Abstract
Investigating the mechanobiology of chondrocytes is challenging due to the complex micromechanical environment of cartilage tissue. The innate zonal differences and poroelastic properties of the tissue combined with its heterogeneous composition create spatial- and temporal-dependent cell behavior, which further complicates the investigation. Despite the numerous challenges, understanding the mechanobiology of chondrocytes is crucial for developing strategies for treating cartilage related diseases as chondrocytes are the only cell type within the tissue. The effort to understand chondrocyte behavior under various mechanical stimuli has been ongoing over the last 50 years. Early studies examined global biosynthetic behavior under unidirectional mechanical stimulus. With the technological development in high-speed confocal imaging techniques, recent studies have focused on investigating real-time individual and collective cell responses to multiple / combined modes of mechanical stimuli. Such efforts have led to tremendous advances in understanding the influence of local physical stimuli on chondrocyte behavior. In addition, we highlight the wide variety of experimental techniques, spanning from static to impact loading, and analysis techniques, from biochemical assays to machine learning, that have been utilized to study chondrocyte behavior. Finally, we review the progression of hypotheses about chondrocyte mechanobiology and provide a perspective on the future outlook of chondrocyte mechanobiology.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The central physiological role of cartilage is purely mechanical. Cartilage cushions mechanical joint loading to facilitate smooth movement. The composition and structure of cartilage tissue have evolved to accommodate the complex in vivo multiaxial loading that the tissue experiences. Such composition and structure generate a unique micromechanical environment for chondrocytes during mechanical loading. Over the last 50 years, researchers have been investigating the relationship between the unique structure and the mechanical response of cartilage at multiple scales. Understanding how chondrocytes respond to such complex micromechanical environment under load is crucial for describing the bulk mechanical behavior of the tissue. As such, the field of chondrocyte mechanobiology, which seeks to understand how mechanically driven physical stimuli influence cell behavior, emerged as an important area of biomedical engineering about 30 years ago.
Mechanobiology is particularly important for chondrocytes and cartilage because chondrocytes are the only cell type to generate cartilage without blood vessels or nerves. In addition, the sole purpose of the tissue is to bear in vivo mechanical loads. Therefore, mechanical damage to cartilage is detrimental as the tissue continuously degenerates, ultimately leading to debilitating joint movements. Thus, investigating the influence of local physical stimuli on chondrocytes is crucial for understanding cartilage-related diseases, such as osteoarthritis, and for developing potential treatments to prevent or stop cartilage degeneration.
Studying cartilage mechanobiology is exceptionally challenging due to the three-dimensional chondrocyte-matrix interaction and the innate electrochemical-mechanical properties of the tissue. Even a simple compressive boundary condition can generate interstitial fluid flow, change in fixed charge density, and heterogenous matrix deformation, which are all coupled. Such coupled local physical stimuli are sensed by chondrocytes and influence the cells’ behavior.
This chapter aims to review the history of mechanobiology studies in chondrocytes and describe experimental techniques that have been utilized. In addition, we describe the progress of hypotheses and important local physical factors that can influence chondrocyte behavior and provide an outlook for the future of chondrocyte mechanobiology.
2 Static Stimulus
Initial studies on the effects of mechanical forces on the chondrocyte mechanism were performed under static loading conditions. Because cartilage is poroelastic, static loading must be applied by imposing weight or displacement on the sample and waiting for hydrostatic pressure and fluid flow to be dissipated [1,2,3,4,5,6]. Under constant load conditions, cartilage experiences poroelastic creep or stress relaxation [7, 8]. Such poroelastic behavior is caused by interstitial fluid flow. In addition, confined and unconfined boundary conditions can be imposed on the samples [9]. A confined boundary condition is accomplished by placing a cartilage sample in an impermeable and enclosed chamber with permeable porous platen compressing the tissue, while an unconfined boundary condition is achieved using an open chamber with an impermeable platen. These boundary conditions affect the direction of interstitial fluid flow caused by the imposed static compression. Under confined compression, fluid escapes against the loading direction through the permeable porous platen. Meanwhile, unconfined compression (Fig. 2.1a, d) forces the fluid to escape radially.
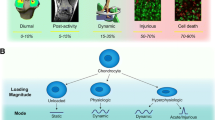
Modes of mechanical stimulation to study cartilage mechanobiology. (a) Compression. (b) Shear. (c) Tension. Regimes of loading rates. (d) Static, εi represents imposed strain. (e) Dynamic, εi represents imposed strain, εa represents strain amplitude, and f represents frequency. (f) Injurious loading. εi represents imposed strain, and \( \dot{?} \) represents strain rate
The effects of these static compressive loading methods and variable boundary conditions on chondrocyte biosynthetic activities have been thoroughly studied for more than 30 years [1,2,3,4,5,6, 10]. At the tissue level, cartilage biosynthetic activity, defined by proline and sulfate tissue intake, is suppressed monotonically with increasing stress and strain [1] (Fig. 2.1 and Table 2.1). Notably, this is the first study to observe biosynthetic change in cartilage under load. Given the complexity of the mechanical response of cartilage, this topic has remained an important area of investigation for the past three decades. The first mechanism of compression-induced biosynthetic change explored was changes in pH that occur under static compression. Compressing cartilage concentrates negative charges within the matrix, which requires increase in interstitial counterions, including H+ and K+. An increase in H+ concentration reduces interstitial pH, and the reduction of interstitial pH reduces the biosynthesis level. Remarkably, lowering the media’s pH produces the comparable interstitial pH of compressed tissue and reduces biosynthetic levels. Overall, this highlights the important role of counterions in chondrocyte biosynthesis.
