Abstract
Melioidosis in an infectious disease of humans and animals caused by the bacterium Burkholderia pseudomallei. Melioidosis is often considered a disease of the tropics, but recent data suggests that B. pseudomallei is distributed worldwide and the disease is likely largely underestimated. B. pseudomallei is inherently resistant to many antibiotics, which complicates treatment, particularly in low-resource countries. There is currently no licensed vaccine to prevent melioidosis. Fortunately, there has been significant progress over the last decade in our understanding of B. pseudomallei pathogenesis and host immunity. This has been paralleled by the discovery and testing of promising vaccine candidates against melioidosis. Collectively, these scientific advances spark optimism that licensure of a safe and effective vaccine is achievable.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Melioidosis
Melioidosis, also known as Whitmore’s disease, is caused by Burkholderia pseudomallei, a Gram-negative aerobic bacillus capable of infecting both animals and humans. The bacterium has an impressive collection of virulence factors encoded on two chromosomes, an unusual feature for most bacteria [1]. B. pseudomallei can cause a wide variety of disease manifestations, ranging from asymptomatic infection to life-threatening pneumonia or sepsis, which may be influenced by the route of infection and underlying human risk factors or immune status [2].
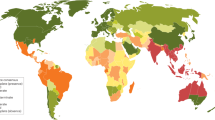
Melioidosis is most common in subtropical areas, with the highest incidence in Southeast Asia and Northern Australia. B. pseudomallei is also found in parts of Africa and has recently been detected in the US Virgin Islands and other Caribbean islands, including Puerto Rico [3]. While B. pseudomallei is not considered endemic in Europe or North America, four cases of melioidosis were recently reported in the USA. The four cases occurred in Kansas, Texas, Georgia, and Minnesota in individuals who had not traveled outside of the USA. Genetic analyses of the isolates linked the infection to a common source—a contaminated imported aromatherapy spray [4, 5]. There have been case reports of endemic melioidosis in Central and South America, pointing to a more global spread of B. pseudomallei than previously appreciated [6].
B. pseudomallei is a soil saprophyte, and routes of exposure may include inoculation, inhalation, or ingestion from a contaminated source. The incubation time to disease onset is 1–21 days from exposure with an average of 9 days, depending on the infectious dose and host factors. Melioidosis is considered an acute infection if the symptoms manifest for less than 2 months, whereas symptoms persisting longer than 2 months constitute a chronic infection. Latent infection with reactivation disease can also occur [7]. The outcomes of infection depend on several factors including B. pseudomallei strain, route of infection and dose, and host immune status. The most common host risk factor in development of severe melioidosis is diabetes mellitus [8], but other risk factors include chronic kidney, lung, and liver diseases and other conditions that impair the immune system [9]. Nonetheless, severe sickness and death can also occur in otherwise healthy individuals [10]. The case fatality rate percentage (CFR%) ranges from 8% in Europe up to 60% in Africa. In endemic areas, such as Australia, the CFR% is approximately 41% [11].
Melioidosis may result in a broad range of symptoms, such as pneumonia, fever, pain, cough, and headache. The disease often mimics the manifestations of more common pathogens leading to misdiagnosis and delayed treatment [2, 12]. Diagnosis based only on clinical manifestations is sometimes possible, but bacterial culture is the gold standard for proper diagnosis. Because of the nature of Burkholderia species, it may sometimes be dismissed as a contaminant, so appropriate training of clinical laboratory staff in endemic areas is important. In a presumed melioidosis case, clinical samples should be sent to laboratories with experience in identifying the bacterium. Common samples include blood, throat or wound swabs, and urine. Repeated sampling is needed as samples may initially test negative. B. pseudomallei grows slowly on most agar plates and may take a few days for visible detection. Antibiotic resistance profiles are helpful for identification, especially in settings with scarce resources. B. pseudomallei is resistant to gentamicin and polymyxin and sensitive to amoxicillin-clavulanic acid. This sensitivity pattern in a Gram-negative, oxidase-positive bacillus can be helpful for diagnosis. Molecular diagnostics are not common in most endemic areas. Proper clinical management must be initiated early in every suspect case. B. pseudomallei is susceptible to β-lactam antibiotics such as imipenem, meropenem, amoxicillin-clavulanic acid, and ceftazidime. In the eradication phase, trimethoprim/sulfamethoxazole is recommended [13].
