Abstract
Terms such as retrosternal, substernal, and intrathoracic have been used to define an extension of >50% of the thyroid gland beyond the thoracic inlet. The reported incidence of retrosternal goiters is highly variable, ranging from 1% to 45% of thyroidectomies in studies with different defining criteria. Forgotten goiter lacks any parenchymatous or vascular connection with the cervical thyroid and may represent remnants of a partially resected cervical goiter or perhaps the result of a separate and autonomously functioning thyroid tissue that becomes hypertrophic after the removal of the cervical gland. A recurrent goiter is the regrowth of thyroid tissues after thyroidectomy for benign disease or malignancy of the thyroid. Computed tomography and/or magnetic resonance imaging are essential in surgical decision-making for retrosternal goiters, since they provide a clear definition of the multidimensional size and of the morphology of the disease, defining the relevant and at-risk anatomical structures. Total thyroidectomy represents the treatment of choice for retrosternal goiters, and reoperative thyroid surgery should be performed in case of symptomatic recurrent or forgotten goiter, in case of suspected malignancy or in some cases of recurrent thyrotoxicosis; the surgery is achieved through a cervical or extracervical approach, which requires multidisciplinary collaboration.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Retrosternal goiter
- Forgotten goiter
- Recurrent goiter
- Cervical approach
- Extracervical approach
- Substernal
- Intrathoracic
- Mediastinal
- Redo surgery
1 Retrosternal Goiter
Retrosternal goiter (RG) was first described by Haller [1]. The clinical significance of this condition increases progressively due to clinical features that include impingement on surrounding structures and onset of compressive symptoms. Goiter development is a multifactorial event, iodine deficiency being considered as one of the major environmental factors [2]. The diagnosis of RG is usually established in the fifth or sixth decade of life, with a female-to-male ratio of approximately 3–4:1 [2].
There is no standard definition for RGs [3]. Some authors have considered an RG as one that extends up to the fourth thoracic vertebra on chest x-ray, or reaches the aortic arch; others have defined a mediastinal thyroid as one in which most of the gland extends below the thoracic inlet with the patient in surgical position [3]. Terms such as retrosternal, substernal, and intrathoracic have been used to define an extension of >50% of the thyroid gland beyond the thoracic inlet [4]. The reported incidence of RG is highly variable, ranging from 1% to 45% of thyroidectomies in studies with different defining criteria [2, 3, 5].
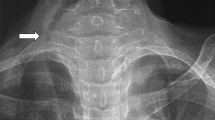
The classification of RGs as primary or secondary is of surgical relevance because of differences in the blood supply. Primary RG is rare (1%), arising from ectopic thyroid tissue in the mediastinum, and is supplied by non-anatomic mediastinal vessels [2, 3, 5]. The majority of RGs are secondary, originating from extension of the cervical gland into the chest, with a blood supply arising from the cervical vessels (Fig. 5.1) [3].
RGs are located in the anterior mediastinum in 80–90% of cases and less frequently (10–15%) in the posterior compartment, posterior to the trachea or esophagus [2]. In the latter, the recurrent laryngeal nerve (RLN) may be severely anteriorly displaced, placing it at higher risk of accidental injury [6].
The Huins et al. [7] classification of RGs categorizes them as retroclavicular, upper border of the aortic arch or below the aortic arch. Goiters have also been classified according to their shape on coronal imaging with the Simo classification: “iceberg” or “conical”, “tubular” or “oval” [8].
Evaluation of a suspected RG involves full history taking and examinations, including fiberoptic nasendoscopy, baseline thyroid function tests, thyroid autoantibody screen, and neck ultrasound [3, 9]. Ultrasound-guided fine-needle aspiration cytology is not routinely applied in RGs [3, 10]. Therefore, computed tomography (CT) and/or magnetic resonance imaging (MRI) are essential in the surgical decision-making for RGs, since they provide a clear definition of the multidimensional size and morphology of the disease, defining the relevant and at-risk anatomical structures.
Most goiters grow very slowly over many decades. Therefore, most patients with RG are asymptomatic. If a cervical mass is palpated on physical examination but the examining physician is unable to palpate the most inferior portion of the mass at the thoracic inlet, the presence of an intrathoracic extension of the goiter should be strongly suspected. Airway compression is one of the most common findings in patients with RG. Other reported symptoms include exertional dyspnea, stridor or wheezing (when the luminal diameter is below 5 mm), unproductive cough, choking sensation due to dysphagia, hoarseness, vascular (e.g., superior vena cava syndrome) and neurologic symptoms (e.g., vocal cord paresis/paralysis, hoarseness, and Horner’s syndrome) [2, 11, 12].