The second mechanism of compression-induced regulation of chondrocyte biosynthesis is alteration of molecular transport. Compression with an impermeable boundary condition causes solute transport to occur radially and leads to a decrease in the pore size of the matrix. Further research [2, 10] has shown that biosynthetic activity under static compression is location-dependent. In general, increased static compression decreases location-specific biosynthetic activities uniformly across the construct. Tissue at the radial edge consistently expresses a higher synthetic level, but static compression decreases the overall biosynthesis. The biosynthetic activity forms a radial gradient as the level decreases gradually towards the center. More interestingly, the synthesis level at the radial edge of the free swelling sample is slightly higher than in the rest of the sample. These phenomena were thought to be caused by the limitation of molecular transport due to the compression-induced decrease in tissue diffusivity. The compaction of the matrix around chondrocytes reduces the characteristic pore size, hindering the delivery of macromolecules to the cells [11]. Therefore, at the center of the sample, nutrients are not as readily available as at the radial edge, leading to a radial gradient of the biosynthetic level.
However, chondrocytes embedded in agarose respond differently to those present in native tissue [12]. Agarose-chondrocyte constructs held at 5% static strain do not display a statistically significant difference in biosynthetic levels compared to those of free-swelling constructs. This indicates that other factors might play a more important role than molecular transport in depression of biosynthesis. Notably, agarose gel is significantly more diffusive than the native cartilage matrix. Therefore, 5% static strain may not hinder the diffusivity of agarose-chondrocyte constructs as much as that of native cartilage matrix. Collectively, these data highlight the unique nature of cartilage with respect to the consequences of static compression. In the absence of transport restriction and mechanochemical effects, the primary effect of static compression on chondrocytes is due to deformation. Interestingly, biosynthetic activities seem to depend on the deformation of cells in agarose systems.
At the length scale of a single cell, chondrocyte biosynthesis is primarily concentrated in the pericellular matrix [13]. Biosynthetic levels are approximately uniformly distributed around the cell in the absence of any physical stimuli [6]. Chondrocytes undergo morphological changes under static compression [3]. Cell volume and surface area decrease as higher levels of compression are imposed. The cell radius decreases in the direction of compression, while the radius in the direction perpendicular to compression remains unchanged. This deformation creates directional strain within the chondrocyte, resulting in the highest levels of biosynthesis in directions perpendicular to that of the applied compression. The directional dependence of biosynthesis becomes even more pronounced in the radial edge of the tissue compared to the center. On the other hand, the deformation pattern and magnitude of each cell remain relatively uniform across the construct.
Studies performed at the tissue and cell level suggest that alterations to chemical composition, diffusivity, and cell volume due to applied physical stimuli are the leading factors that influence chondrocyte behavior. Under static compression, cartilage tissue volume decreases due to compaction of collagen matrix. Such decrease in volume forces co-ions such as sulfate and proline to escape, increasing the concentration of counterions such as K+ and H+. Changes in electrochemical composition cause a decrease in interstitial pH, leading to a reduction in chondrocyte biosynthesis. In addition, molecular transport into the tissue is slowed due to a compression-induced decrease in pore size [14, 15]. As pore size decreases, transport of nutrients needed for biosynthesis becomes limited at the center of the tissue. Such limitation generates a spatially dependent biosynthetic pattern in which the radial edge displays a consistently higher biosynthetic level than the center. Furthermore, changes in cell volume in response to applied stimuli create directionally dependent cell biosynthetic activity. These phenomena explain observed changes in biosynthesis levels in cartilage tissue under static compression.
2.1 Mechanical Anchoring and Substrate Stiffness
Even in the absence of external stimuli, chondrocytes are sensitive to the mechanics of the surrounding extracellular matrix (ECM). Chondrocyte adhesion indicated by phenotype increases dramatically over substrate stiffness ranging from 25 kPa to 150 kPa [16]. These effects are dependent on interactions with integrins, suggesting that the cells are actively probing the matrix mechanics. Active mechanical sensing is further reiterated by studies in which cell behavior is altered by mechanical anchoring of the substrate. Static compression studies indicate that alterations to molecular transport, chemical composition, and cell volume are the major factors that influence chondrogenic biosynthesis. However, a recent study [17] has shown that mechanical properties of ECM, such as stress relaxation time and stiffness, have a significant impact on chondrocyte behavior. Chondrocytes embedded in hydrogel with faster stress relaxation can produce up to 3 times more interconnected cartilage matrix volume and proliferate up to 6 times more than those in hydrogel with slower stress relaxation time. The effects of ECM stiffness on chondrocyte biosynthesis are not yet clear due to conflicting results [17, 18]. In addition, tissue-engineered menisci constructs that were mechanically anchored during the culture are 3 times stiffer, and collagen fibers were 50% more aligned than those that were not anchored [19].
Passive physical stimuli, such as substrate stiffness, do not alter the physicochemical properties of the tissue or chondrocyte, yet they still influence the behavior of chondrocytes. These results indicate that the chondrocyte-matrix interaction is another significant factor that impacts chondrocyte behavior, complementing static compression studies that demonstrate the importance of molecular transport, interstitial pH level, and cell deformation.
3 Dynamic Stimuli
3.1 Dynamic Compression
Studies of static stimuli on cartilage provide insights into chondrocyte behavior, but dynamic stimulus is a more physiologically realistic representation of in vivo loading. Superimposing cyclic loading on top of static load introduces different factors such as fluid flow, hydrostatic pressure, and streaming potential. Previous in vivo joint loading studies have suggested that dynamic loading may play a critical role in proteoglycan synthesis and content [20, 21]. Dynamic loading experiments (Fig. 2.1a, e) can mimic the in vivo loading environment of the articular cartilage and better simulate chondrocyte behavior in vitro. These loading conditions inherently impose both static and cyclic components where the tissue would experience the magnitude of strain and frequency. Utilizing the base knowledge and hypotheses formed from static compression studies, the influence of frequency on chondrocyte biosynthesis can be differentiated from the static component of the dynamic physical stimuli.
