Abstract
Osteoarthritis is a major source of pain, disability, and economic cost worldwide. For nearly a century, there has been a debate about the causes of hip osteoarthritis and the role that structural abnormalities may play as a causative factor. Recent advances in open and minimally invasive techniques such as the periacetabular osteotomy, surgical hip dislocation and arthroscopic approaches have allowed us safe access into the joint to not only improve the abnormal bony structure and repair damaged tissue but also to gain clinical insights into the cause of joint damage. At present, structural abnormalities such as acetabular dysplasia and CAM deformities of the proximal femur are thought to be a major factor causing premature hip OA. Over the past 30 years, our understanding of the function and biology of articular cartilage has evolved from a relatively acellular lubricating cushion to a metabolically active tissue that can modulate its tissue composition in response to mechanical loading. Using advanced biochemical MR imaging technique called delayed Gadolinium Enhanced MRI of Cartilage (dGEMRIC), it has been shown that alteration in the mechanical environment of the hip with a pelvic osteotomy in acetabular dysplasia can alter the articular cartilage composition. This further demonstrates the importance of mechanics in development of joint damage and the potential for surgical correction to prevent or slow down the progression of OA.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Osteoarthritis is a major source of pain, disability, and economic cost worldwide. At present it is accepted that this disease is caused by multiple factors including genetic, biologic, and mechanical factors. Over the decades, the view of this condition has evolved from a wear and tear phenomena of the articular cartilage due to mechanical factors to that of a complex condition affecting the whole joint [1]. Similarly, the initial studies of articular cartilage focused on its material properties as it was thought to be relatively inactive biologically due to its acellular nature. However, it became increasingly clear that articular cartilage is not only biologically active but also mechanosensitive and is a mechanical/biological factor in the maintenance of the synovial joint and the disease process [2]. The surgical care of patients with hip osteoarthritis has undergone a similar evolution over time. Prior to arthroplasty, both femoral and pelvic osteotomies were performed to correct the underlying structural abnormalities. In addition, repositioning osteotomies were performed for osteoarthritic hips to relieve pain; however, due to the more predictable clinical outcomes with an arthroplasty, in most countries much of the osteotomy approaches were abandoned. The exception has been in the pediatric setting where arthroplasty is inappropriate and in countries such as Germany and Japan where they had a high incidence of acetabular dysplasia. The improved pelvic osteotomy [3] and hip surgical dislocation approach technique, and the understanding that subtle acetabular and femoral deformities are a causative factor in early joint damage, have renewed interests in hip preservation surgical techniques [4].
There are many parallels and interplay between the evolution in our understanding of osteoarthritis epidemiology, cartilage mechanics and physiology, and surgical care of our hip patients over the past 30 years. Clinical observations have informed our basic understanding and vice versa and I suspect will continue to do so in the future.
2 Osteoarthritis or Osteoarthrosis – What Is the Role of Biological vs Mechanical Factors in the Development and Progression of Hip OA?
Starting in the 1930s, clinical observations were made that hip deformity such as acetabular dysplasia [5] and then subsequently “tilt deformity” of the proximal femur [6] can lead to osteoarthritis. Murray used the term “tilt deformity” to describe an abnormal relationship between the femoral head and neck and in order to distinguish this asymptomatic development of hip deformity from a slipped capital femoral epiphysis, which often presents with a limp or an inability to walk. In fact, Murray hypothesized that perhaps abnormal stresses in adolescence may cause a minor degree of epiphysiolysis which can cause this deformity to develop which leads to damage to the joint. In 1975, Solomon [7] published a prospective study looking at the association between proximal femoral and acetabulum shape on radiographs and the pathological findings at time of joint replacement and made similar inferences as Murray. He even postulated that perhaps early intervention should be done to prevent further damage to the joint.
However, the hip joint is a dynamic organ that can remodel its shape in response to inflammation and injury. In the 1970s, Resnick made the observation that as part of the normal osteoarthritic process, the femoral head shape can remodel into a similarly abnormal shape as described by Murray and Solomon [8]. In fact, he specifically refuted that the observations made by Murray were simply due to the bony remodeling during osteoarthritis, i.e. a secondary effect, and not a cause of osteoarthritis [9].