Prevention may be achieved in endemic areas by avoiding high-risk activities and through additional protective measures for those at occupational risk. Often, this is difficult to achieve in poor countries with high B. pseudomallei endemicity. There is currently no licensed vaccine to prevent melioidosis. A vaccine could mitigate the public health burden of B. pseudomallei in areas of the world with high caseloads. In addition, B. pseudomallei has been classified as a Tier 1 select agent by the US Department of Health and Human Services and the US Department of Agriculture, due to its potential threat as a bioweapon. A vaccine to safeguard against B. pseudomallei misuse is therefore also highly desired.
2 Pathogenesis and Host Response
B. pseudomallei utilizes multiple virulence factors to invade human cells. This bacterium can enter mammalian cells via adherence using adhesion proteins such as fimbriae, type IV pili, flagellin [14,15,16], and components of the type V secretion system that work as autotransporters, such as BcaA, and the trimeric autotransporter adhesins BoaA and BoaB [17]. The type III secretion system proteins are used to invade and escape the endosome and are required for intracellular survival [18]. Type II, type III, and type VI secretion systems are required for the bacteria to exit the cells by membrane lysis [19, 20]. Autophagy is avoided by the effector protein BopA and translocator protein BipD [21,22,23]. Actin-based motility is mediated by BimA and leads to cell-to-cell fusion and the formation of multinucleated giant cells that are associated with granulomatous diseases, such as sarcoidosis and tuberculosis, complicating clinical diagnosis [24].
Immune responses to B. pseudomallei infection are triggered by pathogen-associated molecular patterns (PAMP) activation upon recognition of bacterial lipoproteins, lipopolysaccharide (LPS), and flagella by Toll-like receptor (TLR)2, TLR4, and TLR5, respectively, leading to a pro-inflammatory response mediated via NF-κΒ activation [25, 26]. Activation of complement pathway and destruction via the membrane attack complex is impaired by the presence of capsular polysaccharide (CPS), a major virulence determinant [27]. Activation of caspase 1 via the inflammasome leads to pyroptosis, releasing IL-1β and IL-18 [28, 29]. IL-18 promotes the induction of IFN-γ, helping to recruit additional macrophages. Macrophages that are activated via INF-γ produce nitric oxide that can kill sensitive strains [30]. However, some strains have developed resistance to oxidative stress by expressing superoxide dismutase C (sodC), alkyl hydroperoxide reductase C (ahpC), and a nonspecific DNA-binding protein known as dpsA [31, 32]. The release of IL-1β contributes to pathogenesis by recruiting neutrophils, which leads to tissue damage [33]. As commonly observed in other diseases, uncontrolled pro-inflammatory responses are associated with poor outcomes. An exacerbated release of IL-1β, IL-6, IL-12, TNF, and IFN-γ can cause severe melioidosis disease [27]. Both humoral and cell-mediated immune responses have been shown to protect against disease. Antibodies against LPS, O-polysaccharide (OPS), and Hcp-1 of B. pseudomallei are associated with better outcomes in melioidosis [27, 34]. CD4+ T cells that secrete IFN-γ are found in acute melioidosis patients and are thought to play a role in protection [35, 36]. However, further research is required to elucidate the innate or adaptive immune responses that are responsible for full protection against B. pseudomallei.
3 Vaccine Development
Over the last decade, several promising vaccine candidates have emerged using various approaches, and the most efficacious vaccines appear to be multivalent in nature. This is not entirely surprising, considering the sophisticated intracellular pathogenesis of B. pseudomallei that may require both arms of the immune response to target multiple antigens for complete protection. The various vaccine platforms include, but are not limited to, live-attenuated or inactivated whole-cell bacteria and multivalent subunit or conjugate vaccines. These are summarized below and listed in Table 15.1. The target bacterial antigens and their respective functions are described in Table 15.2.