During a single compression-release cycle, the interstitial fluid escapes during the compression and enters the tissue during the release [22]. On this short time scale, consistent with the static physical stimulus, the proline and sulfate content in chondrocytes decreases down to 50% during the compression. However, during the release, the uptake increases up to 100%, indicating that the biosynthesis rate exceeds the pre-compression level following applied stimulus. These phenomena led to an interest in studying the effect of prolonged cyclic compression on cartilage metabolism.
Consistent and prolonged dynamic loading has different effects than single or couple compression release cycles. Sub-physiologic (0.0001 Hz) to physiologic (1 Hz) frequencies are often used for prolonged experiments (Table 2.1). Cyclic compression studies suggest that stimulus-induced amplification of biosynthesis displays a strain and frequency threshold. Frequencies of 0.01–1 Hz combined with strain amplitude of 1–5% stimulated biosynthesis levels up to 40%. Furthermore, a spatially dependent biosynthesis level is also present in dynamically stimulated tissues. At a lower frequency of 0.01 Hz with 4–7% strain, the biosynthesis rate is uniformly distributed across the tissue [23]. However, at a higher frequency of 0.1 Hz, the cartilage tissue at the radial edge has a 50% higher biosynthetic level than at the center, consistent with the observation of statically compressed samples [10]. At frequencies lower than 0.1 Hz, the interstitial pressure is uniformly distributed across the construct, creating a uniform fluid flow from the center to the outer ring of the explant. As the frequency increases, the interstitial fluid does not have adequate time to escape, and the center of the tissue becomes incompressible, causing the fluid flow to concentrate in the outer ring. Spatially dependent fluid flow creates a spatially dependent biosynthesis level. Concentration of fluid flow is further confirmed by an increase in the streaming potential in response to an increase in frequency [24]. Dynamic compression induces counterion separation, and co-ions from the separation are transported out of the tissue leading to increase in streaming potential. Collectively, these results indicate that biosynthesis stimulation is highly correlated with local interstitial fluid flow.
At the cell level, the biosynthesis level increases with dynamic loading compared to cells under free swelling condition. Frequency and spatially dependent chondrocyte biosynthesis levels are consistent with the tissue level data [6]. Chondrocytes under 0.01 Hz compression display a relatively uniform increase in biosynthesis level across constructs [10]. On the other hand, chondrocytes under 0.1 Hz display a 50% increase in biosynthesis at the radial edge, while no change is observed at the center. This trend in the biosynthetic level matches the theoretical interstitial fluid velocity and is consistent with the findings from static compression cell-level data.
Dynamic compression data collected at both the tissue and cell level indicate that interstitial fluid flow might be the most important factor in stimulating the biosynthesis of cartilage tissue. In general, dynamic compression induces interstitial fluid flow, resulting in increased streaming potential and ultimately accelerates the chondrocyte biosynthesis. There is evidence that dynamic compression helps molecular incorporation into constructs [25, 26]. In addition, biosynthesis stimulation through dynamic compression is temporally dependent [27]. Tissues under alternate day loading display up to a 30% increase in proteoglycan synthesis and a suppression of proline synthesis down to 40% compared to a continuous loading regime. This indicates that proteoglycan and proline synthesis are differently stimulated under dynamic compression. Such finding is extremely valuable as proteoglycan provides compressive mechanical strength to cartilage while collagen provides shear strength. Furthermore, biosynthesis levels vary significantly depending on the type of matrix in which chondrocytes are embedded, pointing toward the importance of chondrocyte-matrix interaction. Collectively, dynamic compression studies reveal that fluid flow is an important stimulus of chondrocyte biosynthesis.
3.2 Oscillatory Shear and Tension
Static and dynamic compression studies suggest that interstitial fluid flow and matrix deformation are the prominent factors that influence chondrocyte biosynthesis. These two factors are coupled under dynamic compression, as the volume change that occurs under compression generates interstitial fluid flow. In contrast, dynamic shear generates high matrix deformation with minimal interstitial fluid flow [28]. As such, imposing dynamic simple shear (Fig. 2.1b, e) can be used to differentiate the effects of matrix deformation and interstitial fluid flow on the biosynthetic activity of chondrocytes. Indeed, dynamic shear influences the biosynthetic activity of chondrocytes differently than compression. Notably, dynamic shear strain stimulates collagen synthesis two-fold more than proteoglycan synthesis. Further, tissue biosynthetic activity does not show spatial dependence [28,29,30], unlike the static and dynamic compressive stimuli. Importantly, dynamic shear does not promote molecular transport within the tissue, as observed under dynamic compression [30]. Collectively, these findings suggest that (1) the shear-induced ECM deformation stimulates collagen synthesis and (2) fluid flow induced by compression stimulates proteoglycan synthesis and enhances molecular transport.
Studies of the effects of dynamic tension on chondrocyte behavior have utilized a hydrogel culture system, partly due to the challenges in imposing tension on intact cartilage. Based on findings from applications of compression and shear stimuli on intact tissue, chondrocytes exposed to oscillatory tension (Fig. 2.1c, e) are expected to express an increase in collagen and proteoglycan synthesis, as this type of loading generates both matrix deformation and interstitial fluid flow [31]. Surprisingly, chondrocytes embedded in fibrin hydrogels experience a stimulation in proteoglycan synthesis with no change in collagen synthesis under dynamic tension [32]. In addition, chondrocytes harvested from different regions (superficial, middle, and deep) display different levels of biosynthetic activity in response to the same physical stimulus. Furthermore, recent studies have suggested that the mechanical properties of cartilage zones are depth-dependent [26,27,28,29]. The zone-specific mechanical properties generate a unique micromechanical environment for chondrocytes in each zone. In fact, these differences in mechanical properties lead to differences in local strain [33], which are directly related to chondrocyte behavior [34, 35]. Differences in chondrocyte behavior, both in various cartilage zones and in native tissue versus fibrin hydrogels, confirm that chondrocytes sense matrix density and mechanics. Collectively, these findings underscore the importance of the micromechanical environment on the response of chondrocytes to external mechanical stimuli.