Subsequent studies by Murphy et al. [10] and Harris [11] extended Wiberg and Murray’s original hypotheses. The primary underlying mechanism by which joint damage occurs was thought to be mechanical in nature and the preferred term used to describe this condition was osteoarthrosis. Harris specifically made the argument that many of these femoral and acetabular deformities are seen prior to the onset of severe osteoarthritis. However, this was a period of rapid advancement in total hip arthroplasty. Much interest in understanding the etiology of hip osteoarthritis waned and we would have to wait for further advances in surgical technique to occur in the late 1990s that would allow additional clinical insights as well as improved ability to alter the underlying hip deformity.
For the hip, the importance of underlying mechanics as an initiator of joint damage was revitalized with the advent of advanced surgical techniques in the 1990s that allowed direct observations of damage within the joint even the early stages of joint damage. Traditionally, intraarticular joint damage was observed mostly in specimens at time of joint replacement, which by its very nature was in the advanced stage of disease. The safe surgical dislocation technique of the hip was developed by Ganz and co-workers, which allowed complete exposure of the hip joint without the risk of avascular necrosis and with little or no morbidity [12]. This technique not only allowed direct observation of the joint damage pattern in pre-arthritic hips but also provided a surgical approach to repair damaged tissue as well as to address underlying structural abnormalities that may be the cause of the damage. Two distinct types of femoroacetabular impingement have been identified. The first type of impingement is caused by a direct contact between the acetabular rim and the femoral neck – pincer impingement. This is often caused by the acetabulum being retroverted or over covered. The second and more common type of impingement would occur due to a non-spherical extension of the femoral head into the acetabulum - the so-called cam impingement. The two types of impingements can be mixed but when they occur in isolation, they can cause distinctive patterns of intra-articular damage. The cam type impingement is most like the tilt deformity described by Murray but now there are multiple studies demonstrating that a subset of the cam deformity is developmental in nature with the formation occurring during the end of growth during adolescence [13, 14]. Furthermore, some of the cam deformity is caused by an extension of the femoral epiphysis (Fig. 9.1) and clearly not due to new appositional bone formation as described by Resnick [15].
Acetabular dysplasia (Fig. 9.2) has been a more accepted structural cause of hip OA. A more contemporary cohort study by Murphy [10] demonstrated the relationship between the extent of femoral head under coverage and the eventual development of osteoarthritis. With the use of advanced biochemical MRI techniques such as delayed Gadolinium Enhanced MRI of Cartilage (dGEMRIC) [16], the relationship between the femoral head under coverage and the extent of chondral damage in the early stages of joint injury has been confirmed [17]. In addition, a recent systematic review and meta-analysis of prospective and cross-sectional studies have shown that cam deformity (alpha angle > 60 deg; OR = 2.52, 95% CI: 1.83–3.46) and acetabular dysplasia (LCEA < 25 deg; OR = 2.38, 95% CI: 1.84–3.07) are risk factors for the development of hip osteoarthritis [18].
It seems now evident, that hip mechanics plays an important role in the initiation of joint damage. However, we also know that this is not a simple wear and tear phenomenon. The cartilage is not simply being worn away. During the past 30 years when the role of mechanics in the development of osteoarthritis was become clearer, the role of mechanics and the modulation of articular cartilage metabolism and initiation of cartilage degradation was also being elucidated.
3 Articular Cartilage – Paradigm Shift from Inert Lubricating Cushion to Biologically Active and Mechanosensitive Tissue
Articular cartilage is a relatively acellular tissue that provides a near frictionless lubricating surface in synovial joints. It is also avascular and aneural; hence, initial concept of osteoarthritis was that of a mechanically induced wear and tear process. However, the opposite is true where the articular cartilage tissue is often very active and reactive in the disease process [19, 20].
During the 1980s and 1990s, the interplay between the mechanical forces on cartilage and its metabolism and well as disease states was being elucidated. Sah et al.[21] demonstrated using calf cartilage explant system that static compression will inhibit glycosaminoglycan and protein synthesis (Fig. 9.3) while gentle dynamic compression will stimulate biosynthetic activity in a frequency dependent manner. Higher frequency (>0.001 Hz) small strain (1–5%) compression produced a stimulatory effect while lower frequency did not (Fig. 9.4). This study provided a framework for identifying the physical and biological mechanisms by which dynamic compression can modulate chondrocyte biosynthetic activity. Further studies using the radially unconfined compression explant system demonstrated that the increase in biosynthetic activity of glycosaminoglycans during dynamic small strain compression was confined to the radial periphery where there would be increased fluid flow. This only occurred in the high frequency range where the increased fluid flow was predicted to occur (Fig. 9.5) [22].