3.1 Live-Attenuated Vaccines
A successful vaccine against B. pseudomallei will likely require both humoral and cellular immune responses for complete protection. Live-attenuated vaccines have long been considered the gold standard for achieving both arms of the immune response. Live-attenuated vaccines created by mutation of tonB and hcp1, genes belonging to a siderophore complex and the T6SS, respectively, induced robust immunity and significant protection in multiple studies [57, 58]. Deletion of purM created a highly attenuated strain (Bp82) that was avirulent in mice, Syrian hamsters, and immune incompetent mice [interferon (IFN)-γ−/−, severe combined immunodeficiency (SCID)] [59]. Immunization of mice with Bp82 conferred 100% survival over 60 days against pulmonary melioidosis [60]. Deletion of relA-spoT produced a B. pseudomallei double mutant that displayed defects in stationary-phase survival and replication in murine macrophages and was attenuated in acute and chronic mouse models of melioidosis. Immunization of mice with the ΔrelA ΔspoT live-attenuated strain resulted in full protection against infection with B. pseudomallei [39]. An asd mutant of B. pseudomallei 1026b was avirulent in BALB/c mice, and animals vaccinated with the mutant were protected against acute inhalation melioidosis [40]. Immunization with an aroC mutant also significantly protected mice against challenge [38]. Immunization with B. thailandensis strain E555, which possesses CPS, provided significant protection against B. pseudomallei challenge [42]. Collectively, these studies demonstrate the safety and protective efficacy of live-attenuated vaccines in animal models of melioidosis.
3.2 Inactivated Whole-Cell Vaccines
Historically, whole-cell, inactivated vaccines have not provided the same level of protection as live vaccines in rodent models of melioidosis. This has prompted novel approaches to improve their protective efficacy. Incorporation of cationic liposomes complexed with noncoding plasmid DNA (CLDC) to a heat-killed B. pseudomallei vaccine conferred 100% survival of immunized mice that lasted greater than 40 days [37]. In another study, synthetic microparticles composed of acetylated dextran with encapsulated B. pseudomallei cell lysate induced significant protection against a lethal challenge in mice [56].
3.3 Subunit Vaccines
One of the vaccine targets that have been extensively studied is the CPS, a known protective antigen and virulence determinant for B. pseudomallei. The CPS is a homopolymer of unbranched 1 → 3 linked 2-O-acetyl-6-deoxy-β-d-manno-heptopyranose. Immunization with B. pseudomallei CPS linked to CRM197, a nontoxic mutant of diphtheria toxin, elicited IgG and opsonizing antibody responses. Immunization with a combination of CPS-CRM197 and recombinant Hcp1 protected 100% of mice following an otherwise lethal inhalation challenge with B. pseudomallei [46]. In another study, immunization with chemically synthesized CPS conjugated to tetanus toxoid induced serum IgM and IgG antibodies. While the CPS-specific antibody titers were considerably less than that induced by native CPS, 66% of BALB/c mice survived challenge with B. pseudomallei [48].
Immunization with lipopolysaccharide (LPS) conjugated to the Hc fragment of tetanus toxoid produced a better survival outcome (81%) compared to immunization with LPS alone (62%) [44]. B. pseudomallei O-polysaccharide (OPS II) conjugated to a carrier protein AcrA from Campylobacter jejuni elicited similar levels of protection to killed bacteria against a highly lethal challenge [49]. LPS glycoconjugates have also been successfully incorporated onto the surface of gold nanoparticles (AuNP). Mice immunized with AuNP-FlgL-LPS alone or with a protein combination (FlgL, Hcp1, and hemagglutinin) demonstrated up to 100% survival and reduced lung colonization following a lethal challenge with B. pseudomallei [51].
Other protein subunits have been identified as possible candidates due to their immunogenicity, such as FliC, BopE, AhpC, and several outer membrane proteins (OMP) [62,63,64,65,66,66]. A DNA vaccine composed of flagellin reduced bacterial burdens, lung inflammation, and pathology. Levels of plasma TNF-α, IFN-γ, and MCP1 cytokines were also reduced, signaling a reduction in systemic inflammation. Vaccinated mice displayed 53% survival compared to 15% survival in control mice [45]. Mice immunized with a single dose of parainfluenza virus 5 (PIV5) expressing BatA, a Burkholderia autotransporter protein, displayed 60% overall survival, with 78% of surviving mice clearing bacteria in the lungs and 44% clearing bacteria in the spleen [67]. A synthetic biology approach was used to predict the antigenicity and toxicity of a recombinant collagen-like 8 protein that is conserved among Burkholderia species [68]. Two formulations were created, one with a recombinant protein and another one using the β-barrel portion of the protein. Use of β-barrel peptides from Buc (Bucl8) adjuvanted with an oil-in-water nano-emulsion produced a strong Th2 response in C57BL/6 mice.