Dynamic stimulation studies highlight the complexity of the micromechanical environment and chondrocyte biological responses to external stimuli. In general, chondrocyte biosynthesis depends heavily on the local physical environment (Fig. 2.2a). Tissue regions that experience high levels of compression and associated interstitial fluid flow tend to show stimulated proteoglycan synthesis, while regions with high matrix deformation tend to display stimulated collagen synthesis. In fact, chondrocytes within the same construct have shown differential matrix synthesis depending on the local physical stimulus (Fig. 2.2b) [36]. In these studies, chondrocytes under local tensile strain synthesize more collagen with organized fibers. Those under the local compressive strain synthesize more proteoglycans than collagen, and the formed collagen does not contain organized fibers. Overall, oscillatory tension and shear data further confirm the critical influence of local physical environment on chondrocyte behavior.
Bulk mechanical behavior and biosynthetic analyses for cartilage. (a) Physical stimuli are applied to whole cartilage samples, which induce many changes including: cell deformation; increase in hydrostatic pressure; and interstitial fluid/coion flow. Changes in ECM content are determined through bulk biochemical assays and radio labeling (b), and changes in chondrocyte gene expression can be analyzed via Western Blot or in situ hybridization (c). Media contents of ECM components can be quantified by using biochemical assays or radio labeling (d)
3.3 Impact/Injurious Loading
It is well known that the avascular nature of cartilage hinders the tissue’s natural repair capabilities. Such innate limitation in natural repair results in continuous cartilage degeneration following injuries, ultimately leading to osteoarthritis [37, 38]. Previous studies of dynamic compression reveal that compressive strain rate increases both hydrostatic pressure and matrix synthesis [10, 23, 24, 39]. In addition, chondrocytes contained within different zones react differently to the same physical stimuli [32, 40]. These findings, coupled with the ability of chondrocytes to probe the micromechanical environment, suggest that impact loading (Fig. 2.1a, f) can offer a unique perspective on the role of chondrocytes in cartilage degeneration following injurious loading.
At the tissue level, chondrocyte survivability depends heavily on the strain rate. Physical stimuli resulting in a strain rate higher than the matrix diffusion rate causes chondrocytes death at the superficial zone [41, 42], while at a strain rate lower than the matrix diffusion rate, cell death is distributed throughout the tissue [43]. Interestingly, a higher relative strain rate decreases tissue biosynthesis by 33% compared to that of a lower strain rate [44]. In addition, a higher strain rate causes surface fissures and disrupts the collagen network. Such damage results in GAG loss in tissue within 24 h following the impact [45]. During the impact, the superficial zone acts as a protective layer, where tissue without superficial zone loses three times more GAG than that with superficial zone [46]. The relationship between peak stress and total GAG loss is still unclear [45, 47]. Despite GAG loss within the tissue, impact does not affect proteoglycan synthesis. However, collagen synthesis is most likely stimulated by collagen network disruption [44]. Other factors such as insulin-like growth factor and synoviocyte co-culture can reduce GAG loss and collagen network disruption (Fig. 2.2c) [48, 49], while cytokines can accentuate tissue damage [50]. Overall, the results suggest that the injurious impact disrupts and damages the collagen network, resulting in GAG loss (Fig. 2.2d).
At the length scale of a single cell, strain imposed by impact loading is highly correlated with cell death [42]. In addition, chondrocyte death develops within 2 h after the impact and is concentrated at the superficial zone of the tissue. When the surface region is removed, chondrocyte death is distributed towards the deeper zone. This data is consistent with tissue level data [43] and further confirms the protective role of the superficial zone [46]. Numerous studies demonstrate that such high-speed impact induces cell death over time, mostly through apoptosis [44, 51,52,53,54,55]. At 1 s−1 strain rate, 5–20% of the total cells undergo apoptosis depending on the age of the subject [56], and up to 97% of the dead cells undergo apoptosis [57], demonstrating that preventing apoptosis can potentially stop the development of post-traumatic osteoarthritis. Various factors can influence the apoptotic process. Following injury, the immune system produces pro-inflammatory cytokines, like tumor necrosis factor alpha (TNF-α), and such cytokines can induce further GAG loss [55]. On the contrary, the response to impact injury can also induce expression of several factors such as vascular endothelial growth factor, hypoxia-inducible factor, and matrix metalloproteinase [38, 52, 54, 58]. Additionally, anti-inflammatory cytokines like interleukin-10 can reduce GAG loss and apoptosis [59]. Furthermore, estrogen and antioxidants significantly reduce impact-induced cell death [46, 56], suggesting potential effects from gender and age. Despite numerous injury studies [38, 58], the mechanisms by which impact induces apoptosis are not clear.
Recent technological developments in high-speed confocal microscopy and soft tissue impact testing devices (Fig. 2.3a) enable further investigation of phenomena upstream of apoptosis. Additionally, these techniques facilitate the assessment of spatially dependent behavior of single cells on physiologic time scales (Fig. 2.3b, c). With such advances, a recent study [60] demonstrates that impact-induced chondrocyte apoptosis is caused by mitochondrial dysfunction, and mitoprotective therapy can prevent chondrocytes from undergoing apoptosis [61]. Further investigation reveals that calcium signaling, inter- and intra-cellular communication that activates mitochondrial dysfunction in response to physical stimuli, occurs within milliseconds after the impact [62]. The impact-induced chondrocyte death mechanism remains under active investigation and developing a greater understanding of this phenomenon could inform therapeutic options to prevent post-traumatic osteoarthritis.