Total thyroidectomy represents the treatment of choice for all RGs and is achieved through a cervical (CA) or extracervical approach (ECA). In most cases, thyroidectomy can be performed using a CA (up to 90%), but a manubriotomy, sternotomy, or thoracotomy is always necessary for primary RG, when the gland is predominantly intrathoracic or when preoperative assessment with CT scan suggests infiltration into surrounding structures [2,3,4,5,6,7,8, 10]. Therefore, RGs with giant intrathoracic extension, recurrence, or posterior extension to both trachea and esophagus, or between trachea and esophagus, and a diameter greater than the thoracic inlet diameter should be treated with an ECA [2,3,4,5,6,7,8, 10, 13]. Extension beyond the aortic arch and an “iceberg shape” are the strongest predictors of the need for sternotomy [2,3,4,5,6,7,8, 10].
RGs affecting both anterior and posterior mediastinum, moreover, ride over the brachiocephalic vascular bundle causing distortion of these vessels and possibly of the RLN [10]. Therefore, an anterior approach via midline sternotomy is preferred over a cranio-caudal one, as the former provides for a direct view (as shown in the Video 5.1).
The classic ECAs to an anterior mediastinal mass are median sternotomy, cervical mediastinoscopy and video-assisted thoracoscopic surgery [6], which is an excellent option for an ECA, with fewer side effects than median sternotomy.
Thyroid lobectomy can also be sufficient in selected high-risk patients, in whom a conservative strategy may resolve the symptoms, while reducing the risk of complications such as bilateral RLN paralysis, tracheostomy and permanent hyperparathyroidism [10].
Intraoperative nerve monitoring is a risk-reducing tool used to verify RLN integrity during thyroid surgery, and the literature supports its use in revision surgery, invasive malignancy, and large RGs [14].
The indications for surgery in symptomatic RGs take into account that radioiodine ablation implies a possible acute risk of radiation thyroiditis causing airway obstruction, that most RGs tend to grow, and that up to 25% of RGs harbor malignancy [2, 3].
A non-surgical approach, involving monitoring with serial CT scans, is preferred in older patients and poor surgical candidates, but also in asymptomatic patients with normal flow-volume loops [2].
Radioiodine, alone or following rhTSH stimulation to increase radioiodine efficacy, is a reasonable option for patients who cannot undergo surgery or refuse it.
Other minimally invasive local treatment modalities have been described, such as radiofrequency ablation and laser microfilament ablation, but these methods have yet to be validated in larger studies.
Literature data differ on the increased incidence of malignancy in cases of RG compared to cervical goiter; some authors reported a range from 10% to 35% [3, 8, 10], while others reported that malignancy is less common in RG than in cervical goiter [15].
In addition, the impossibility to perform fine-needle aspiration cytology in the presence of mediastinal disease, due to risks of bleeding or thoracic organ damage, limits the preoperative diagnosis of malignancy [16].
The literature shows an increased incidence of permanent hypoparathyroidism and definitive RLN palsy after total thyroidectomy for RG, rather than for cervical goiter [3, 5, 17,18,19,20,21].
The side effects of a CA include: transient (2–5.4%) or permanent (1–2%) unilateral RLN injury, transient (33.9%) or permanent (2.1%) hypocalcemia [2, 3, 19,20,21] and tracheomalacia (3%) [2].
A lesion that occupies space in the mediastinum has greater potential to distort the normal anatomy and, when coupled with an increase in gland size, involves a technically challenging procedure where the RLN may not be easily identifiable in its anatomic location. Bilateral RLN palsy is the main causative factor for tracheostomy in the RG patient, with tracheostomy rates of approximately 2–3% in this population [6].
Although the parathyroid glands can be identified and structurally preserved, an even more significant challenge arises in the RG patient in order to maintain an uninterrupted blood supply. Specifically in RG, while the inferior parathyroids are more likely to be displaced and have a truly intrathoracic location, the position of the superior ones is often unaffected [6].
Postoperative bleeding is rare but potentially fatal, occurring in 0.3–2% of cases [3]. Intraoperative bleeding complicates surgical dissection by staining and hiding important structures and causing indirect RLN- and parathyroid-related morbidity due to blind surgical maneuvers. Therefore, innovative devices such as the harmonic scalpel [22] and topical hemostatic agents [23] are commonly used.