B. pseudomallei outer membrane vesicles (OMVs) contain numerous protective antigens associated with the bacterial outer membrane including OmpA, CPS, and LPS. B. pseudomallei OMV vaccines have demonstrated safety, immunogenicity, and protection against pneumonic and septicemic disease in mice and nonhuman primates [53, 54, 69]. When OMVs are produced under macrophage mimicking growth conditions, they contain proteins that are associated with intracellular survival, conferring strong protection against inhalational melioidosis and eliciting both humoral and cellular immune responses [55]. Due to the sophisticated intracellular pathogenesis of B. pseudomallei, inclusion of antigens expressed during the chronic stage of infection may be important for obtaining complete vaccine protection. Three proteins, BPSL1897, BPSL2287, and BPSL3369, which were expressed in chronically infected mice, were combined with the surface protein OmpA (BPSL2765) to create a multicomponent subunit vaccine formulation. Mice immunized with the chronic antigens plus LolC or CPS displayed increased survival compared to mice immunized with only the individual subunits, LolC, or CPS [43].
4 Conclusions
In recent years, several promising vaccine candidates have emerged to combat melioidosis, which is considered an emerging and expanding infectious disease. Adjuvants are also being evaluated as part of vaccine development as they can drive different protective components of humoral and cellular immune responses. Going forward, it will be important to elucidate the immune mechanisms of protection to better inform vaccine design and to establish immune correlates of vaccine protection. Based on recent success in animal models, human clinical trials are currently being planned for more than one vaccine candidate. These include a phase 1 clinical trial of vaccine candidate CPS-CRM197/Hcp1 adjuvanted with alum/CpG that was developed at the University of Nevada, Reno, USA [46]. The trial will take place in Oxford in 36 healthy human subjects with a follow-up phase 1b planned in Ubon Ratchathani, Thailand. An OMV vaccine, developed at Tulane University, Louisiana, USA, is also being considered for phase 1 clinical trials planned in Australia [55]. For any vaccine, larger phase 2 and 3 clinical trials would be necessary to demonstrate vaccine safety and efficacy. Nonetheless, initiation of human vaccine trials for a disease once largely ignored sparks hope that licensure of human vaccines to prevent melioidosis may soon be realized.
References
Tumapa S, Holden MTG, Vesaratchavest M, Wuthiekanun V, Limmathurotsakul D, Chierakul W, et al. Burkholderia pseudomallei genome plasticity associated with genomic island variation. BMC Genomics. 2008;9:190. https://pubmed.ncbi.nlm.nih.gov/18439288/.
Wiersinga WJ, Virk HS, Torres AG, Currie BJ, Peacock SJ, Dance DAB, et al. Melioidosis. Nat Rev Dis Primers. 2018;4:17108.
Stone NE, Hall CM, Browne AS, Sahl JW, Hutton SM, Santana-Propper E, et al. Burkholderia pseudomallei in soil, US Virgin Islands, 2019. Emerg Infect Dis. 2020;26(11):2773.
2021 Multistate outbreak of melioidosis | Melioidosis | CDC [Internet]. https://www.cdc.gov/melioidosis/outbreak/2021/index.html. Accessed 29 Oct 2021.
Walmart Recalls Better Homes and Gardens Essential Oil Infused Aromatherapy Room Spray with Gemstones Due to Rare and Dangerous Bacteria; Two Deaths Investigated | CPSC.gov [Internet]. https://www.cpsc.gov/Recalls/2022/Walmart-Recalls-Better-Homes-and-Gardens-Essential-Oil-Infused-Aromatherapy-Room-Spray-with-Gemstones-Due-to-Rare-and-Dangerous-Bacteria-Two-Deaths-Investigated. Accessed 29 Oct 2021.