Microscale behavior and cellular responses for cartilage. Physical stimuli are imposed (a) and cellular responses (b) and local micromechanical environment (c) can be measured in real-time via microscopy. Comparing physical stimuli and cellular response (d) enables high throughput assessment of chondrocyte mechanobiology (adapted from [42])
4 Future Direction
4.1 Combined Loading
Cartilage experiences a complicated in vivo mechanical environment wherein mixed modes of loading are applied to the tissue. Unidirectional mechanical testing, such as compression, tension, and shear, grants only a limited understanding of the influence of local physical stimuli on chondrocyte behavior. In addition, cartilage tissue has shown that the consequence of a mode of loading can affect the tissue behavior under another mode of loading. For example, impact loading increases the surface roughness of cartilage tissue two-fold, causing the friction coefficient to increase [63], and dynamic shear can increase the secretion of lubricating molecules [35]. Understanding chondrocyte behavior under combined loading is particularly important to halt the development and progression of osteoarthritis.
Several studies have investigated the effect of combined loading on the tissue level [41, 44, 50, 64]. In general, dynamic compression followed by an injurious impact slightly promotes biosynthesis [44, 50], but only up to a threshold amplitude of 20%. In addition, injured cartilage displays elevated shear strain [64], and dynamic shear after an injury exacerbates the apoptotic behavior [41]. Chondrocyte behavior under combined loading is most likely spatially and temporarily dependent, as indicated by previous studies [3, 23, 62]. Understanding the temporally and spatially dependent chondrocyte response to combined loading could identify the mechanism of osteoarthritis progression and enable development of therapeutic options to stop the progression of osteoarthritis.
4.2 Big Data/Machine Learning
Recent technological development in high-speed confocal imaging techniques has enabled the capture of individual and collective cell responses to multiple modes of physical stimuli at a higher frame rate. This development has led to an explosion in the number of collectable data sets. In the late 1980s, a single cartilage explant could provide only two data points, sulfate and proline uptake [1, 22, 24]. With the advent of high-speed confocal imaging, a single sample can provide more than 2000 individual cell data points [42]. This exponential increase in collectable data sets makes individual data analysis inefficient. Utilizing machine learning would enable efficient data analysis and the categorization of cellular behavior under various types of loading. In fact, a recent study has shown the efficacy of machine learning in analyzing cell signaling and mitochondrial depolarization [62]. The combination of machine learning algorithms and mechanobiology is an uncharted territory. The innate complexity of chondrocyte behavior makes machine learning an attractive candidate for data analysis.
5 Conclusion
Five decades of research have led to a much greater understanding of the influence of local physical stimuli on chondrocyte behavior. The innate zonal differences and poroelastic properties of cartilage tissue create spatial- and temporal-dependent cell behavior under various types of loads. This chapter covered the progression of hypotheses for chondrocyte behavior under load and the development of associated experimental techniques. Early studies investigated cartilage biosynthesis at the tissue level under static stimulus. Long-term biosynthesis was suppressed the most at the center and the least at the edge of the tissue, revealing a spatially dependent response. Dynamic stimulus tends to increase the biosynthetic level. The spatially dependent response still exists, but only when the stimulus is at a high frequency (>0.1 Hz). Findings from studies with static and dynamic stimuli generally indicate that the micromechanical environment plays a critical role in chondrocyte behavior. Along with the development of imaging and mechanical loading techniques for soft tissues, further understanding of chondrocyte behavior has been achieved. Impact loading, combined with advanced confocal imaging techniques, indicates that chondrocyte behavior is not only spatially dependent, but also possesses temporal characteristics. Under impact loading, most of the chondrocyte death is concentrated at the superficial zone, and the apoptosis process starts within 2 h after the applied stimulus. Further research in combined loading accompanied by machine learning is required to understand chondrocyte behavior during the onset and progression of osteoarthritis. Such understanding will give insight into prevention and treatment possibilities for post-injury cartilage degeneration.
References
Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC (1988) Mechanical and physicochemical determinants of the chondrocyte biosynthetic response. J Orthop Res 6:777–792. https://doi.org/10.1002/jor.1100060602
Buschmann MD, Hunziker EB, Kim YJ, Grodzinsky AJ (1996) Altered aggrecan synthesis correlates with cell and nucleus structure in statically compressed cartilage. J Cell Sci 109:499–508. https://doi.org/10.1242/jcs.109.2.499
Wong M, Wuethrich P, Buschmann MD, Eggli P, Hunziker E (1997) Chondrocyte biosynthesis correlates with local tissue strain in statically compressed adult articular cartilage. J Orthop Res 15:189–196. https://doi.org/10.1002/jor.1100150206
