Abstract
Thyroid cancer is the most common endocrine malignancy, with an overall good prognosis, despite an increasing incidence and a notable recurrence and metastases rate. A portion of thyroid cancers progresses to aggressive and refractory disease. Unfortunately, no test is available to accurately differentiate before surgery patients that requiring total thyroidectomy with or without lymph node removal from those that can be cured with hemithyroidectomy or deserve active surveillance. Understanding how angiogenesis turns into a deregulated process, leading to the angiogenic switch that causes the formation of new vessels, local invasion, proliferation, and metastases, is one of the critical sectors of research in the field of thyroid cancer behavior. Studying several molecular pathways, and characterizing the angiogenic microenvironment of thyroid cancer, allows researchers to identify more than one mediator that can be used as risk stratification markers and targets for new therapies. Therefore, the scientific community aims to dissect the angiogenic microenvironment of thyroid cancer with multi-omic and translational approaches to discover how to predict de-differentiation and radioiodine resistance preoperatively, to limit diagnostic thyroidectomies, as well as to find new molecules to treat advanced cases.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Thyroid cancer
- Angiogenic microenvironment
- Risk stratification
- Prognostic markers
- Antiangiogenic therapy
1 Introduction
Thyroid cancer is the most common endocrine malignancy, and it has been characterized in recent decades by the fastest increase in incidence among all neoplasms [1, 2]. Among differentiated thyroid cancer (DTC), papillary thyroid cancer (PTC) is the most common and accounts for 90% of all histotypes. Usually, PTCs are slow-growing and indolent tumors associated with lymph node metastases in 30–90% of cases [3, 4].
The cornerstone of the PTC treatment is the surgical removal of the tumor with or without radioiodine therapy. Despite an overall good prognosis, a portion of cases progresses to aggressive and refractory disease, showing neck recurrence or distant metastases. The angiogenic switch is the critical process that leads a tumor to local invasion and distant metastases through the growth of new vessels [5]. A deep understanding of the molecular mechanisms that transform the thyroid cancer microenvironment into an angiogenic microenvironment is at the center of the research into new prognostic markers and therapeutic targets [6]. Examining these processes could improve many steps of thyroid cancer management: identifying cytologically indeterminate nodules needing surgical removal, thus reducing diagnostic thyroidectomies; differentiating aggressive cancers to modulate surgical removal extent and approach and the follow-up strategies; improving the targeted therapy of metastatic patients with refractory disease.
Therapeutic options for thyroid cancers, indeed, encompass the unilateral or total surgical removal of the thyroid, followed or not by thyroid hormone suppression and ablative therapy with radioactive iodine (RAI) treatment. In addition, a lateral or central neck dissection is also performed in the case of intra- or preoperative evidence of lateral or central neck lymph node involvement. Distant metastatic patients need further therapies since their tumors are often refractory to T4-mediated thyroid-stimulating hormone (TSH) suppression and RAI therapy [7, 8]. Research in the field of new molecules for treating such patients is focused on the angiogenic processes.
The network of angiogenic cytokines and other mediators prompts the interaction between thyroid cancer cells, thyroid normal and stromal cells. Among these factors, there is evidence that genomic, transcriptomic and proteomic variables can be linked to prognosis and therapeutic purposes at different levels. Systems biology and multi-omic approaches are the new research strategies that trace the link between all these variables.
2 The Thyroid Cancer Microenvironment and Angiogenesis
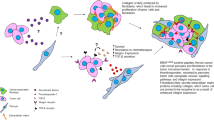
The combination of stromal cells (SCs) and extracellular matrix (ECM) components is the core of the thyroid cancer microenvironment [9]. The interaction between SCs and ECM components gives support and nutrition to cancer cells through a series of mediators, such as growth factors and cytokines [10].
Cancer-associated fibroblasts (CAFs) produce mediators and drive inflammation, immune response, metabolism, and drug resistance [11]. Indeed, poor survival and lymphatic spread characterize a subtype of papillary thyroid cancer with peculiar CAF-related proteins associated with the mutation V600E of the BRAF gene (BRAFV600E) and the loss of PTEN [12,13,14].