Chewapreecha C, Holden MTG, Vehkala M, Välimäki N, Yang Z, Harris SR, et al. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat Microbiol. 2017;2:16263. https://pubmed.ncbi.nlm.nih.gov/28112723/.
Currie BJ, Fisher DA, Anstey NM, Jacups SP. Melioidosis: acute and chronic disease, relapse and re-activation. Trans R Soc Trop Med Hyg. 2000;94(3):301–4. https://academic.oup.com/trstmh/article/94/3/301/1918300.
Kronsteiner B, Chaichana P, Sumonwiriya M, Jenjaroen K, Chowdhury FR, Chumseng S, et al. Diabetes alters immune response patterns to acute melioidosis in humans. Eur J Immunol. 2019;49(7):1092. /pmc/articles/PMC6618312/.
Wiersinga WJ, Currie BJ, Peacock SJ. Medical progress: melioidosis. N Engl J Med. 2012;367(11):1035–44. https://pubmed.ncbi.nlm.nih.gov/22970946/.
White NJ. Melioidosis. Lancet. 2003;361(9370):1715–22. https://pubmed.ncbi.nlm.nih.gov/12767750/.
Fong JH, Pillai N, Yap CG, Jahan NK, Fong JH, Pillai N, et al. Incidences, case fatality rates and epidemiology of melioidosis worldwide: a review paper. Open Access Libr J. 2021;8(6):1–20. http://www.scirp.org/journal/PaperInformation.aspx?PaperID=110072.
Meumann EM, Cheng AC, Ward L, Currie BJ. Clinical features and epidemiology of melioidosis pneumonia: results from a 21-year study and review of the literature. Clin Infect Dis. 2012;54(3):362. /pmc/articles/PMC3258273/.
Dance D. Treatment and prophylaxis of melioidosis. Int J Antimicrob Agents. 2014;43(4):310. /pmc/articles/PMC4236584/.
Lafontaine ER, Balder R, Michel F, Hogan RJ. Characterization of an autotransporter adhesin protein shared by Burkholderia mallei and Burkholderia pseudomallei. BMC Microbiol. 2014;14(1):92. https://pubmed.ncbi.nlm.nih.gov/24731253/.
Nithichanon A, Rinchai D, Gori A, Lassaux P, Peri C, Conchillio-Solé O, et al. Sequence-and structure-based immunoreactive epitope discovery for Burkholderia pseudomallei flagellin. PLoS Negl Trop Dis. 2015;9(7):1–20. https://pubmed.ncbi.nlm.nih.gov/26222657/.
Stone JK, DeShazer D, Brett PJ, Burtnick MN. Melioidosis: molecular aspects of pathogenesis. Expert Rev Anti Infect Ther. 2014;12(12):1487. /labs/pmc/articles/PMC4409121/.
Balder R, Lipski S, Lazarus JJ, Grose W, Wooten RM, Hogan RJ, et al. Identification of Burkholderia mallei and Burkholderia pseudomallei adhesins for human respiratory epithelial cells. BMC Microbiol. 2010;10:250. https://pubmed.ncbi.nlm.nih.gov/20920184/.
Vander Broek CW, Stevens JM. Type III secretion in the melioidosis pathogen Burkholderia pseudomallei. Front Cell Infect Microbiol. 2017;7:255. /pmc/articles/PMC5471309/.
French CT, Bulterys PL, Woodward CL, Tatters AO, Ng KR, Miller JF. Virulence from the rhizosphere: ecology and evolution of Burkholderia pseudomallei-complex species. Curr Opin Microbiol. 2020;54:18–32. https://pubmed.ncbi.nlm.nih.gov/32028234/.
Burtnick MN, Brett PJ, DeShazer D. Proteomic analysis of the Burkholderia pseudomallei type ii secretome reveals hydrolytic enzymes, novel proteins, and the deubiquitinase TssM. Infect Immun. 2014;82(8):3214–26. https://pubmed.ncbi.nlm.nih.gov/24866793/.
Selvam K, Khalid MF, Mustaffa KMF, Harun A, Aziah I. Bipd of Burkholderia pseudomallei: structure, functions, and detection methods. Microorganisms. 2021;9(4):711. https://pubmed.ncbi.nlm.nih.gov/33808203/.