Chen AC, Sah RL (1998) Effect of static compression on proteoglycan biosynthesis by chondrocytes transplanted to articular cartilage in vitro. J Orthop Res 16:542–550. https://doi.org/10.1002/jor.1100160504
Ragan PM, Chin VI, Hung H-HK, Masuda K, Thonar EJ-MA, Arner EC, Grodzinsky AJ, Sandy JD (2000) Chondrocyte extracellular matrix synthesis and turnover are influenced by static compression in a new alginate disk culture system. Arch Biochem Biophys 383:256–264. https://doi.org/10.1006/abbi.2000.2060
Quinn TM, Grodzinsky AJ, Buschmann MD, Kim YJ, Hunziker EB (1998) Mechanical compression alters proteoglycan deposition and matrix deformation around individual cells in cartilage explants. J Cell Sci 111:573–583. https://doi.org/10.1242/jcs.111.5.573
Nia HT, Han L, Li Y, Ortiz C, Grodzinsky A (2011) Poroelasticity of cartilage at the nanoscale. Biophys J 101:2304–2313. https://doi.org/10.1016/j.bpj.2011.09.011
Han L, Frank EH, Greene JJ, Lee H-Y, Hung H-HK, Grodzinsky AJ, Ortiz C (2011) Time-dependent nanomechanics of cartilage. Biophys J 100:1846–1854. https://doi.org/10.1016/j.bpj.2011.02.031
Eisenberg SR, Grodzinsky AJ (1985) Swelling of articular cartilage and other connective tissues: electromechanochemical forces. J Orthop Res 3:148–159. https://doi.org/10.1002/jor.1100030204
Kim YJ, Sah RLY, Grodzinsky AJ, Plaas AHK, Sandy JD (1994) Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys 311:1–12. https://doi.org/10.1006/abbi.1994.1201
Bonassar LJ, Grodzinsky AJ, Srinivasan A, Davila SG, Trippel SB (2000) Mechanical and physicochemical regulation of the action of insulin-like growth factor-I on articular cartilage. Arch Biochem Biophys 379:57–63. https://doi.org/10.1006/abbi.2000.1820
Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB (1995) Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci 108:1497–1508. https://doi.org/10.1242/jcs.108.4.1497
Quinn TM, Maung AA, Grodzinsky AJ, Hunziker EB, Sandy JD (1999) Physical and biological regulation of proteoglycan turnover around chondrocytes in cartilage explants: implications for tissue degradation and repair. Ann N Y Acad Sci 878:420–441. https://doi.org/10.1111/j.1749-6632.1999.tb07700.x
Quinn TM, Morel V, Meister JJ (2001) Static compression of articular cartilage can reduce solute diffusivity and partitioning: implications for the chondrocyte biological response. J Biomech 34:1463–1469. https://doi.org/10.1016/S0021-9290(01)00112-9
Quinn TM, Kocian P, Meister J-J (2000) Static compression is associated with decreased diffusivity of dextrans in cartilage explants. Arch Biochem Biophys 384:327–334. https://doi.org/10.1006/abbi.2000.2077
Genes NG, Rowley JA, Mooney DJ, Bonassar LJ (2004) Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch Biochem Biophys 422:161–167. https://doi.org/10.1016/j.abb.2003.11.023
Lee H, Gu L, Mooney DJ, Levenston ME, Chaudhuri O (2017) Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat Mater 16:1243–1251. https://doi.org/10.1038/nmat4993
Lee CR, Grodzinsky AJ, Spector M (2001) The effects of cross-linking of collagen-glycosaminoglycan scaffolds on compressive stiffness, chondrocyte-mediated contraction, proliferation and biosynthesis. Biomaterials 22:3145–3154. https://doi.org/10.1016/S0142-9612(01)00067-9
Puetzer JL, Koo E, Bonassar LJ (2015) Induction of fiber alignment and mechanical anisotropy in tissue engineered menisci with mechanical anchoring. J Biomech 48:1436–1443. https://doi.org/10.1016/j.jbiomech.2015.02.033
Bricca A, Juhl CB, Grodzinsky AJ, Roos EM (2017) Impact of a daily exercise dose on knee joint cartilage – a systematic review and meta-analysis of randomized controlled trials in healthy animals. Osteoarthr Cartil 25:1223–1237. https://doi.org/10.1016/j.joca.2017.03.009
Kiviranta I, Tammi M, Jurvelin J, Säämänen A-M, Helminen HJ (1988) Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res 6:188–195. https://doi.org/10.1002/jor.1100060205
Sah RL-Y, Kim Y-J, Doong J-YH, Grodzinsky AJ, Plass AHK, Sandy JD (1989) Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res 7:619–636. https://doi.org/10.1002/jor.1100070502
Buschmann MD, Kim Y-J, Wong M, Frank E, Hunziker EB, Grodzinsky AJ (1999) Stimulation of Aggrecan synthesis in cartilage explants by cyclic loading is localized to regions of high interstitial fluid Flow1. Arch Biochem Biophys 366:1–7. https://doi.org/10.1006/abbi.1999.1197
Kim Y-J, Bonassar LJ, Grodzinsky AJ (1995) The role of cartilage streaming potential, fluid flow and pressure in the stimulation of chondrocyte biosynthesis during dynamic compression. J Biomech 28:1055–1066. https://doi.org/10.1016/0021-9290(94)00159-2
Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB (2001) The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. J Orthop Res 19:11–17. https://doi.org/10.1016/S0736-0266(00)00004-8
DiDomenico CD, Xiang Wang Z, Bonassar LJ (2017) Cyclic mechanical loading enhances transport of antibodies into articular cartilage. J Biomech Eng 139:011012. https://doi.org/10.1115/1.4035265
Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ (2004) Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech 37:595–604. https://doi.org/10.1016/j.jbiomech.2003.10.005
Jin M, Frank EH, Quinn TM, Hunziker EB, Grodzinsky AJ (2001) Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch Biochem Biophys 395:41–48. https://doi.org/10.1006/abbi.2001.2543
Frank EH, Jin M, Loening AM, Levenston ME, Grodzinsky AJ (2000) A versatile shear and compression apparatus for mechanical stimulation of tissue culture explants. J Biomech 33:1523–1527. https://doi.org/10.1016/S0021-9290(00)00100-7