Cartilage explants were statically compressed in a radially unconfined manner in a culture chamber. The glycosaminoglycan (sulfate) and protein (proline) synthesis decreased with increasing static compression. (Reprinted with permission from Sah et al. [21])
Cartilage explants were dynamically compressed with small displacement sinusoidal manner with fixed frequencies. The glycosaminoglycan synthesis increased in a frequency dependent manner. No stimulation at very low frequencies of stimulation. (Reprinted with permission from Sah et al. [21]; Kim et al. [22])
The cartilage explants were separated from the radial (ring) vs center part of the disc and the biosynthetic rate assessed. The biosynthetic rate of glycosaminoglycans (b) only increased in the ring at high frequency stimulation (0.1 Hz) where the fluid flow is increased according to the poroelastic modeling data (a). (Reprinted with permission from Kim et al. [22])
Compared to gentle dynamic compression, direct mechanical injury to the articular cartilage can not only disrupt the tissue structure but it can also induce matrix degradation mediated by chondrocytes via expression of matrix degrading enzymes (ADAM-TS5, MMP-1, MMP-2, MMP-3, MMP-9, MMP-13). In addition, the biosynthetic activity will decrease and will also induce chondrocyte death by necrosis and apoptosis [2].
These and other studies have confirmed that articular cartilage is an active tissue that can modulate its composition in response to mechanical loads and when mechanically injured the tissue can degrade itself and will contribute to the development of osteoarthritis.
4 Bench to Bedside – Use of dGEMRIC in Understanding the Effect of Pelvic Osteotomy on Hip Articular Cartilage
Acetabular dysplasia is a natural model of mechanically induced cartilage damage that leads to osteoarthritis. The articular cartilage and labral damage start at the acetabular edge where the increased mechanical loads are predicted to occur. We have surgical interventions to correct the acetabular dysplasia, which would lead to normalization of the mechanical environment in the joint [23]. Furthermore, acetabular dysplasia is an important cause of premature osteoarthritis in young women.
A clinically important question in patients with acetabular dysplasia is what is happening to the joint? Are we preventing or slowing down the inevitable onset of osteoarthritis? Traditionally, these patients would be followed for long term (~20–30 years) to be able to detect radiographically the onset of osteoarthritis. However, we have sought to see if we can gain insights sooner using a biochemical MR imaging technique call delayed Gadolinium Enhanced MRI of Cartilage (dGEMRIC) [16], which estimates the charge density of the articular cartilage and hence the tissue composition and potential health of the joint.
This imaging technique was validated for use as a clinical imaging tool in the mature hip [17, 24, 25]. We performed a prospective cohort study of subjects [26] with acetabular dysplasia that were about to undergo surgical correction using the Bernese periacetabular osteotomy. Thirty-seven patients with no or minimal radiographic evidence of osteoarthritis were treated with a periacetabular osteotomy for symptomatic acetabular dysplasia. All patients had a pre-operative, one-year and two-year dGEMRIC scans. Standard radiographic and clinical follow-ups were performed at regular intervals.
As expected, we had clinical improvement in symptoms in this cohort and there were no major complications. Radiographically, we had good correction of the acetabular dysplasia and there were no significant radiographic progression of osteoarthritis. What we did find is a decrease in the dGEMRIC index from a preoperative measure of 561.6 msec to 515.2 msec at the 1 year postoperative scans and subsequently recovered to 529.2 msec at the two-year post-operative scan. dGEMRIC index is correlated with charge density; therefore, a decrease in the dGEMRIC index may suggest a worsening of the articular tissue quality.
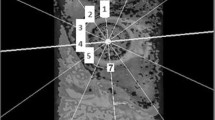
However, upon closer inspection, it was noted that the most pronounced change in articular cartilage dGEMRIC index occurred at the superior aspect of the acetabulum which is where the increased mechanical load seen prior to surgery would decrease after correction of the acetabular dysplasia (Fig. 9.6). The MRI imaging data was acquired in the same scanner and using the same imaging protocol. However, due to the reorientation of the acetabulum, the 3D imaging dataset was reformatted in a rotating radial frame and realigned to the original orientation for comparison before and after periacetabular osteotomy (Fig. 9.7) [28]. The articular cartilage was manually segmented between the acetabular and femoral cartilages (Fig. 9.8) and when we looked at the change in dGEMRIC values in the acetabular cartilage at various locations around the joint, what we found is a decrease in the dGEMRIC values in the superior-anterior and superior part of the joint that sees increased mechanical load prior to the operation, and a decrease down to a range that is within normal range after osteotomy (Fig. 9.9). This suggests that in these pre-arthritic hips, the articular cartilage in the overloaded acetabulum is adapted to the increased mechanical load pre-operatively but is able to “normalize” its matrix composition after surgical correction. This suggests that a periacetabular osteotomy for acetabular dysplasia will modulate the mechanical environment in the joint, which in turn affects the biosynthetic activity and the tissue composition of the articular cartilage.
