Abstract
Devascularization of the parathyroid glands (PGs) during total thyroidectomy (TT) is the cause of post-surgical parathyroprival hypocalcemia. Safeguarding the anatomical integrity and vascularity of the PGs primarily requires an experienced surgeon. The combined use of magnification loupes (2.5× or 3.5×) with an ultrasound/radiofrequency device or bipolar forceps is also helpful. Near-infrared autofluorescence imaging may provide additional support in the recognition of the PGs during TT. Finally, the intravenous injection of indocyanine green (ICG) can be useful to verify the good vascularization of the PGs, even though there are still conflicting views on the clear clinical benefit that may justify routine use of this technique. A PG that remains accidentally devascularized must be reimplanted. The gland should be fragmented into 1 × 2-mm segments to maximize its contact surface with the muscle pocket. The most commonly used sites for reimplantation are the brachioradial muscle and, preferably, the sternocleidomastoid muscle. PG transplantation is an effective solution for ensuring post-thyroidectomy normocalcemia; however, the main objective is to preserve the anatomical and vascular integrity of all the PGs by means of an accurate and gentle surgical gesture.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Parathyroid autotransplantation
- Thyroidectomy
- Autofluorescence
- Indocyanine green
- Near-infrared fluorescence imaging
1 Introduction
Recognition of the “glandulae parathyroideae” in humans has been defined as the last anatomical discovery. It dates back to 1877 and it is owed to Ivor Sandström, a medical student at the Uppsala Department of Anatomy [1].
In 1883, Emil Theodor Kocher observed, as suggested by Jacques-Louis Reverdin, that patients undergoing total thyroidectomy (TT) always presented myxedema and at times even tetany. On the contrary, those who underwent subtotal thyroidectomy, following Theodor Billroth’s preferred surgical technique, never manifested myxedema but often developed tetany. Therefore, Kocher empirically decided to adopt the following surgical strategy: only lobectomy in the first place, and subtotal resection of the contralateral lobe only in the case of relapse [2].
In 1891, Marcel Eugène Gley realized that post-thyroidectomy tetany in dogs was due to the concurrent removal of the parathyroid glands (PGs) [3]. However, even though this proved the causal link, it was not sufficient to explain the phenomenon. In 1908, William G. MacCallum and Carl Voegtlin discovered that the PGs regulate the blood level of calcium, and they started to treat post-thyroidectomy tetany by administering this electrolyte [4]. In 1923, Harald Salvesen demonstrated that post-surgical tetany was caused by the removal of the PGs. Since then, it was clear that, when performing TT, the PGs had to be identified and safeguarded so as to prevent parathyroprival tetany.
Thyroid surgery has been aptly termed surgery of the recurrent laryngeal nerves and PGs. In fact, these are the only anatomical structures that can suffer even significant damage after a TT. Hypocalcemia due to hypoparathyroidism is the most frequent complication. After TT, about 50% of patients have altered serum calcium values, which are associated with clinical signs of hypocalcemia in 14% of cases but which normalize in the vast majority of patients within a couple of weeks or within the first year after surgery at the latest [5]. In 2–3% of cases, hypocalcemia may persist throughout life, accompanied by very low or null values of parathyroid hormone (PTH) [6]. Multicenter studies have published much higher incidence rates of up to 16.7% which, if more uniform criteria to define hypocalcemia are applied, result in a 7.9% incidence of persistent hypoparathyroidism [7].
Even more evident is the incidence of forensic litigation for permanent hypocalcemia, which reaches 9% of all claims for post-thyroidectomy complications [8]. The epidemiological aspects and the repercussions, also in terms of the financial burden of chronic hypoparathyroidism on the expenditure of private individuals and national health services, remain to be assessed [9].
2 The Issue of Maintaining the Functionality of the Parathyroid Glands
How many PGs need to be preserved to maintain a normal serum calcium level? The PG is functionally unique, even though divided into four portions of about 30–40 mg each for a total of 120–160 mg. It follows that either the residual parathyroid tissue replaces the missing tissue to produce the necessary amount of PTH or parathyroid function will remain deficient. However, each individual parathyroid has its own calcium set point (which can be defined as the level of extracellular calcium that causes a 50% reduction of the maximum secretion of PTH) and can respond in a unique way to calcemic stimuli. Therefore, the number of PGs needed to ensure parathyroid function is not definable.
The arterial vascularization of the PGs is of the terminal type and any surgical maneuver that damages it will cause partial or total ischemia of the affected gland. The upper and lower PGs are supplied by the lower thyroid artery. In about 20% of cases, the upper PGs are supplied by the posterior branch of the superior thyroid artery [10], which should therefore not be tied and interrupted during thyroidectomy. The PGs must be carefully separated from the thyroid capsule and each small peripheral arterial branch will be interrupted distal to the PGs to preserve the blood supply.
