Abstract
There are many nature-based antimicrobial solutions that could be used to decrease food spoilage and increase food safety. The use of bacteriophages (phages), viruses that infect bacteria but not human, animal or plant cells, is an example of a biotechnological approach for food preservation. Bacteriophage P100, marketed as LISTEX, was the first bacteriophage product to be Generally Recognized As Safe (GRAS) by the US FDA. This phage is active against the foodborne pathogen Listeria monocytogenes, responsible for a severe infection in the elderly, neonates and the immunocompromised. In this article, ECJ Case T-568/19, Micreos Food Safety BV vs European Commission, is analysed as a starting point for a discussion on whether a novel legal approach to the use of phages in the European Union is needed.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The World Health Organization (WHO) estimates that each year food contaminated with bacteria, parasites, toxins and allergens accounts for 600 million cases of illness and 420,000 deaths worldwide (Havelaar et al. 2015).
Improving food safety is essential if the United Nations Sustainable Development Goals are to be successfully achieved, particularly Goal 2 (“There is no food security without food safety”), Goal 3 (“Food safety has a direct impact on people’s health and nutritional intake”) and Goal 12 (“knowledge and solutions for better control of foodborne pathogens reduce the number of food recalls contributing to a reduction in food waste”).Footnote 1
While it is generally accepted that food has never been safer than it is today,Footnote 2 it is also recognised that consumers have never been so demanding and so concerned about their diet.Footnote 3 Yet consumer demands are hard to meet. While, on the one hand, consumers expect nutritious, safe and convenient food with a long shelf life, on the other hand they favour less preserved food—low in sugar, low in salt, and with no synthetic chemical additives—with minimal processing. It is within this framework that nature-based antimicrobial solutions find their place.
There are many naturally occurring antimicrobials (of plant, animal or microbial origin) that could be used in food preservation systems. Nevertheless, only a few have been commercially exploited. The lack of knowledge about their antimicrobial mechanisms, efficacy and safety prevents their use from being approved by official bodies.
2 Microbial Biocontrol Agents
Biological control by microorganisms and/or their metabolites, i.e., the use of harmless microbes to inhibit or destroy microbial pathogens or spoilers, is one of the oldest ways of using biotechnology in food systems.
Fermentation, a process relying on the activity of microorganisms, mainly lactic acid bacteria or yeasts, has been used to preserve milk, meat, fruits, vegetables and cereals since ancient times.Footnote 4 Lactic acid bacteria produce different inhibitory compounds such as organic acids, hydrogen peroxide, diacetyl, carbon dioxide and bacteriocins, and this arsenal can inhibit undesirable microbes. As a result, it is possible to make perishable products available all year round instead of only seasonally, and to increase diversity in diets.
Bacteriocins are antimicrobial peptides and proteins produced by bacteria. These inhibitory proteins are commonly produced by lactic acid bacteria and have demonstrated great efficiency in the control of some pathogens, namely Listeria monocytogenes in different food products (Pinto et al. 2008; Borges and Teixeira 2016; Peng et al. 2017; Ramos et al. 2020). So far, nisin, a bacteriocin produced by Lactococcus lactis, is the only bacteriocin to be licensed as a biopreservative. It was first licensed for use as a food preservative in England in the 1950s and its use has subsequently been approved in many other countries. Nisin was granted international authorisation by the Joint Food and Agriculture Organization/World Health Organization (FAO/WHO) Expert Committee on Food Additives in 1969. Nisin was also added to the European food additive list, where it was assigned the number E234.Footnote 5
Despite the great potential of bacteriocins (Borges and Teixeira 2016), their use also has recognised limitations, namely, a narrow spectrum of activity, loss of activity in particular food matrices, sensitivity to proteolytic enzymes, a high dosage requirement and uneven distribution in food products. To overcome these drawbacks, the use of bacteriocins in combination with other environmentally friendly treatments has been investigated with very promising results. High pressure processing and bacteriophages are examples of some of these treatments (Komora et al. 2021).
