al. Robotic versus laparoscopic elective colectomy for left side diverticulitis: a propensity score–matched analysis of the NSQIP database. Int J Colorectal Dis. 2019;34(8):1385–92.
Xiong B, Ma L, Zhang C, Cheng Y. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a meta-analysis. J Surg Res. 2014;188(2):404–14.
Ackerman SJ, Daniel S, Baik R, et al. Comparison of complication and conversion rates between robotic-assisted and laparoscopic rectal resection for rectal cancer: which patients and providers could benefit most from robotic-assisted surgery? J Med Econ. 2017;21(3):254–61.
Katsuno H, Hanai T, Masumori K, et al. Robotic surgery for rectal cancer: operative technique and review of the literature. J Anus Rectum Colon. 2020;4(1):14–24.
Simillis C, Lal N, Thoukididou SN, et al. Open versus laparoscopic versus robotic versus transanal mesorectal excision for rectal cancer: a systematic review and network meta-analysis. Ann Surg. 2019;270(1):59–68.
Esposito S, Formisano G, Giuliani G, et al. Update on robotic surgery for rectal cancer treatment. Ann Laparosc Endosc Surg. 2017;2(8):132.
Kowalewski KF, Seifert L, Ali S, et al. Functional outcomes after laparoscopic versus robotic-assisted rectal resection: a systematic review and meta-analysis. Surg Endosc. 2021;35(1):81–95.
Milone M, Manigrasso M, Velotti N, et al. Completeness of total mesorectum excision of laparoscopic versus robotic surgery: a review with a meta-analysis. Int J Colorectal Dis. 2019;34(6):983–91.
Kim MJ, Park SC, Park JW, et al. Robot-assisted versus laparoscopic surgery for rectal cancer: a phase II open label prospective randomized controlled trial. Ann Surg. 2018;267(2):243–51.
Park SY, Lee SM, Park JS, et al. Robot surgery shows similar long-term oncologic outcomes as laparoscopic surgery for mid/lower rectal cancer but is beneficial to ypT3/4 after preoperative chemoradiation. Dis Colon Rectum. 2021;64(7):812–21.
Cho MS, Baek SJ, Hur H, et al. Short and long-term outcomes of robotic versus laparoscopic total mesorectal excision for rectal cancer: a case-matched retrospective study. Medicine (Baltimore). 2015;94(11):e522.
Kim J, Baek SJ, Kang DW, et al. Robotic resection is a good prognostic factor in rectal cancer compared with laparoscopic resection: long-term survival analysis using propensity score matching. Dis Colon Rectum. 2017;60(3):266–73.
Higgins RM, Frelich MJ, Bosler ME, Gould JC. Cost analysis of robotic versus laparoscopic general surgery procedures. Surg Endosc. 2016;31(1):185–92.
Khorgami Z, Li WT, Jackson TN, et al. The cost of robotics: an analysis of the added costs of robotic-assisted versus laparoscopic surgery using the National Inpatient Sample. Surg Endosc. 2019;33(7):2217–21.
Cleary RK, Mullard AJ, Ferraro J, Regenbogen SE. The cost of conversion in robotic and laparoscopic colorectal surgery. Surg Endosc. 2018;32(3):1515–24.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Video 10.1
Robotic nerve sparing total mesorectal excision: rectal resection with indocyanine green use (MP4 1254954 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Bugiantella, W., De Rosa, M., Mariani, L., Rondelli, F., Scabini, S., Ceccarelli, G. (2024). Robotic Nerve-Sparing Total Mesorectal Excision. In: Ceccarelli, G., Coratti, A. (eds) Robotic Surgery of Colon and Rectum. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-33020-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-33020-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-33019-3
Online ISBN: 978-3-031-33020-9
eBook Packages: MedicineMedicine (R0)