Druar C, Yu F, Barnes JL, Okinaka RT, Chantratita N, Beg S, et al. Evaluating Burkholderia pseudomallei Bip proteins as vaccines and Bip antibodies as detection agents. FEMS Immunol Med Microbiol. 2008;52(1):78–87. https://pubmed.ncbi.nlm.nih.gov/17995960/.
Yu D, Yin Z, Jin Y, Zhou J, Ren H, Hu M, et al. Evolution of bopA gene in Burkholderia: a case of convergent evolution as a mechanism for bacterial autophagy evasion. Biomed Res Int. 2016;2016:6745028. https://pubmed.ncbi.nlm.nih.gov/28018913/.
Stockton JL, Torres AG. Multinucleated giant cell formation as a portal to chronic bacterial infections. Microorganisms. 2020;8:1–10. https://pubmed.ncbi.nlm.nih.gov/33113944/.
Amemiya K, Dankmeyer JL, Bearss JJ, Zeng X, Stonier SW, Soffler C, et al. Dysregulation of TNF-α and IFN-γ expression is a common host immune response in a chronically infected mouse model of melioidosis when comparing multiple human strains of Burkholderia pseudomallei. BMC Immunol. 2020;21(1):5. /pmc/articles/PMC6998218/.
Weehuizen TAF, Prior JL, van der Vaart TW, Ngugi SA, Nepogodiev SA, Field RA, et al. Differential toll-like receptor-signalling of Burkholderia pseudomallei lipopolysaccharide in murine and human models. PLoS One. 2015;10(12):e0145397. /pmc/articles/PMC4687033/.
Mariappan V, Vellasamy KM, Barathan M, Girija ASS, Shankar EM, Vadivelu J. Hijacking of the host’s immune surveillance radars by Burkholderia pseudomallei. Front Immunol. 2021;12:718719. /pmc/articles/PMC8384953/.
Bast A, Krause K, Schmidt IHE, Pudla M, Brakopp S, Hopf V, et al. Caspase-1-dependent and -independent cell death pathways in Burkholderia pseudomallei infection of macrophages. PLoS Pathog. 2014;10(3):1003986. /pmc/articles/PMC3953413/.
Lichtenegger S, Stiehler J, Saiger S, Zauner A, Kleinhappl B, Bernecker C, et al. Burkholderia pseudomallei triggers canonical inflammasome activation in a human primary macrophage-based infection model. PLoS Negl Trop Dis. 2020;14(11):e0008840. /pmc/articles/PMC7605897/.
Miyagi K, Kawakami K, Saito A. Role of reactive nitrogen and oxygen intermediates in gamma interferon- stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect Immun. 1997;65(10):4108–13. https://pubmed.ncbi.nlm.nih.gov/9317015/.
Vanaporn M, Wand M, Michell SL, Sarkar-Tyson M, Ireland P, Goldman S, et al. Superoxide dismutase C is required for intracellular survival and virulence of burkholderia pseudomallei. Microbiology. 2011;157(8):2392–400. https://pubmed.ncbi.nlm.nih.gov/21659326/.
Loprasert S, Whangsuk W, Sallabhan R, Mongkolsuk S. Regulation of the Katg-dpsA operon and the importance of KatG in survival of Burkholderia pseudomallei exposed to oxidative stress. FEBS Lett. 2003;542(1–3):17–21. https://pubmed.ncbi.nlm.nih.gov/12729890/.
Sahoo M, del Barrio L, Miller MA, Re F. Neutrophil elastase causes tissue damage that decreases host tolerance to lung infection with Burkholderia species. PLoS Pathog. 2014;10(8):e1004327. /pmc/articles/PMC4148436/.
Pumpuang A, Phunpang R, Ekchariyawat P, Dulsuk A, Loupha S, Kwawong K, et al. Distinct classes and subclasses of antibodies to hemolysin co-regulated protein 1 and O-polysaccharide and correlation with clinical characteristics of melioidosis patients. Sci Rep. 2019;9(1):1–14. https://www.nature.com/articles/s41598-019-48828-4.