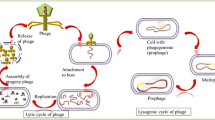
Bacteriophages (or phages)—literally meaning “bacteria eaters”—are viruses that only infect bacteria. The first descriptions of what might have been bacteriophages date back to the end of the eighteenth century, but it was only in 1917 that D'Herelle reported “an invisible, antagonistic microbe of the dysentery bacillus” (D'Hérelle 1917, 2007).Footnote 6 The first documented clinical use of phages is dated 1919 and was also conducted by D'Herelle, at the Hôpital des Enfants, to treat four children with bacterial dysentery. Since then, bacteriophages have been the subject of intense research with various purposes. Remarkably, in 1969, Max Delbrück, Alfred Hershey and Salvador Luria were awarded the Nobel Prize in Physiology and Medicine for their discoveries on “the replication mechanism and the genetic structure of viruses” using bacteriophages as models.Footnote 7
Despite the promising use of phages in the treatment of infections, research on phage therapy slowed down in the U.S. and Western Europe in the 1940s with the advent of antibiotics. However, research continued in Eastern European countries, especially in Poland and in the former Soviet Union, and phages are now being reconsidered as therapeutic options for infections caused by antibiotic resistant bacteria. According to the World Health Organization, “There is no time to wait. Unless the world acts urgently, antimicrobial resistance will have disastrous impact within a generation.”.Footnote 8 Currently, phage therapies are commonly used for the treatment of infections in Georgia, Poland and Russia, and these treatments are sought by patients from all over the world as there are still many legal limitations to their use in jurisdictions outside the former Eastern Bloc countries (Kutter et al. 2020). The ‘compassionate use’ of phages has allowed their use in exceptional situations in patients across EU and US (Patey et al. 2018).
Although controversial, the use of phages has been approved in many jurisdictions for other purposes, namely for the control of foodborne pathogens, and phages are emerging as biocontrol agents in food production (Hudson et al. 2005; Ribeiro et al. 2016; Endersen and Coffey 2020; Vikram et al. 2021).
In 2006, the FDA approved ListShield™ (a cocktail of phages active against L. monocytogenes) as an additive for ready-to-eat foods.Footnote 9 Later in the same year, LISTEX™ (phage P100 active against L. monocytogenes) was the first US FDA ‘GRAS’ (Generally Recognized as Safe) approved phage product.Footnote 10 Since then, other bacteriophages active against L. monocytogenes and other important foodborne pathogens (e.g., Salmonella spp., Escherichia coli) have also been approved and are commercially available.Footnote 11
Phages have the ideal characteristics to be used as biocontrol agents in food and in the food processing environment, as they:
-
are the most abundant organisms on Earth and can be isolated from natural environments;
-
are self-replicating agents;
-
only infect bacteria;
-
are highly specific, “magic bullets” that only destroy the target bacteria;
-
are not known to produce adverse or toxic effects on eukaryotic cells;
-
do not alter the sensory characteristics of foods;
-
can be produced at industrial scale.
However, these characteristics do not dispense with the need for careful selection of phages with potential food applications. Among other characteristics, they cannot harbour genes encoding for virulence determinants or antimicrobial resistance.
Furthermore, no phages have been approved at European-wide level, although the use of phages is permitted in some countries under national law.
3 Phages Targeting Listeria monocytogenes
Listeria monocytogenes is one of the most feared foodborne pathogens and is the most frequent cause of death due to contaminated food in developed countries (EFSA and ECDC 2021). Despite worldwide efforts by research organisations and the food industry to reduce the incidence of listeriosis, this pathogen remains a serious threat to human health and the food supply.