The von Mises stress in hips with normal coverage (a) and varying severity of acetabular dysplasia were estimated using finite element modeling technique (b–d). After simulated pelvic osteotomy to normalize coverage, the mechanical stress in the joint decreased (e–g). (Reprinted with permission from Zhao et al. [27])
The isotopically acquired 3D MRI data was reformatted in a rotating frame around the femoral neck axis. The dGEMRIC data was realigned back to the original acetabular position so same regions of interests could be compared before and after periacetabular osteotomy. (Reprinted with permission from Bittersohl et al. [28])
The acetabular dGEMRIC value as a function of position within the joint. In the superior-anterior to superior-posterior regions the dGEMRIC values were high prior to periacetabular osteotomy. The values decrease down but not below the normal levels after osteotomy. (Modified graph based on data presented in Hingsammer et al. [26])
5 Summary
Over the past decades, the role of mechanics in the initiation and progression of osteoarthritis, nature of articular cartilage, and surgical treatment paradigm for patients with joint pain and early joint damage have evolved greatly. Clinical insights and basic science knowledge have informed each other and will continue to advance over time. It is our goal that in the near future, we will have the ability to prevent the development and progression of osteoarthritis with appropriate surgical interventions in the case of OA caused by structural abnormalities, the ability to replace or repair damaged tissue to prolong the function of the native joint in the case of joints with limited pre-existing damage, and finally continued advancements in joint replacement technology will allow us to restore hip joint function in the older population with predictable and long term outcomes.
References
Glyn-Jones S, Palmer AJR, Agricola R et al (2015) Osteoarthritis. Lancet 386(9991):376–387. https://doi.org/10.1016/S0140-6736(14)60802-3
Kurz B, Lemke AK, Fay J, Pufe T, Grodzinsky AJ, Schünke M (2005) Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat Anat Anz Off Organ Anat Ges 187(5-6):473–485. https://doi.org/10.1016/j.aanat.2005.07.003
Ganz R, Klaue K, Vinh TS, Mast JW (1988) A new periacetabular osteotomy for the treatment of hip dysplasias. Technique and preliminary results. Clin Orthop 232:26–36
Leunig M, Ganz R (2014) The evolution and concepts of joint-preserving surgery of the hip. Bone Jt J 96-B(1):5–18. https://doi.org/10.1302/0301-620X.96B1.32823
Wiberg G (1939) Studies on Dysplasic Acetabula and Congenital Subluxation of the Hip Joint. Acata Chirgrgica Scand 83:5–131
Murray RO (1965) The aetiology of primary osteoarthritis of the hip. Br J Radiol 38(455):810–824. https://doi.org/10.1259/0007-1285-38-455-810
Solomon L (1975) Proceedings: osteoarthritis of the hip: pathological changes and their implications for treatment. J Bone Joint Surg (Br) 57(2):258
Resnick D (1975) Patterns of migration of the femoral head in osteoarthritis of the hip. Roentgenographic-pathologic correlation and comparison with rheumatoid arthritis. Am J Roentgenol Radium Ther Nucl Med 124(1):62–74. https://doi.org/10.2214/ajr.124.1.62
Resnick D (1976) The “tilt deformity” of the femoral head in osteoarthritis of the hip: a poor indicator of previous epiphysiolysis. Clin Radiol 27(3):355–363. https://doi.org/10.1016/s0009-9260(76)80089-x
Murphy SB, Ganz R, Müller ME (1995) The prognosis in untreated dysplasia of the hip. A study of radiographic factors that predict the outcome. J Bone Joint Surg Am 77(7):985–989. https://doi.org/10.2106/00004623-199507000-00002
Harris WH (1986) Etiology of osteoarthritis of the hip. Clin Orthop 213:20–33
Lavigne M, Parvizi J, Beck M, Siebenrock KA, Ganz R, Leunig M (2004) Anterior femoroacetabular impingement: part I. Techniques of joint preserving surgery. Clin Orthop (418):61–66