Sengyee S, Yarasai A, Janon R, Morakot C, Ottiwet O, Schmidt LK, et al. Melioidosis patient survival correlates with strong IFN-γ secreting T cell responses against Hcp1 and TssM. Front Immunol. 2021;12:698303. https://pubmed.ncbi.nlm.nih.gov/34394091/.
Jenjaroen K, Chumseng S, Sumonwiriya M, Ariyaprasert P, Chantratita N, Sunyakumthorn P, et al. T-Cell responses are associated with survival in acute melioidosis patients. PLoS Negl Trop Dis. 2015;9(10):e0004152. https://pubmed.ncbi.nlm.nih.gov/26495852/.
Henderson A, Propst K, Kedl R, Dow S. Mucosal immunization with liposome-nucleic acid adjuvants generates effective humoral and cellular immunity. Vaccine. 2011;29(32):5304–12. https://pubmed.ncbi.nlm.nih.gov/21600950/.
Srilunchang T, Proungvitaya T, Wongratanacheewin S, Strugnell R, Homchampa P. Construction and characterization of an unmarked aroC deletion mutant of Burkholderia pseudomallei strain A2. Southeast Asian J Trop Med Public Health. 2009;40(1):123–30. https://pubmed.ncbi.nlm.nih.gov/19323044/.
Müller CM, Conejero L, Spink N, Wand ME, Bancroft GJ, Titball RW. Role of RelA and SpoT in Burkholderia pseudomallei virulence and immunity. Infect Immun. 2012;80(9):3247–55. https://pubmed.ncbi.nlm.nih.gov/22778096/.
Norris MH, Propst KL, Kang Y, Dow SW, Schweizer HP, Hoang TT. The Burkholderia pseudomallei Δasd mutant exhibits attenuated intracellular infectivity and imparts protection against acute inhalation melioidosis in mice. Infect Immun. 2011;79(10):4010–8. https://pubmed.ncbi.nlm.nih.gov/21807903/.
Khakhum N, Bharaj P, Myers JN, Tapia D, Kilgore PB, Ross BN, et al. Burkholderia pseudomallei Δ tonB Δ hcp1 live attenuated vaccine strain elicits full protective immunity against aerosolized melioidosis infection. mSphere. 2019;4(1):e00570–18. https://pubmed.ncbi.nlm.nih.gov/30602524/.
Scott AE, Laws TR, D’Elia RV, Stokes MGM, Nandi T, Williamson ED, et al. Protection against experimental melioidosis following immunization with live Burkholderia thailandensis expressing a manno-heptose capsule. Clin Vaccine Immunol. 2013;20(7):1041–7. https://pubmed.ncbi.nlm.nih.gov/23677322/.
Champion OL, Gourlay LJ, Scott AE, Lassaux P, Conejero L, Perletti L, et al. Immunisation with proteins expressed during chronic murine melioidosis provides enhanced protection against disease. Vaccine. 2016;34(14):1665–71. https://pubmed.ncbi.nlm.nih.gov/26917010/.
Scott AE, Ngugi SA, Laws TR, Corser D, Lonsdale CL, D’Elia RV, et al. Protection against experimental melioidosis following immunisation with a lipopolysaccharide-protein conjugate. J Immunol Res. 2014;2014:1. https://pubmed.ncbi.nlm.nih.gov/24892035/.
Lankelma JM, Wagemakers A, Birnie E, Haak BW, Trentelman JJA, Weehuizen TAF, et al. Rapid DNA vaccination against Burkholderia pseudomallei flagellin by tattoo or intranasal application. Virulence. 2017;8(8):1683–94. https://pubmed.ncbi.nlm.nih.gov/28323523/.
Burtnick MN, Shaffer TL, Ross BN, Muruato LA, Sbrana E, DeShazer D, et al. Development of subunit vaccines that provide high-level protection and sterilizing immunity against acute inhalational melioidosis. Infect Immun. 2018;86(1):e00724–17. /pmc/articles/PMC5736816/.
Scott AE, Burtnick MN, Stokes MGM, Whelan AO, Williamson ED, Atkins TP, et al. Burkholderia pseudomallei capsular polysaccharide conjugates provide protection against acute melioidosis. Infect Immun. 2014;82(8):3206. /pmc/articles/PMC4136211/.