In addition to causing suffering, functional disability and death, L. monocytogenes and listeriosis have a huge economic impact, severely affecting not only health systems but also the food industry. Listeria is a common cause of major food recalls—for example, it was the 2nd largest cause in the USA in 2019. As a result, tons of foods are wasted as recalled foods are normally destroyed, and food manufacturers suffer great losses due to damage to their brand reputation. Recalls also prompt further food waste due to a matter of consumer perception—“just in case, better to throw it away”. In addition, land used, human labour, water, energy and other resources that went into producing that food are also wasted. Although recalled foods only account for a fraction of food waste, food recalls are very high profile and lead to huge amounts of waste so, to the extent that we can minimize them…absolutely we should be putting the effort in”.Footnote 12
The ubiquitous nature of L. monocytogenes and its ability to grow in harsh conditions—including refrigeration temperatures, high salt concentration and low pH values—makes it difficult for the food industry to control this pathogen. Some strains (particular molecular subtypes) may be repeatedly isolated over time in the same plant for several months/years—recognised as persistent strains. This represents a major challenge for the food sector as cross-contamination by the equipment and general food processing environment is one of the most important sources of food contamination (Ferreira et al. 2014). “This is an unavoidable risk that food producers have when they are making their products, because listeria is everywhere and can pop up at any time despite all the rigorous hygiene measures that they undertake.”Footnote 13
However, in the EU, as mentioned previously, we appear to have access to a powerful weapon to fight L. monocytogenes:
Even at a senior official level, it is not understood why, since 2007, the Commission has not been able to form an opinion on the way in which the use of Listex P100 in the production of food can be approved in the EU. (…) Listeria bacteria as such are more dangerous than COVID-19: a much higher percentage of Listeria victims die, experience miscarriages or persistent complaints such as paralysis. Of course, the important difference is that a virus is an epidemic disease that occasionally pops up; Listeria bacteria are not transferable from person to person, but they are latently present. A faster procedure for Listex P100 creates hope that the more than 1000 recent Listeria victims, especially in Spain, Germany and the Netherlands, don’t get any ‘successors’.Footnote 14
4 The (Lack of a) European Legal Regulation on Phages
From a European legal point of view, in the absence of specific regulation on phages, the question arises whether bacteriophages used on food must be classified as decontaminants, additives or processing aids.
This is relevant in order to determine whether phages fall within the scope of application of EU Regulation No 853/2004, of 29 April 2004,Footnote 15 on the hygiene of foodstuffs, and, therefore, if a European authorisation is needed for such products to be placed on the market. In fact, according to Article 3 of that Regulation, “Food business operators shall not use any substance other than potable water or (…) clean water, to remove surface contamination from products of animal origin, unless use of the substance has been approved by the Commission”.
On the other hand, according to Article 3 of Regulation (EC) No 1333/2008, of 16 December 2008,Footnote 16 on food additives, these “are substances that are not normally consumed as food itself but are added to food intentionally for a technological purpose described in this Regulation, such as the preservation of food”. In order to ensure harmonisation, the risk assessment and approval of food additives must be carried out in accordance with the procedure laid down in Regulation (EC) No 1331/2008 of the European Parliament and of the Council of 16 December 2008, establishing a common authorisation procedure for food additives, food enzymes and food flavourings,Footnote 17 which ends with the Commission deciding whether to include a certain substance in the list of substances admitted in the European Union (Szajkowska 2012; Estorninho 2013; Estorninho and Macieirinha 2014; Meulen and Wernaart 2020)
Unlike the case of decontaminants and additives, use of processing aids does not require European authorisation. In fact, according to Article 2 of Regulation (EC) No 1333/2008, of 16 December 2008,Footnote 18 on food additives, processing aids are excluded from its scope of application. The Regulation defines a ‘processing aid’ as any substance which:
(i) | is not consumed as a food by itself; |
(ii) | is intentionally used in the processing of raw materials, foods or their ingredients, to fulfil a certain technological purpose during treatment or processing; and |
(iii) | may result in the unintentional but technically unavoidable presence in the final product of residues of the substance or its derivatives provided they do not present any health risk and do not have any technological effect on the final product. |
5 ECJ Case T-568/19, Micreos Food Safety BV vs European Commission
On 19 June 2015, Micreos Food Safety BV, established in Netherlands, lodged an application before the EU Commission, for approval of the use of Listex™ P100, a phage-based product that can be used against the contamination by Listeria of ready-to-eat food, as a decontaminant to reduce the presence of L. monocytogenes in animal-derived ready-to-eat food.
On July 2016, following the adoption by the European Food Safety Authority (EFSA) of a scientific opinion on Listex™ P100, the Commission adopted a draft regulation authorising the use of Listex™ P100 for the reduction of L. monocytogenes in animal-derived ready-to-eat food, under Article 3(2) of Regulation No 853/2004. The draft was the subject of public consultation in 2017, but it was never approved. In the SCoPAFF meeting of July 2018 the Commission and the Member States failed to reach agreement on the question of whether Listex™ P100 used on animal-derived ready-to-eat food must be classified as a decontaminant, an additive, or a processing aid. This question was once again discussed at the meeting of April 2019 of DG Health and Food Safety. Belgium raised the point that there could be a distortion of the internal market, if Member States were to classify the product Listex™ P100 differently. The Netherlands asked for clarification as to whether the product was a processing aid (national authorisation needed), a food additive or a decontaminant according to hygiene rules. Some Member States indicated that they could accept it as a food additive (Germany, France). Germany and Austria highlighted that the safety of this product remained unclear. Following discussions, the chair indicated a willingness to seek legal advice on whether an authorisation in accordance with Article 3(2) of Regulation (EC) No 853/2004 was applicable, although repeating that in his understanding this was the case.