Tumor-associated macrophages (TAMs) are another component of the thyroid cancer microenvironment that show high production of IL-10 and low production of IL-12 [15,16,17]. TAMs allow angiogenesis through the secretion of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF) and cause remodeling of the extracellular matrix through the synthesis of matrix metalloproteases (MMPs) [18, 19].
Tryptase-positive mast cells are another cellular marker of thyroid cancer invasiveness and extrathyroidal extension, showing a higher presence than adenoma [20]. Mast cells produce IL-6, TNF-α, CXCL8/IL-8, CCL25/TECK, CXCL10/IP-10, CXCL1/GRO-α, and VEGF, all involved in the transition from epithelium to mesenchyme, angiogenesis, and lymphangiogenesis [21].
Granulocyte colony-stimulating factor and CXCL8/IL-8, produced by thyroid cancer cells, recruit neutrophils that release oncostatin-M and VEGF-A, as well as granules containing elastase, promoting angiogenesis and cancer proliferation and invasiveness [22].
A differential gene expression characterizes the stroma of thyroid cancer compared to normal thyroid. Different genes, indeed, implied in the control of apoptosis, metabolism, and cell response to hypoxia and proliferation, trigger these cellular and proteomic differences in the SCs and ECM components [23].
Neovascularization in the cancer microenvironment includes three different mechanisms [24]:
-
1.
angiogenesis, the sprouting of new vessels from pre-existing ones;
-
2.
vasculogenesis, the formation of new vessels starting from endothelial precursor cells (hemangioblasts);
-
3.
vasculogenic mimicry, the ability of tumor cells or other non-endothelial cells to complete or form new vessels without vascular endothelial cells.
The first mechanism is the most important and accounts for the longitudinal splitting of existing vasculature into two functional vessels (intussusceptive angiogenesis) and the loop-shaped expansion of the vessel (looping angiogenesis) [25].
Multinodular goiter, Graves’ disease, and cancer show increased vascularity. On the other hand, microvascular density (MVD) has been shown to correlate with disease-free survival in thyroid cancers, particularly in PTC and follicular thyroid carcinoma (FTC) [26]. The metastatic spread pattern differs among tumor types, probably because of the influence of proangiogenic and antiangiogenic factors on the phenotype, as well as the expression of receptors, extracellular matrix components and, finally the differential gene expression profile. Consequently, adenomas, microcarcinomas, PTCs, FTCs, undifferentiated, and medullary thyroid carcinomas show considerable differences in metastatic spread.
The following processes (and genes) are involved in the activity of the stromal cells surrounding thyroid cancer: cell survival (RIPK5), proliferation (PTGS2, DUSP5), apoptosis (ZFP36L1, IER3), metabolism (SLCA2A3), organization (RAB7B), response to hypoxia (HIF1A, TUFT1, BHLHB2), and protein degradation (SKP1, KLK-4) [23].
As already explained, a network of processes develops from these differentially expressed genes, allowing the components of stroma and matrix to communicate with cancer cells, inducing angiogenesis and aggressiveness. The release of exosomes by the thyroid cancer cells is another crucial moment of this communication [27].
From a transcriptomic point of view, dysregulation of miRNA influences the features of different types of thyroid cancers through the effects on proliferative signals, resistance to apoptosis, and epithelial-mesenchymal transition [28].
Thyroid hormones, T3 and T4, ligate their receptor site on the ανβ3 integrin and modulate, together with HIF-1α, the activity of VEGF and bFGF on endothelial and other vascular cells [26]. TSH also shows a proangiogenic activity because it stimulates VEGF production through a protein C kinase pathway [24, 29].
In addition, thyroid microvascular activation depends on cAMP- and mTOR-mediated pathways, linked to the iodine deprivation and reactive oxygen species (ROS) production that causes VEGF release [30].
VEGF is not a single factor, but it encompasses a family of proteins, VEFG-A, -B, -C, -D, -E and PDFG, each with a receptor differentially expressed in various cells [31]. Angiogenesis is mediated by VEGF-A, -E and VEFGR-2-neuropilin (NRP)-1, -2, while lymphangiogenesis by VEGF-C, -D and VEGFR-2, -3 [31]. Moreover, cells different from vascular ones, can undergo the influence of VEGF through autocrine and paracrine phenomena, including the following pathways: ROS production, lysophosphatidic acid signaling, c-Jun N-terminal kinase, NF-kB, PI3K/Akt signaling, AP-1 and SP-1 [32]. VEFG-C, angiopoietin-2, VEGFR-2 and -3 over-expression correlates with increased tumor size, aggressiveness and metastasis formation of thyroid cancer [33]. bFGF and FGFR, independently from VEFG, promote thyroid cancer angiogenesis [34].