Jin M, Emkey GR, Siparsky P, Trippel SB, Grodzinsky AJ (2003) Combined effects of dynamic tissue shear deformation and insulin-like growth factor I on chondrocyte biosynthesis in cartilage explants. Arch Biochem Biophys 414:223–231. https://doi.org/10.1016/S0003-9861(03)00195-4
Grodzinsky AJ, Roth V, Myers E, Grossman WD, Mow VC (1981) The significance of electromechanical and osmotic forces in the nonequilibrium swelling behavior of articular cartilage in tension. J Biomech Eng 103:221–231. https://doi.org/10.1115/1.3138284
Vanderploeg EJ, Wilson CG, Levenston ME (2008) Articular chondrocytes derived from distinct tissue zones differentially respond to in vitro oscillatory tensile loading. Osteoarthr Cartil 16:1228–1236. https://doi.org/10.1016/j.joca.2008.02.016
Wong BL, Bae WC, Chun J, Gratz KR, Lotz M, Sah RL (2008) Biomechanics of cartilage articulation: effects of lubrication and degeneration on shear deformation. Arthritis Rheum 58:2065–2074. https://doi.org/10.1002/art.23548
Bonnevie ED, Delco ML, Bartell LR, Jasty N, Cohen I, Fortier LA, Bonassar LJ (2018) Microscale frictional strains determine chondrocyte fate in loaded cartilage. J Biomech 74:72–78. https://doi.org/10.1016/j.jbiomech.2018.04.020
Nugent GE, Aneloski NM, Schmidt TA, Schumacher BL, Voegtline MS, Sah RL (2006) Dynamic shear stimulation of bovine cartilage biosynthesis of proteoglycan 4. Arthritis Rheum 54:1888–1896. https://doi.org/10.1002/art.21831
Puetzer JL, Bonassar LJ (2016) Physiologically distributed loading patterns drive the formation of zonally organized collagen structures in tissue-engineered meniscus. Tissue Eng A 22:907–916. https://doi.org/10.1089/ten.tea.2015.0519
Varady NH, Grodzinsky AJ (2016) Osteoarthritis year in review 2015: mechanics. Osteoarthr Cartil 24:27–35. https://doi.org/10.1016/j.joca.2015.08.018
Kurz B, Lemke AK, Fay J, Pufe T, Grodzinsky AJ, Schünke M (2005) Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat 187:473–485. https://doi.org/10.1016/j.aanat.2005.07.003
Orozco GA, Tanska P, Florea C, Grodzinsky AJ, Korhonen RK (2018) A novel mechanobiological model can predict how physiologically relevant dynamic loading causes proteoglycan loss in mechanically injured articular cartilage. Sci Rep 8:1–16. https://doi.org/10.1038/s41598-018-33759-3
Jones ARC, Chen S, Chai DH, Stevens AL, Gleghorn JP, Bonassar LJ, Grodzinsky AJ, Flannery CR (2009) Modulation of lubricin biosynthesis and tissue surface properties following cartilage mechanical injury. Arthritis Rheum 60:133–142. https://doi.org/10.1002/art.24143
Ayala S, Delco ML, Fortier LA, Cohen I, Bonassar LJ (2021) Cartilage articulation exacerbates chondrocyte damage and death after impact injury. J Orthop Res 39:2130–2140. https://doi.org/10.1002/jor.24936
Bartell LR, Fortier LA, Bonassar LJ, Cohen I (2015) Measuring microscale strain fields in articular cartilage during rapid impact reveals thresholds for chondrocyte death and a protective role for the superficial layer. J Biomech 48:3440–3446. https://doi.org/10.1016/j.jbiomech.2015.05.035
Morel V, Quinn TM (2004) Cartilage injury by ramp compression near the gel diffusion rate. J Orthop Res 22:145–151. https://doi.org/10.1016/S0736-0266(03)00164-5
Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ (2001) Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res 19:1140–1146. https://doi.org/10.1016/S0736-0266(01)00033-X
DiMicco MA, Patwari P, Siparsky PN, Kumar S, Pratta MA, Lark MW, Kim Y-J, Grodzinsky AJ (2004) Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum 50:840–848. https://doi.org/10.1002/art.20101
Imgenberg J, Rolauffs B, Grodzinsky AJ, Schünke M, Kurz B (2013) Estrogen reduces mechanical injury-related cell death and proteoglycan degradation in mature articular cartilage independent of the presence of the superficial zone tissue. Osteoarthr Cartil 21:1738–1745. https://doi.org/10.1016/j.joca.2013.07.007
Patwari P, Cheng DM, Cole AA, Kuettner KE, Grodzinsky AJ (2007) Analysis of the relationship between peak stress and proteoglycan loss following injurious compression of human post-mortem knee and ankle cartilage. Biomech Model Mechanobiol 6:83–89. https://doi.org/10.1007/s10237-006-0037-y
Lee CM, Kisiday JD, McIlwraith CW, Grodzinsky AJ, Frisbie DD (2013) Synoviocytes protect cartilage from the effects of injury in vitro. BMC Musculoskelet Disord 14:54. https://doi.org/10.1186/1471-2474-14-54
Li Y, Wang Y, Chubinskaya S, Schoeberl B, Florine E, Kopesky P, Grodzinsky AJ (2015) Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: relevance to post-traumatic osteoarthritis. Osteoarthr Cartil 23:266–274. https://doi.org/10.1016/j.joca.2014.11.006
Li Y, Frank EH, Wang Y, Chubinskaya S, Huang H-H, Grodzinsky AJ (2013) Moderate dynamic compression inhibits pro-catabolic response of cartilage to mechanical injury, tumor necrosis factor-α and interleukin-6, but accentuates degradation above a strain threshold. Osteoarthr Cartil 21:1933–1941. https://doi.org/10.1016/j.joca.2013.08.021
Loening AM, James IE, Levenston ME, Badger AM, Frank EH, Kurz B, Nuttall ME, Hung H-H, Blake SM, Grodzinsky AJ, Lark MW (2000) Injurious mechanical compression of bovine articular cartilage induces chondrocyte apoptosis. Arch Biochem Biophys 381:205–212. https://doi.org/10.1006/abbi.2000.1988
Lee JH, Fitzgerald JB, DiMicco MA, Grodzinsky AJ (2005) Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum 52:2386–2395. https://doi.org/10.1002/art.21215
Kisiday JD, Vanderploeg EJ, McIlwraith CW, Grodzinsky AJ, Frisbie DD (2010) Mechanical injury of explants from the articulating surface of the inner meniscus. Arch Biochem Biophys 494:138–144. https://doi.org/10.1016/j.abb.2009.11.022