Later, 2018, Micreos submitted that Listex™ P100 should be regarded not as a decontaminant but as a processing aid, which does not fall within the scope of application of Regulation No 853/2004.
On 17 June 2019, the Commission informed Micreos that it did not intend to pursue the evaluation of the request to approve Listex™ P100 on the basis of Article 3(2) of Regulation No 853/2004 and also, regarding the new request by the applicant for Listex™ P100 to be regarded as a processing aid outside the scope of Regulation No 853/2004, that “even if Listex™ P100 were to be classified as a processing aid, it would fall within the scope of Regulation No 853/2004, to the extent that it is used for decontaminating purposes”.
Micreos brought an action pursuant to Article 263 TFEU seeking the annulment of the alleged decisions of the Commission of 17 June 2019 by which the Commission (i) would have rejected its original application for the approval of Listex™ P100 as a decontaminant in animal-derived ready-to-eat food, or re-examined that application (‘the first alleged decision’); and (ii) would have rejected its alternative application to regard Listex™ P100 as a non-decontaminating processing aid (‘the second alleged decision’); and, (iii) would have prohibited the placing on the European Union market of that product as a processing aid for that food (‘the third alleged decision’).
The Commission contended that the application was inadmissible because the alleged decision prohibiting the placing on the market of Listex™ P100, contested in the main application, did not exist. In essence, the Commission submitted that the contested acts were not decisions or acts that were open to challenge and annulment. According to the Commission, they were merely informative.
By order of 26 September 2019, (T-568/19 R), the President of the General Court dismissed the application for interim measures as inadmissible and the main action was also dismissed as inadmissible, on 18 December 2020.
An analysis of ECJ Case T-568/19, Micreos Food Safety BV vs European Commission, allows us to conclude that, at EU level, neither has an authorisation for the placing on the market of Listex™ P100 in accordance with Article 3(2) of Regulation No 853/2004 been granted, nor has a decision prohibiting its use been taken. Furthermore, the Commission also stated it had no intention to propose a specific regulation on phages.
6 Conclusion and Perspectives
Traditional food preservation methods rely heavily on thermal processes. In fact, high temperature kills microbes, ensuring food safety and shelf-life extension. Nevertheless, thermal processes also alter the nutritional and organoleptic properties of food products. Moreover, with the need to intensify food production due to the increase in the world population, sustainability and mitigation of environmental impacts are key issues to consider in food processing. Biotechnology is uniquely positioned to address such challenges and microbiological biocontrol approaches are promising safe, environmentally friendly and chemical-free alternatives to ensure food safety and prevent food spoilage, in addition to the benefits associated with their minimal effects on the nutritional and sensory properties of foods. Further research is still needed to overcome some of the limitations and maximise all the potential benefits of these sustainable approaches, thus providing authorities with the knowledge they require to take decisions.
The COVID crisis has shown not only the need for deeper and stronger European health policies, but also that in cases of urgency, public health reasons allow the European Commission to use mechanisms of urgent response (for instance, the urgent authorisation for COVID vaccines). As regards the use of bacteriophages to fight Listeria, it is time to put an end to hesitations, lack of transparency and bureaucracy. The precautionary principle advocates a cautious approach in cases of uncertainty Fisher et al. (2006), but in the case of phages, extensive research demonstrates that they are natural, safe and green alternative solutions. The principle of sustainability implies a novel legal approach to the use of phages in the European Union.
Notes
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
Archived from the original on 2010-12-04. http://www.webcitation.org/5uicsPk41.
- 7.
- 8.
- 9.
- 10.