MMPs, zinc-endopeptidases, contribute to angiogenesis because their degradation of ECM components stimulates the release of angiogenic factors stored with heparan sulphate [35]. MMP-9, in particular, is regulated by epidermal growth factor (EFG) through focal adhesion kinase (FAK) phosphorylation. The balance between MMPs and tissue inhibitors of metalloproteinases (TIMPs), produced by the tumor microenvironment, is at the basis of a switch towards an invasive phenotype, mainly due to increased MMP production [36].
3 Angiogenic Mediators and their Prognostic Value
The introduction of gene classifiers, primarily based on BRAF mutations, on the fine-needle aspiration specimen improved the selection of patients with cytologically indeterminate nodules affected by malignant disease [37]. However, even if every genomic variant of thyroid cancer were described, this information would not be fully useful in clinical practice: many mutations can even be found in normal cells, and many thyroid cancers do not show any mutation [38].
Aggressive DTC shows BRAF mutated, galectin-3, HBME-1, CK19 and estrogen receptor beta. In particular, BRAFV600E is typical of the tall cell variant of PTC, and the same is true for the following hub genes: COL5A1, COL1A1, COL10A1, COL11A1, CCL20, and CXCL5 [39]. BRAF is also altered in the radioiodine refractory DTC and cases with central neck nodal metastases, mainly associated with miR-146b-3p, miR-146b-5p, and miR-222 [40]. Moreover, BRAFV600E identifies cases with a high risk of recurrence. In the case of anaplastic thyroid cancer, BRAFV600E reduces TSP-1 expression, inducing the stabilization of pericytes, which contributes to the secretion of VEFG, PDGF and other factors by vascular and stromal cells, with migration and invasion of neoplastic cells into the new vessels [41].
VEGF-A, -C, PDGF-BB and angiopoietin-2, although higher in patients with the neoplastic and benign disease compared with healthy patients, cannot identify aggressive disease, metastatic disease or large cancers [36]. Nevertheless, VEGF-C, angiopoietin-2, KDR, Flt-4, and TEK, are highly expressed during the angiogenic shift of thyroid cancer and correlate with tumor size, nodal and distant metastases. Both mechanisms, reduced TSP-1 expression induced by BRAFV600E and VEGF overexpression, are associated with an increased microvascular count [24].
Angiogenic stimulation accompanies cell cycle activation in thyroid cancer, which is proven by the association of VEGF with FAL1 and cyclin D1 [42]. However, a regulatory effect of VEGF production pertains to the effects of TSH, opening perspectives for therapies based on recombinant human TSH.
Among circulating markers, miRNAs play a predominant role: in addition to the already cited miR-146b-3p, miR-146b-5p, and miR-222, the exosomes containing miR-21-5p produced by thyroid cancer cells show a significant proangiogenic effect [27, 43].
Medullary thyroid carcinoma (MTC) with metastases is characterized by overexpression of VEGFR-2 and EGFR. Its adverse prognosis correlates with HIF-1α, induced by hypoxia and other signals that coordinately induce VEGF expression [44].
Prostate-specific membrane antigen (PSMA) is a favorable prognostic marker of MTC when found in its neo-vessels, and it could be used as a target for radio-guided imaging and therapy [45]. Moreover, the MMP-2/TIMP-2 ratio is another prognostic factor for MTC [46].
PTEN loss and BRAFV600E seem to drive collagen deposition from an increased number of fibroblasts and its cross-linking, conferring an aggressive behavior to thyroid cancer [14, 47]. Meanwhile, the role of other factors, like macrophages and T lymphocytes, is still uncertain [9].
References
Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21.
Li M, Maso LD, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol. 2020;8(6):468–70.
Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646–53.
Jensen CB, Saucke MC, Francis DO, et al. From overdiagnosis to overtreatment of low-risk thyroid cancer: a thematic analysis of attitudes and beliefs of endocrinologists, surgeons, and patients. Thyroid. 2020;30(5):696–703.