Utmost care must be taken to safeguard PG vascularity. PGs, like all endocrine glands, do not have an excretory duct as hormone secretion occurs through the venous network. Therefore, ensuring integrity of the arterial supply, although necessary, is not sufficient to guarantee gland function, which requires as much care in safeguarding venous vascularity [11] as well. Preservation of the venous branches of the PGs that flow into the lower thyroid veins reduces the risk of postoperative hypocalcemia and allows for a faster recovery of normal calcium levels [12].
If the PGs are anatomically well protected within the parathyroid capsule, it will be easier for the surgeon to avoid damaging them. If, on the other hand, they adhere tenaciously to the thyroid capsule and take the venous vascularization largely or even exclusively, as occurs in 7–8% of cases, from the thyroid, they will have to be carefully isolated, trying as much as possible to safeguard the residual venous vascularity [13]. In the case of venous congestion or glandular hemorrhagic infarction, an incision of the parathyroid capsule is needed for decompression purposes. Scrupulous surgical compliance with the anatomical and vascular integrity of the PGs during TT will not, however, always correspond to sufficient parathyroid function [11].
3 Requirements for Adequate Localization of the Parathyroid Glands
A surgeon experienced in thyroid and parathyroid surgery able to visually recognize the PGs is the first requirement. However, even experienced surgeons might not always be able to identify all the PGs during TT and they may be unable to identify any of them with certainty [14].
To this end, it is useful to repeatedly touch with the tip of a blunt instrument what is presumed to be parathyroid tissue since this, which is very similar in color to adipose tissue, will take on a characteristic chamois color within seconds, thus facilitating its identification. If the PGs do not manifest easily, one should not persist in the search owing to the risk of devascularizing them. It is important, however, to make sure that the glands are not attached to the removed thyroid capsule [15].
The combined use of magnification loupes (2.5× or 3.5×) with an ultrasound/radiofrequency device or bipolar forceps is recommended. Use of magnification loupes allows for a clearer and sharper view of the anatomy. The associated use of an appropriate device allows for more effective hemostasis, improving precision of the surgical gesture with a bloodless operating field and a significant reduction in operating times [16, 17].
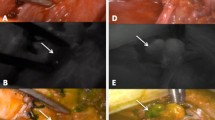
To further minimize the risk of parathyroid lesions, it may be helpful to support naked eye recognition with near-infrared autofluorescence imaging (NIR-AF). As demonstrated by a pilot study published in 2011, the fluorescence intensity of PGs is consistently greater than that of the thyroid and all other neck tissues [18]. This allows recognition of the PGs, which display autofluorescence when imaged by the camera. At the end of the TT, after intravenous injection of indocyanine green (ICG), the good vascularity of the PGs can be verified by identifying their vessels. ICG is an inert, hydrosoluble organic dye containing 5% sodium iodine and which, when administered intravenously, binds to the plasma lipoproteins. It has a very short half-life (3.4 ± 0.7 minutes). Considering the presence of iodine, it must not be administered to those who are allergic to the substance.
NIR-AF imaging of the PGs during thyroidectomy can indeed help identify and preserve the PGs. This is particularly important if we consider that the incidence of involuntary removal of the PGs and the finding of parathyroid tissue on the pathology report is relatively frequent, being noted in almost 25% of cases [7, 19, 20].
However, NIR-AF does not significantly reduce the incidence of parathyroid hypocalcemia [21].
The combined use of NIR-AF to detect PGs and infusion of ICG after TT to verify preservation of PG vascularity is a promising technique. In fact, it appears to reduce the risk of transient postoperative hypocalcemia and it allows safer preservation of the PGs by evaluating their perfusion, thus avoiding unnecessary reimplantation [22].
However, recent studies show no significant difference in the number of autotransplanted PGs, which is what we would expect [23, 24]. To date, in fact, conflicting opinions persist on a clear clinical benefit that might justify the routine use of this method [25, 26].
4 Reimplantation of Parathyroid Glands
A PG that accidentally remains devascularized should be reimplanted. Parathyroid autotransplantation during thyroidectomy was first described in 1926 by Frank H. Lahey [27]. Since then, many studies have shown that autotransplantation is an effective procedure in preserving against permanent [28, 29] and temporary [30] postoperative hypoparathyroidism.