Patwari P, Cook MN, DiMicco MA, Blake SM, James IE, Kumar S, Cole AA, Lark MW, Grodzinsky AJ (2003) Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum 48:1292–1301. https://doi.org/10.1002/art.10892
Sui Y, Lee JH, DiMicco MA, Vanderploeg EJ, Blake SM, Hung H-H, Plaas AHK, James IE, Song X-Y, Lark MW, Grodzinsky AJ (2009) Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor α in immature bovine and adult human articular cartilage. Arthritis Rheum 60:2985–2996. https://doi.org/10.1002/art.24857
Kurz B, Lemke A, Kehn M, Domm C, Patwari P, Frank EH, Grodzinsky AJ, Schünke M (2004) Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum 50:123–130. https://doi.org/10.1002/art.11438
Patwari P, Gaschen V, James IE, Berger E, Blake SM, Lark MW, Grodzinsky AJ, Hunziker EB (2004) Ultrastructural quantification of cell death after injurious compression of bovine calf articular cartilage. Osteoarthr Cartil 12:245–252. https://doi.org/10.1016/j.joca.2003.11.004
Pufe T, Lemke A, Kurz B, Petersen W, Tillmann B, Grodzinsky AJ, Mentlein R (2004) Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. Am J Pathol 164:185–192. https://doi.org/10.1016/S0002-9440(10)63109-4
Behrendt P, Preusse-Prange A, Klüter T, Haake M, Rolauffs B, Grodzinsky AJ, Lippross S, Kurz B (2016) IL-10 reduces apoptosis and extracellular matrix degradation after injurious compression of mature articular cartilage. Osteoarthr Cartil 24:1981–1988. https://doi.org/10.1016/j.joca.2016.06.016
Delco ML, Bonnevie ED, Bonassar LJ, Fortier LA (2018) Mitochondrial dysfunction is an acute response of articular chondrocytes to mechanical injury. J Orthop Res 36:739–750. https://doi.org/10.1002/jor.23651
Delco ML, Bonnevie ED, Szeto HS, Bonassar LJ, Fortier LA (2018) Mitoprotective therapy preserves chondrocyte viability and prevents cartilage degeneration in an ex vivo model of posttraumatic osteoarthritis. J Orthop Res 36:2147–2156. https://doi.org/10.1002/jor.23882
Zheng J, Jackson T, Fortier LA, Bonassar LJ, Delco ML, Cohen I (2020) Establishing the peracute relationship between calcium signaling and mitochondrial depolarization after impact injury to articular cartilage. ORS 2020 Annual Meeting No 0191
Bonnevie ED, Delco ML, Galesso D, Secchieri C, Fortier LA, Bonassar LJ (2017) Sub-critical impact inhibits the lubricating mechanisms of articular cartilage. J Biomech 53:64–70. https://doi.org/10.1016/j.jbiomech.2016.12.034
Wong BL, Kim SHC, Antonacci JM, McIlwraith CW, Sah RL (2010) Cartilage shear dynamics during tibio-femoral articulation: effect of acute joint injury and tribosupplementation on synovial fluid lubrication. Osteoarthr Cartil 18:464–471. https://doi.org/10.1016/j.joca.2009.11.008
Gray ML, Pizzanelli AM, Lee RC, Grodzinsky AJ, Swann DA (1989) Kinetics of the chondrocyte biosynthetic response to compressive load and release. Biochim Biophys Acta Gen Subj 991:415–425. https://doi.org/10.1016/0304-4165(89)90067-6
Davisson T, Kunig S, Chen A, Sah R, Ratcliffe A (2002) Static and dynamic compression modulate matrix metabolism in tissue engineered cartilage. J Orthop Res 20:842–848. https://doi.org/10.1016/S0736-0266(01)00160-7
Grodzinsky AJ, Levenston ME, Jin M, Frank EH (2000) Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng 2:691–713. https://doi.org/10.1146/annurev.bioeng.2.1.691
Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB (1992) Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res 10:745–758. https://doi.org/10.1002/jor.1100100602
Thibault M, Robin Poole A, Buschmann MD (2002) Cyclic compression of cartilage/bone explants in vitro leads to physical weakening, mechanical breakdown of collagen and release of matrix fragments. J Orthop Res 20:1265–1273. https://doi.org/10.1016/S0736-0266(02)00070-0
Hunter CJ, Mouw JK, Levenston ME (2004) Dynamic compression of chondrocyte-seeded fibrin gels: effects on matrix accumulation and mechanical stiffness. Osteoarthr Cartil 12:117–130. https://doi.org/10.1016/j.joca.2003.08.009
Mouw JK, Connelly JT, Wilson CG, Michael KE, Levenston ME (2007) Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells 25:655–663. https://doi.org/10.1634/stemcells.2006-0435
Lee B, Han L, Frank EH, Chubinskaya S, Ortiz C, Grodzinsky AJ (2010) Dynamic mechanical properties of the tissue-engineered matrix associated with individual chondrocytes. J Biomech 43:469–476. https://doi.org/10.1016/j.jbiomech.2009.09.053
Zhang L, Miramini S, Smith DW, Gardiner BS, Grodzinsky AJ (2015) Time evolution of deformation in a human cartilage under cyclic loading. Ann Biomed Eng 43:1166–1177. https://doi.org/10.1007/s10439-014-1164-8
Lee CM, Kisiday JD, McIlwraith CW, Grodzinsky AJ, Frisbie DD (2013) Development of an in vitro model of injury-induced osteoarthritis in cartilage explants from adult horses through application of single-impact compressive overload. Am J Vet Res 74:40–47. https://doi.org/10.2460/ajvr.74.1.40
Rolauffs B, Muehleman C, Li J, Kurz B, Kuettner KE, Frank E, Grodzinsky AJ (2010) Vulnerability of the superficial zone of immature articular cartilage to compressive injury. Arthritis Rheum 62:3016–3027. https://doi.org/10.1002/art.27610
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Kim, B., Bonassar, L.J. (2023). Understanding the Influence of Local Physical Stimuli on Chondrocyte Behavior. In: Connizzo, B.K., Han, L., Sah, R.L. (eds) Electromechanobiology of Cartilage and Osteoarthritis. Advances in Experimental Medicine and Biology, vol 1402. Springer, Cham. https://doi.org/10.1007/978-3-031-25588-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-25588-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25587-8
Online ISBN: 978-3-031-25588-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)