Even considering the compressive pathological process of the RG, tracheomalacia is rarely described in the literature as a clinically significant concern. Significantly powered studies have shown little evidence, with rates reported as being <1.5% [6]. In patients with suspected tracheomalacia other causes of airway obstruction should immediately be ruled out. Generally, reintubation is uneventful even in confirmed tracheomalacia [6].
However, division of the sternum increases the morbidity of the treatment for benign disease. Complications of median sternotomy include deep space wound infections, dehiscence, arrhythmias, and sternal instability. Risks of cervical mediastinoscopy include severe bleeding due to the need for median sternotomy, RLN injury specifically on the patient’s left and thoracic duct injury [6].
Mortality in RG surgical series ranges from 0% to 15.3% and is higher in patients who have malignant disease or when surgery becomes urgent due to severe worsening of respiratory symptoms [10]. The causes of death are related to severe postoperative complications such as sternotomy dehiscence and tracheobronchial fistula.
Therefore, strict preoperative diagnosis through proper imaging is important to avoid hazardous digital dissection and simultaneously improper sternal division. This can be explained by the fact that the sternal division provides the surgeon with control of all the structures of the neck and upper mediastinum. In contrast, during digital dissection of the mediastinal component, the removal of the intrathoracic part of the goiter through the CA forces the surgeon to perform blind maneuvers, placing the inferior parathyroids and the RLNs at risk of injury [3, 24].
When the upper mediastinum is occupied by a goiter, it could be considered a ‘no man’s land’ [3]. Indeed, the endocrine surgeon is not usually familiar with the course of the RLNs and their anatomical variability in this district, and the cardiothoracic surgeon is not familiar with the endocrine-surgical challenges. Therefore, the ECA requires multidisciplinary collaboration.
2 Forgotten Goiter
Forgotten goiter (FG) was first described by Massard et al. in 1992 [25].
FG lacks any parenchymatous or vascular connection to the cervical thyroid and may represent remnants of an incompletely resected cervical goiter or perhaps the result of completely separate and autonomously functioning thyroid tissue that becomes hypertrophic following removal of the cervical gland [26]. Compensatory hypertrophy of residual thyroid tissue following partial thyroidectomy was first described by Wagner in 1884 and was confirmed in the succeeding decades by several authors [27]. Therefore, any thyroid tissue remaining after cervical thyroidectomy can grow or migrate inferiorly [28].
Furthermore, based on the embryology of the thyroid, although rarely, some anomalies may occur, and the gland can be located in the mediastinum, the so-called autonomous intrathoracic goiter (AIG) which must be distinguished from migratory goiters in partially resected glands after thyroidectomy. The gland arises from a saclike entodermal diverticulum, which appears in the midline of the ventral surface of the pharynx. This sac will form the parenchyma of the thyroid and is connected to the ventral floor of the pharynx via the thyroglossal duct. As the thyroglossal duct atrophies, the thyroid progressively relocates anterior to the trachea [29].
FG most likely represents a clinically silent goiter left behind at the time of original thyroidectomy, and the diagnosis is an incidental finding. Less commonly, patients may become symptomatic either from hormonally active thyroid tissue within the mediastinum or from mass effect causing tracheal compression and deviation.
In the literature there is no clear consensus as to the optimal approach for resection. The literature reaffirms that an FG, like the majority of RGs, can be safely and completely resected using a standard CA, after prompt diagnosis with MRI and, more recently, multidetector CT scan to provide better anatomical assessment [25,26,27,28,29]. The size, location, and benign characteristics remain the most relevant considerations in determining a cervical rather than a trans-sternal access, as in RG.
3 Recurrent Goiter
A recurrent goiter is the regrowth of thyroid tissues after thyroidectomy for benign disease or malignancy of the thyroid. In the literature, the recurrence rates after an incomplete thyroidectomy vary from 2% to 42%, and are mainly influenced by the definition of recurrence and the follow-up, with a peak of recurrence between 10 and 20 years after primary surgery [30]. On the other hand, in the case of total thyroidectomy, the recurrence rate is <1% [31].
While recurrence after surgery for benign disease should be preventable, recurrence after malignant disease depends on many factors. The causes of recurrence can be broadly attributed to inadequate surgery or postoperative levothyroxine therapy, missed embryological remnants, and recurrence of a thyroid cancer.
The three embryological remnants of thyroid at risk of regrowth are the pyramidal lobe, the tubercle of Zuckerkandl, and thyrothymic tissue; these may develop as isolated or combined recurrences [31, 32].
Reoperative thyroid surgery should be performed in case of symptomatic recurrent goiter, in case of suspected malignancy or in some cases of recurrent thyrotoxicosis [33].