GRN No. 218 Bacteriophage P100 preparation from Listeria innocua https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=218&sort=GRN_No&order=DESC&startrow=1&type=basic&search=218.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
References
Borges S, Teixeira P (2016) Application of bacteriocins in food and health care. In: Padilla T (ed) Bacteriocins: production, applications and safety. Nova Publishers, New York, pp 47–76
D'Hérelle F (1917) Sur un microbe invisible antagoniste des bacilles dysentériques. C R Acad Sci Paris 165:373–375
D'Herelle F (2007) On an invisible microbe antagonistic toward dysenteric bacilli: brief note by Mr. F. D'Herelle, presented by Mr. Roux. 1917. Res Microbiol 158:553–554
EFSA and ECDC (2021) The European Union one health 2020 zoonoses report. EFSA J 19:6971
Endersen L, Coffey A (2020) The use of bacteriophages for food safety. Curr Opin Food Sci 36:1–8
Estorninho MJ (2013) Direito da alimentação/food law. AAFDL, Lisboa
Estorninho MJ, Macieirinha T (2014) Direito da saúde. UCP, Lisboa
Ferreira V, Wiedmann M, Teixeira P, Stasiewicz MJ (2014) Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J Food Prot 77:150–170
Fisher EC, Jones JS, von Schomberg R (2006) Implementing the precautionary principle: perspectives and prospects. Edward Elgar Publishing, Cheltenham
Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, de Silva NR, Gargouri N, Speybroeck N, Cawthorne A, Mathers C, Stein C, Angulo FJ, Devleesschauwer B (2015) World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med 12:e1001923
Hudson JA, Billington C, Carey-Smith G, Greening G (2005) Bacteriophages as biocontrol agents in food. J Food Prot 68:426–437
Komora N, Maciel C, Amaral R, Fernandes R, Castro SM, Saraiva J, Teixeira P (2021) Innovative hurdle system towards Listeria monocytogenes inactivation in a fermented meat sausage model - high pressure processing assisted by bacteriophage P100 and bacteriocinogenic Pediococcus acidilactici. Food Res Int 148:110628
Kutter E, Hoyle N, Eisner W, Kuhl S, Alavidze Z, Blasdel BG (2020) 101 - Phage therapy: bacteriophages as natural, self-limiting antibiotics. In: Pizzorno JE, Murray MT (eds) Textbook of natural medicine. Churchill Livingstone, London, UK, pp 777–787.e3
Meulen B, Wernaart B (2020) EU food law handbook. European Institute for Food Law, Amstelveen
Patey O, McCallin S, Mazure H, Liddle M, Smithyman A, Dublanchet A (2018) Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections. Viruses 11:18
Peng C, Borges S, Magalhães R, Carvalheira A, Ferreira V, Casquete R, Teixeira P (2017) Characterization of anti-listerial bacteriocin produced by lactic acid bacteria isolated from traditional fermented foods from Cambodia. Int Food Res J 24:386–393
Pinto AL, Fernandes M, Pinto C, Albano H, Castilho F, Teixeira P, Gibbs PA (2008) Characterization of anti-Listeria bacteriocins isolated from shellfish: potential antimicrobials to control non-fermented seafood. Int J Food Microbiol 129:50–58
Ramos B, Brandão TRS, Teixeira P, Silva CLM (2020) Biopreservation approaches to reduce Listeria monocytogenes in fresh vegetables. Food Microbiol 85:103282
Ribeiro AA, Silva J, Gibbs P, Teixeira P (2016) Isolation and evaluation of the lytic spectrum of bacteriophages active against food-borne bacteria. In: Harrington D (ed) Bacteriophages: an overview and synthesis of a re-emerging field. Nova Science Publishers, New York, pp 71–87
Szajkowska A (2012) Regulating food law: risk analysis and the precautionary principle as general principles of EU food law. Wageningen Academic Publishers, Wageningen
Vikram A, Woolston J, Sulakvelidze A (2021) Phage biocontrol applications in food production and processing. Curr Issues Mol Biol 40:267–302
Acknowledgments
We would like to thank the scientific collaboration under the FCT project UIDB/50016/2020.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Estorninho, M.J., Teixeira, P. (2023). Fighting Listeria monocytogenes with Bacteriophages: Biotechnology for Food Safety. In: Garcia, M.d.G., Cortês, A. (eds) Blue Planet Law. Sustainable Development Goals Series. Springer, Cham. https://doi.org/10.1007/978-3-031-24888-7_21
Download citation
DOI: https://doi.org/10.1007/978-3-031-24888-7_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24887-0
Online ISBN: 978-3-031-24888-7
eBook Packages: Law and CriminologyLaw and Criminology (R0)