Bixby SD, Kienle KP, Nasreddine A, Zurakowski D, Kim YJ, Yen YM (2013) Reference values for Proximal Femoral anatomy in adolescents based on sex, physis, and imaging plane. Am J Sports Med 41(9):2074–2082. https://doi.org/10.1177/0363546513495346
Kienle KP, Keck J, Werlen S, Kim YJ, Siebenrock KA, Mamisch TC (2012) Femoral morphology and epiphyseal growth plate changes of the hip during maturation: MR assessments in a 1-year follow-up on a cross-sectional asymptomatic cohort in the age range of 9–17 years. Skelet Radiol 41(11):1381–1390. https://doi.org/10.1007/s00256-012-1358-9
Siebenrock KA, Wahab KHA, Werlen S, Kalhor M, Leunig M, Ganz R (2004) Abnormal extension of the femoral head epiphysis as a cause of cam impingement. Clin Orthop 418:54–60. https://doi.org/10.1097/00003086-200401000-00010
Burstein D, Velyvis J, Scott KT et al (2001) Protocol issues for delayed Gd(DTPA)(2-)-enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med 6:36–41
Kim YJ, Jaramillo D, Millis MB, Gray ML, Burstein D (2003) Assessment of early osteoarthritis in hip dysplasia with delayed gadolinium-enhanced magnetic resonance imaging of cartilage. J Bone Jt Surg Am Vol 85(10):1987–1992. https://doi.org/10.2106/00004623-200310000-00019
Casartelli NC, Maffiuletti NA, Valenzuela PL et al (2021) Is hip morphology a risk factor for developing hip osteoarthritis? A systematic review with meta-analysis. Osteoarthr Cartil 29(9):1252–1264. https://doi.org/10.1016/j.joca.2021.06.007
Mankin HJ, Lippiello L (1970) Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. J Bone Joint Surg Am 52(3):424–434
Maroudas A (1975) Glycosaminoglycan turn-over in articular cartilage. Philos Trans R Soc Lond Ser B Biol Sci 271(912):293–313. https://doi.org/10.1098/rstb.1975.0054
Sah RLY, Kim YJ, Doong JYH, Grodzinsky AJ, Plass AHK, Sandy JD (1989) Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 7(5):619–636. https://doi.org/10.1002/jor.1100070502
Kim YJ, Sah RL, Grodzinsky AJ, Plaas AH, Sandy JD (1994) Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys 311(1):1–12. https://doi.org/10.1006/abbi.1994.1201
Michaeli DA, Murphy SB, Hipp JA (1997) Comparison of predicted and measured contact pressures in normal and dysplastic hips. Med Eng Phys 19(2):180–186. https://doi.org/10.1016/s1350-4533(96)00051-3
Tiderius CJ, Jessel R, Kim YJ, Burstein D (2007) Hip dGEMRIC in asymptomatic volunteers and patients with early osteoarthritis: the influence of timing after contrast injection. Magn Reson Med 57(4):803–805. https://doi.org/10.1002/mrm.21190
Mamisch TC, Dudda M, Hughes T, Burstein D, Kim YJ (2008) Comparison of delayed gadolinium enhanced MRI of cartilage (dGEMRIC) using inversion recovery and fast T1 mapping sequences. Magn Reson Med 60(4):768–773. https://doi.org/10.1002/mrm.21726
Hingsammer AM, Kalish LA, Stelzeneder D et al (2015) Does Periacetabular Osteotomy for Hip Dysplasia modulate cartilage biochemistry? J Bone Jt Surg Am Vol 97(7):544–550. https://doi.org/10.2106/JBJS.M.01233
Zhao X, Chosa E, Totoribe K, Deng G (2010) Effect of periacetabular osteotomy for acetabular dysplasia clarified by three-dimensional finite element analysis. J Orthop Sci Off J Jpn Orthop Assoc 15(5):632–640. https://doi.org/10.1007/s00776-010-1511-z
Bittersohl B, Hosalkar HS, Werlen S, Trattnig S, Siebenrock KA, Mamisch TC (2011) dGEMRIC and subsequent T1 mapping of the hip at 1.5 Tesla: normative data on zonal and radial distribution in asymptomatic volunteers. J Magn Reson Imaging JMRI 34(1):101–106. https://doi.org/10.1002/jmri.22528
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Kim, YJ. (2023). Hip Osteoarthritis: Bench to Bedside Perspective. In: Connizzo, B.K., Han, L., Sah, R.L. (eds) Electromechanobiology of Cartilage and Osteoarthritis. Advances in Experimental Medicine and Biology, vol 1402. Springer, Cham. https://doi.org/10.1007/978-3-031-25588-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-25588-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25587-8
Online ISBN: 978-3-031-25588-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)