In previous studies [34, 35], the rate of cancer incidence was reported to be higher in recurrent surgery even when the first surgery was performed for benign causes: Menegaux et al. [34] reported a cancer rate of 11.4% in recurrent surgery, while Levin et al. [35] found this rate to be 22%.
Surgery for recurrent goiter is associated with a higher complication rate because of scar tissue which makes it difficult to recognize and preserve neck structures such as the RLNs or the vascular pedicles of the parathyroid gland. A higher risk of complications is described when previous surgery has been performed on both sides and increases with the number of reoperations [33, 36]. In recurrent surgery, the rate of temporary and permanent RLN paralysis is 0–22% and 0–13%, respectively; the rate of transient and permanent hypoparathyroidism is 9–35% and 0–22%, respectively [31, 33, 37]. Thus, considering the increased risk of complications, the indication for recurrent surgery should be well identified.
In redo surgery, the lateral approach should be considered to avoid the infiltration by connective tissue of Kocher’s approach and to identify the nerves in a previously undissected area [34]. The strap muscles are retracted medially followed by entry in a plane anterior to the sternocleidomastoid. The RLN should be always identified, usually in the lower neck, then followed along its cervical course. Intraoperative neuromonitoring is useful in redo surgery and may reduce the morbidity related to RLN injury, because it improves the detection of the nerve in the scar tissue [38, 39].
Little is known about the therapeutic outcome of 131I therapy for recurrent goiters.
References
Haller A. Disputationes Anatomicae Selectae. Gottingen: Vandenhoeck; 1749. p. 96.
Knobel M. An overview of retrosternal goiter. J Endocrinol Investig. 2021;44(4):679–91.
Testini M, Gurrado A, Avenia N, et al. Does mediastinal extension of the goiter increase morbidity of total thyroidectomy? A multicenter study of 19,662 patients. Ann Surg Oncol. 2011;18(8):2251–9.
Katlic MR, Wang CA, Grillo HC. Substernal goiter. Ann Thorac Surg. 1985;39(4):391–9.
Testini M, Nacchiero M, Miniello S, et al. Management of retrosternal goiters: experience of a surgical unit. Int Surg. 2005;90(2):61–5.
Hanson MA, Shaha AR, Wu JX. Surgical approach to the substernal goiter. Best Pract Res Clin Endocrinol Metab. 2019;33(4):101312.
Huins CT, Georgalas C, Mehrzad H, Tolley NS. A new classification system for retrosternal goitre based on a systematic review of its complications and management. Int J Surg. 2008;6(1):71–6.
Tikka T, Nixon IJ, Harrison-Phipps K, Simo R. Predictors of the need for an extracervical approach to intrathoracic goitre. BJS Open. 2018;3(2):174–9.
Rosato L, De Crea C, Bellantone R, et al. Diagnostic, therapeutic and health-care management protocol in thyroid surgery: a position statement of the Italian Association of Endocrine Surgery Units (U.E.C. CLUB). J Endocrinol Investig. 2016;39(8):939–53.
Simó R, Nixon IJ, Vander Poorten V, et al. Surgical management of intrathoracic goitres. Eur Arch Otorhinolaryngol. 2019;276:305–14.
Testini M, Gurrado A, Lissidini G, et al. Emergency surgery for acute respiratory failure secondary to spontaneous thyroid hemorrhage. Int Surg. 2008;93(3):158–62.
Testini M, Logoluso F, Lissidini G, et al. Emergency total thyroidectomy due to non traumatic disease. Experience of a surgical unit and literature review. World J Emerg Surg. 2012;7:9.
Prete FP, Panzera PC, Di Meo G, et al. Risk factors for difficult thyroidectomy and postoperative morbidity do not match: retrospective study from an endocrine surgery academic referral Centre. Updat Surg. 2022;74(6):1943–51.
Prete FP, Sgaramella LI, Di Meo G, et al. Introducing routine intraoperative nerve monitoring in a high-volume endocrine surgery Centre: a health technology assessment. Updat Surg. 2021;73(6):2263–73.
White ML, Doherty GM, Gauger PG. Evidence-based surgical management of substernal goiter. World J Surg. 2008;32(7):1285–300.
Franco IF, Gurrado A, Lissidini G, et al. Floating left innominate vein neoplastic thrombus: a rare case of mediastinal extension of follicular thyroid carcinoma. Phlebology. 2015;30(2):140–4.
Chow TL, Chan TT, Suen DT, et al. Surgical management of substernal goitre: local experience. Hong Kong Med J. 2005;11(5):360–5.