The gland must first be fragmented into 1 × 2-mm segments to maximize its contact surface with the muscle pocket. One fragment is intended for extemporaneous histological examination to verify the diagnosis of parathyroid tissue. Hemostasis of the recipient pocket must be carefully managed to avoid the onset of hematomas that could compromise the engraftment. The muscle pocket is closed with a non-absorbable suture, on which a titanium clip can be affixed to allow recovery of the PG, should it be necessary in the future (hyperparathyroidism) [31].
The most commonly used sites for reimplantation are the brachioradial muscle of the non-dominant forearm or the subcutaneous tissue of the forearm [32], and the ipsilateral sternocleidomastoid muscle. However, the sternocleidomastoid is the preferred site both because it avoids further surgical access and because the risk of having to remove reimplanted parathyroid tissue that has become pathological is extremely low [31].
Already in 1975, Hickey and Wells demonstrated the effectiveness of the PTH production by the autotransplanted tissue [28, 33]. Therefore, any devascularized gland should be reimplanted as autotransplantation is an effective means of restoring parathyroid function and can prevent permanent hypoparathyroidism [29], with a graft survival rate of 93% [28]. It is clearly impossible to demonstrate with certainty the clinical efficacy of parathyroid autotransplantation after TT because the PGs are normally four, but they can also be three (3%) or five (13%) and it is uncommon for all of them to be removed or devascularized at the same time [10].
Reasonably, the greater the number of devascularized glands, the greater the patient’s risk of having post-surgical hypocalcemia, even after reimplantation. In fact, the best guarantee for post-thyroidectomy normocalcemia is to preserve the anatomical and vascular integrity of all the PGs by means of an accurate and gentle surgical gesture.
Although not recently, some authors [34, 35] have recommended the routine prophylactic application of PG autotransplantation in all cases of TT, reporting 0% postoperative hypoparathyroidism, but a significantly higher rate of transient hypocalcemia. Nonetheless, this position does not seem to be embraceable, as the same result can be obtained if we undertake to respect the anatomical integrity and vascularity of the PGs.
References
Sandström IV. On a new gland in man and several mammals – glandulae parathyroideae. Upsala Läk Förenings Förh. 1879-1880;15:441–71.
Hannan SA. The magnificent seven: a history of modern thyroid surgery. Int J Surg. 2006;4(3):187–91.
Gley ME. Sur les functions du corps thyroid. C R Séances Soc Biol Fil. 1891;43:841–3.
MacCallum WG, Voegtlin C. On the relation of tetany to the parathyroid glands and to calcium metabolism. J Exp Med. 1909;11(1):118–51.
Villarroya-Marquina I, Sancho J, Lorente-Poch L, et al. Time to parathyroid function recovery in patients with protracted hypoparathyroidism after total thyroidectomy. Eur J Endocrinol. 2018;178(1):103–11.
Rosato L, Avenia N, Bernante P, et al. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28(3):271–6.
Lončar I, van Kinschot CMJ, van Dijk SPJ, et al. Persistent post-thyroidectomy hypoparathyroidism: a multicenter retrospective cohort study. Scand J Surg. 2022;111(2):14574969221107282.
Padovano M, Scopetti M, Tomassi R, et al. Mapping complications in thyroid surgery: statistical data are useful for medico-legal management of a recurrent safety issue. Updat Surg. 2022;74(5):1725–32.
Bjornsdottir S, Ing S, Mitchell DM, et al. Epidemiology and financial burden of adult chronic hypoparathyroidism. J Bone Miner Res. 2022;37(12):2602–14.
Burger F, Fritsch H, Zwierzina M, et al. Postoperative hypoparathyroidism in thyroid surgery: anatomic-surgical mapping of the parathyroids and implications for thyroid surgery. Sci Rep. 2019;9(1):15700.
Rosato L, De Crea C, Bellantone R, et al. Diagnostic, therapeutic and health-care management protocol in thyroid surgery: a position statement of the Italian Association of Endocrine Surgery Units (U.E.C. CLUB). J Endocrinol Investig. 2016;39(8):939–53.
Lee DY, Cha W, Jeong WJ, et al. Preservation of the inferior thyroidal vein reduces post-thyroidectomy hypocalcemia. Laryngoscope. 2014;124(5):1272–7.
Cui Q, Li Z, Kong D, et al. A prospective cohort study of novel functional types of parathyroid glands in thyroidectomy: in situ preservation or auto-transplantation? Medicine (Baltimore). 2016;95(52):e5810.
Gschwandtner E, Seemann R, Bures C, et al. How many parathyroid glands can be identified during thyroidectomy?: evidence-based data for medical experts. Eur Surg. 2018;50(1):14–21.
Puzziello A, Rosato L, Innaro N, et al. Hypocalcemia following thyroid surgery: incidence and risk factors. A longitudinal multicenter study comprising 2,631 patients. Endocrine. 2014;47(2):537–42.