Ribatti D, Nico B, Crivellato E, et al. The history of the angiogenic switch concept. Leukemia. 2007;21(1):44–52.
Melaccio A, Sgaramella LI, Pasculli A, et al. Prognostic and therapeutic role of angiogenic microenvironment in thyroid cancer. Cancers (Basel). 2021;13(11)
Cabanillas ME, Ryder M, Jimenez C. Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endoc Rev. 2019;40(6):1573–604.
Lim SM, Chung WY, Nam KH, et al. An open label, multicenter, phase II study of dovitinib in advanced thyroid cancer. Eur J Cancer. 2015;51(12):1588–95.
MacDonald L, Jenkins J, Purvis G, et al. The thyroid tumor microenvironment: potential targets for therapeutic intervention and prognostication. Horm Cancer. 2020;11(5):205–17.
Turner HE, Harris AL, Melmed S, Wass JAH. Angiogenesis in endocrine tumors. Endoc Rev. 2003;24(5):600–32.
Östman A. Cancer-associated fibroblasts: recent developments and emerging challenges. Semin Cancer Biol. 2014;25:1–2.
Sun WY, Jung WH, Koo JS. Expression of cancer-associated fibroblast-related proteins in thyroid papillary carcinoma. Tumor Biol. 2016;37(6):8197–207.
Cho JG, Byeon HK, Oh KH, et al. Clinicopathological significance of cancer-associated fibroblasts in papillary thyroid carcinoma: a predictive marker of cervical lymph node metastasis. Eur Arch Otorhinolaryngol. 2018;275(9):2355–61.
Jolly LA, Novitskiy S, Owens P, et al. Fibroblast-mediated collagen remodeling within the tumor microenvironment facilitates progression of thyroid cancers driven by BrafV600E and Pten loss. Cancer Res. 2016;76(7):1804–13.
Crezee T, Rabold K, de Jong L, et al. Metabolic programming of tumor associated macrophages in the context of cancer treatment. Ann Transl Med. 2020;8(16):1028.
Zhang QW, Liu L, Gong CY, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One. 2012;7(12):e50946.
Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416.
Cheng N, Bai X, Shu Y, et al. Targeting tumor-associated macrophages as an antitumor strategy. Biochem Pharmacol. 2021;183:114354.
Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. J Immunol. 2010;184(2):702–12.
Proietti A, Ugolini C, Melillo RM, et al. Higher intratumoral expression of CD1a, tryptase, and CD68 in a follicular variant of papillary thyroid carcinoma compared to adenomas: correlation with clinical and pathological parameters. Thyroid. 2011;21(11):1209–15.
Visciano C, Prevete N, Liotti F, Marone G. Tumor-associated mast cells in thyroid cancer. Int J Endocrinol. 2015;2015:705169.
Galdiero MR, Varricchi G, Loffredo S, et al. Potential involvement of neutrophils in human thyroid cancer. PLoS One. 2018;13(6):e0199740.
Ria R, Simeon V, Melaccio A, et al. Gene expression profiling of normal thyroid tissue from patients with thyroid carcinoma. Oncotarget. 2016;7(20):29677–88.
Rajabi S, Dehghan MH, Dastmalchi R, et al. The roles and role-players in thyroid cancer angiogenesis. Endocr J. 2019;66(4):277–93.
Bugyik E, Renyi-Vamos F, Szabo V, et al. Mechanisms of vascularization in murine models of primary and metastatic tumor growth. Chin J Cancer. 2016;35(1):19.
Mousa SA, Lin HY, Tang HY, et al. Modulation of angiogenesis by thyroid hormone and hormone analogues: implications for cancer management. Angiogenesis. 2014;17(3):463–9.
Feng K, Ma R, Zhang L, et al. The role of exosomes in thyroid cancer and their potential clinical application. Front Oncol. 2020;10:596132.
Santiago K, Chen Wongworawat Y, Khan S. Differential microRNA-signatures in thyroid cancer subtypes. J Oncol. 2020;2020:2052396–14.
Freudenthal B, Williams G. Thyroid stimulating hormone suppression in the long-term follow-up of differentiated thyroid cancer. Clin Oncol. 2017;29(5):325–8.