Zambudio AR, Rodríguez J, Riquelme J, et al. Prospective study of postoperative complications after total thyroidectomy for multinodular goiters by surgeons with experience in endocrine surgery. Ann Surg. 2004;240(1):18–25.
Testini M, Gurrado A, Lissidini G, Nacchiero M. Hypoparathyroidism after total thyroidectomy. Minerva Chir. 2007;62(5):409–15.
Testini M, Gurrado A, Bellantone R, et al. Recurrent laryngeal nerve palsy and substernal goiter. An Italian multicenter study. J Visc Surg. 2014;151(3):183–9.
Gurrado A, Pasculli A, Pezzolla A, et al. A method to repair the recurrent laryngeal nerve during thyroidectomy. Can J Surg. 2018;61(4):278–82.
Testini M, Pasculli A, Di Meo G, et al. Advanced vessel sealing devices in total thyroidectomy for substernal goitre: a retrospective cohort study. Int J Surg. 2016;35:160–4.
Testini M, Marzaioli R, Lissidini G, et al. The effectiveness of FloSeal matrix hemostatic agent in thyroid surgery: a prospective, randomized, control study. Langenbeck’s Arch Surg. 2009;394(5):837–42.
Testini M, Piccinni G, Lissidini G, Nacchiero M. The lifting of substernal goitres using a Fogarty catheter. Ann R Coll Surg Engl. 2005;87(1):63–4.
Massard G, Wihlm JM, Jeung MY, et al. Le goitre médiastinal oublié: sept observations [Forgotten mediastinal goiter: seven cases]. Ann Chir. 1992;46(8):770–3.
Sahbaz A, Aksakal N, Ozcinar B, et al. The “forgotten” goiter after total thyroidectomy. Int J Surg Case Rep. 2013;4(3):269–71.
Patel KM, Parsons CC. Forgotten goiter: diagnosis and management. A case report and literature review. Int J Surg Case Rep. 2016;27:192–4.
Calò PG, Tatti A, Medas F, et al. Forgotten goiter. Our experience and a review of the literature. Ann Ital Chir. 2012;83(6):487–90.
Netter FH, Forsham PH, editors. The Ciba collection of medical illustrations, vol. 4. Section II. West Caldwell, NJ: Ciba; 1974. p. 43–4.
Moalem J, Suh I, Duh QY. Treatment and prevention of recurrence of multinodular goiter: an evidence-based review of the literature. World J Surg. 2008;32(7):1301–12.
Snook KL, Stalberg PL, Sidhu SB, et al. Recurrence after total thyroidectomy for benign multinodular goiter. World J Surg. 2007;31(3):593–8. discussion 599–600
Cirocchi R, Trastulli S, Randolph J, et al. Total or near-total thyroidectomy versus subtotal thyroidectomy for multinodular non-toxic goitre in adults. Cochrane Database Syst Rev. 2015;2015(8):CD010370.
Medas F, Tuveri M, Canu GL, et al. Complications after reoperative thyroid surgery: retrospective evaluation of 152 consecutives cases. Updat Surg. 2019;71(4):705–10.
Menegaux F, Turpin G, Dahman M, et al. Secondary thyroidectomy in patients with prior thyroid surgery for benign disease: a study of 203 cases. Surgery. 1999;126(3):479–83.
Levin KE, Clark AH, Duh QY, et al. Reoperative thyroid surgery. Surgery. 1992;111(6):604–9.
Peix JL, Van Box SP, Olagne E, et al. Résultats des réinterventions pour goitres [Results of reoperations for goiter]. Ann Chir. 1997;51(3):217–21.
Lombardi CP, Raffaelli M, De Crea C, et al. Morbidity of central neck dissection: primary surgery vs reoperation. Results of a case-control study. Langenbeck’s Arch Surg. 2014;399(6):747–53.
Wojtczak B, Barczyński M. Intermittent neural monitoring of the recurrent laryngeal nerve in surgery for recurrent goiter. Gland Surg. 2016;5(5):481–9.
Johnson S, Goldenberg D. Intraoperative monitoring of the recurrent laryngeal nerve during revision thyroid surgery. Otolaryngol Clin N Am. 2008;41(6):1147–51.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
GCM SIC 22 HiRes compact (MP4 62813 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Gurrado, A. et al. (2024). Retrosternal, Forgotten, and Recurrent Goiter. In: Testini, M., Gurrado, A. (eds) Thyroid Surgery. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-31146-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-31146-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-31145-1
Online ISBN: 978-3-031-31146-8
eBook Packages: MedicineMedicine (R0)