Sapalidis K, Papanastasiou A, Fyntanidou V, et al. Comparison between magnification techniques and direct vision in thyroid surgery: a systematic review and meta-analysis. Medicina (Kaunas). 2019;55(11):725.
Suffat LP, Lavorini E, Mondini G, et al. Does the combined use of magnification loupes and harmonic FOCUS improve the outcome of thyroid surgery? World J Endoc Surg. 2020;12(1):18–22.
Paras C, Keller M, White L, et al. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt. 2011;16(6):067012.
Paek SH, Lee YM, Min SY, et al. Risk factors of hypoparathyroidism following total thyroidectomy for thyroid cancer. World J Surg. 2013;37(1):94–101.
Díez JJ, Anda E, Sastre J, et al. Prevalence and risk factors for hypoparathyroidism following total thyroidectomy in Spain: a multicentric and nation-wide retrospective analysis. Endocrine. 2019;66(2):405–15.
Wolf HW, Runkel N, Limberger K, et al. Near-infrared autofluorescence of the parathyroid glands during thyroidectomy for the prevention of hypoparathyroidism: a prospective randomized clinical trial. Langenbeck's Arch Surg. 2022;407(7):3031–8.
Iritani K, Teshima M, Shimoda H, et al. Intraoperative quantitative assessment of parathyroid blood flow during total thyroidectomy using indocyanine green fluorescence imaging – surgical strategies for preserving the function of parathyroid glands. Laryngoscope Investig Otolaryngol. 2022;7(4):1251–8.
Yin S, Pan B, Yang Z, et al. Combined use of autofluorescence and indocyanine green fluorescence imaging in the identification and evaluation of parathyroid glands during total thyroidectomy: a randomized controlled trial. Front Endocrinol (Lausanne). 2022;13:897797.
Barbieri D, Indelicato P, Vinciguerra A, et al. Autofluorescence and indocyanine green in thyroid surgery: a systematic review and meta-analysis. Laryngoscope. 2021;131(7):1683–92.
Di Marco A, Chotalia R, Bloxham R, et al. Does fluoroscopy prevent inadvertent parathyroidectomy in thyroid surgery? Ann R Coll Surg Engl. 2019;101(7):508–13.
Benmiloud F, Godiris-Petit G, Gras R, et al. Association of autofluorescence-based detection of the parathyroid glands during total thyroidectomy with postoperative hypocalcemia risk: results of the PARAFLUO multicenter randomized clinical trial. JAMA Surg. 2020;155(2):106–12.
Lahey FH. The transplantation of parathyroids in partial thyroidectomy. Surg Gynecol Obstet. 1926;62:508–9.
Wells SA Jr, Gunnells JC, Shelbume JD, et al. Transplantation of the parathyroid glands in man: clinical indication and results. Surgery. 1975;78(1):34–44.
Hicks G, George R, Sywak M. Short and long-term impact of parathyroid autotransplantation on parathyroid function after total thyroidectomy. Gland Surg. 2017;6(Suppl 1):S75–85.
Testini M, Rosato L, Avenia N, et al. The impact of single parathyroid gland autotransplantation during thyroid surgery on postoperative hypoparathyroidism: a multicenter study. Transplant Proc. 2007;39(1):225–30.
D’Avanzo A, Parangi S, Morita E, et al. Hyperparathyroidism after thyroid surgery and autotransplantation of histologically normal parathyroid glands. J Am Coll Surg. 2000;190(5):546–52.
Cavallaro G, Iorio O, Centanni M, et al. Parathyroid reimplantation in forearm subcutaneous tissue during thyroidectomy: a simple and effective way to avoid hypoparathyroidism. World J Surg. 2015;39(8):1936–42.
Hickey RC, Samaan NA. Human parathyroid autotransplantation: proved function by radioimmunoassay of plasma parathyroid hormone. Arch Surg. 1975;110(8):892–5.
Zedenius J, Wadstrom C, Delbridge L. Routine autotransplantation of at least one parathyroid gland during total thyroidectomy may reduce permanent hypoparathyroidism to zero. Aust N Z J Surg. 1999;69(11):794–7.
Lo CY, Lam KY. Routine parathyroid autotransplantation during thyroidectomy. Surgery. 2001;129:318–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Rosato, L., Panier Suffat, L. (2024). Autotransplantation of the Parathyroid Glands in Thyroidectomy: The Role of Autofluorescence and Indocyanine Green. In: Testini, M., Gurrado, A. (eds) Thyroid Surgery. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-31146-8_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-31146-8_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-31145-1
Online ISBN: 978-3-031-31146-8
eBook Packages: MedicineMedicine (R0)