Craps J, Joris V, De Jongh B, et al. Involvement of mTOR and regulation by AMPK in early iodine deficiency-induced thyroid microvascular activation. Endocrinology. 2016;157(6):2545–59.
Ria R, Melaccio A, Racanelli V, Vacca A. Anti-VEGF drugs in the treatment of multiple myeloma patients. J Clin Med. 2020;9(6):1765.
Yoshinaga A, Kajihara N, Kukidome D, et al. Hypoglycemia induces mitochondrial reactive oxygen species production through increased fatty acid oxidation and promotes retinal vascular permeability in diabetic mice. Antioxid Redox Signal. 2021;34(16):1245–59.
Lewy-Trenda I, Wierzchniewska-Ławska A. Expression of vascular endothelial growth factor (VEGF) in human thyroid tumors. Pol J Pathol. 2002;53(3):129–32.
Ramsden J. Angiogenesis in the thyroid gland. J Endocrinol. 2000;166(3):475–80.
Quintero-Fabián S, Arreola R, Becerril-Villanueva E, et al. Role of matrix metalloproteinases in angiogenesis and cancer. Front Oncol. 2019;9:1370.
Ria R, Prete F, Melaccio A, et al. Effect of thyroidectomy on circulating angiogenic cytokines in papillary thyroid carcinoma and benign goiter: potential for new biomarkers? Surgery. 2021;169(1):27–33.
Patel KN, Angell TE, Babiarz J, et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surg. 2018;153(9):817–24.
Pagan M, Kloos RT, Lin CF, et al. The diagnostic application of RNA sequencing in patients with thyroid cancer: an analysis of 851 variants and 133 fusions in 524 genes. BMC Bioinform. 2016;17(Suppl 1):6.
Xia F, Jiang B, Chen Y, et al. Prediction of novel target genes and pathways involved in tall cell variant papillary thyroid carcinoma. Medicine. 2018;97(51):e13802.
Aragon Han P, Kim HS, Cho S, et al. Association of BRAFV600E mutation and microRNA expression with central lymph node metastases in papillary thyroid cancer: a prospective study from four endocrine surgery centers. Thyroid. 2016;26(4):532–42.
Huang Y, Qu S, Zhu G, et al. BRAF V600E mutation-assisted risk stratification of solitary intrathyroidal papillary thyroid cancer for precision treatment. J Natl Cancer Inst. 2018;110(4):362–70.
Jeong S, Lee J, Kim D, et al. Relationship of focally amplified long noncoding on chromosome 1 (FAL1) lncRNA with E2F transcription factors in thyroid cancer. Medicine. 2016;95(4):e2592.
Wu F, Li F, Lin X, et al. Exosomes increased angiogenesis in papillary thyroid cancer microenvironment. Endocr Relat Cancer. 2019;26(5):525–38.
Lodewijk L, van Diest P, van der Groep P, et al. Expression of HIF-1α in medullary thyroid cancer identifies a subgroup with poor prognosis. Oncotarget. 2017;8(17):28650–9.
Lodewijk L, Willems SM, Dreijerink KM, et al. The theranostic target prostate-specific membrane antigen is expressed in medullary thyroid cancer. Hum Pathol. 2018;81:245–54.
Mareĉko I, Cvejić D, Tatić S, et al. Expression of matrix metalloproteinase-2 and its tissue inhibitor-2 in fetal and neoplastic thyroid tissue and their significance as diagnostic and prognostic markers in papillary carcinoma. Cancer Biomark. 2012;11(1):49–58.
Boufraqech M, Patel D, Nilubol N, et al. Lysyl oxidase is a key player in BRAF/MAPK pathway-driven thyroid cancer aggressiveness. Thyroid. 2019;29(1):79–92.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Pasculli, A. et al. (2024). The Angiogenic Microenvironment of Thyroid Cancer: An Insight into the Research of New Prognostic Markers. In: Testini, M., Gurrado, A. (eds) Thyroid Surgery. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-31146-8_20
Download citation
DOI: https://doi.org/10.1007/978-3-031-31146-8_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-31145-1
Online ISBN: 978-3-031-31146-8
eBook Packages: MedicineMedicine (R0)