Scott AE, Christ WJ, George AJ, Stokes MGM, Lohman GJS, Guo Y, et al. Protection against experimental melioidosis with a synthetic manno-heptopyranose hexasaccharide glycoconjugate. Bioconjug Chem. 2016;27(6):1435–46. https://pubmed.ncbi.nlm.nih.gov/27124182/.
Garcia-Quintanilla F, Iwashkiw JA, Price NL, Stratilo C, Feldman MF. Production of a recombinant vaccine candidate against Burkholderia pseudomallei exploiting the bacterial N-glycosylation machinery. Front Microbiol. 2014;5:381. https://pubmed.ncbi.nlm.nih.gov/25120536/.
Scott AE, Ngugi SA, Laws TR, Corser D, Lonsdale CL, D’Elia RV, et al. Protection against experimental melioidosis following immunisation with a lipopolysaccharide-protein conjugate. J Immunol Res. 2014;2014:392170. https://pubmed.ncbi.nlm.nih.gov/24892035/.
Muruato LA, Tapia D, Hatcher CL, Kalita M, Brett PJ, Gregory AE, et al. Use of reverse vaccinology in the design and construction of nanoglycoconjugate vaccines against Burkholderia pseudomallei. Clin Vaccine Immunol. 2017;24(11):e00206–17. https://pubmed.ncbi.nlm.nih.gov/28903988/.
Tapia D, Sanchez-Villamil JI, Stevenson HL, Torres AG. Multicomponent gold-linked glycoconjugate vaccine elicits antigen-specific humoral and mixed TH1-TH17 immunity, correlated with increased protection against Burkholderia pseudomallei. MBio. 2021;12(3):e0122721. https://pubmed.ncbi.nlm.nih.gov/34182777/.
Nieves W, Asakrah S, Qazi O, Brown KA, Kurtz J, Aucoin DP, et al. A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine. 2011;29(46):8381–9. https://pubmed.ncbi.nlm.nih.gov/21871517/.
Nieves W, Petersen H, Judy BM, Blumentritt CA, Russell-Lodrigue K, Roy CJ, et al. A Burkholderia pseudomallei outer membrane vesicle vaccine provides protection against lethal sepsis. Clin Vaccine Immunol. 2014;21(5):747–54. https://pubmed.ncbi.nlm.nih.gov/24671550/.
Baker SM, Settles EW, Davitt C, Gellings P, Kikendall N, Hoffmann J, et al. Burkholderia pseudomallei OMVs derived from infection mimicking conditions elicit similar protection to a live-attenuated vaccine. NPJ Vaccines. 2021;6(1):1–10. https://www.nature.com/articles/s41541-021-00281-z.
Schully KL, Bell MG, Prouty AM, Gallovic MD, Gautam S, Peine KJ, et al. Evaluation of a biodegradable microparticulate polymer as a carrier for Burkholderia pseudomallei subunit vaccines in a mouse model of melioidosis. Int J Pharm. 2015;495(2):849–61. https://pubmed.ncbi.nlm.nih.gov/26428631/.
Khakhum N, Bharaj P, Myers JN, Tapia D, Kilgore PB, Ross BN, et al. Burkholderia pseudomallei ΔtonB Δhcp1 live attenuated vaccine strain elicits full protective immunity against aerosolized melioidosis infection. mSphere. 2019;4(1):e00570–18. /labs/pmc/articles/PMC6315081/.
Khakhum N, Bharaj P, Walker DH, Torres AG, Endsley JJ. Antigen-specific antibody and polyfunctional T cells generated by respiratory immunization with protective Burkholderia ΔtonB Δhcp1 live attenuated vaccines. NPJ Vaccines. 2021;6(1):72. https://pubmed.ncbi.nlm.nih.gov/33986290/.
Propst KL, Mima T, Choi KH, Dow SW, Schweizer HP. A Burkholderia pseudomallei deltapurM mutant is avirulent in immunocompetent and immunodeficient animals: candidate strain for exclusion from select-agent lists. Infect Immun. 2010;78(7):3136–43. https://pubmed.ncbi.nlm.nih.gov/20404077/.
Silva EB, Goodyear A, Sutherland MD, Podnecky NL, Gonzalez-Juarrero M, Schweizer HP, et al. Correlates of immune protection following cutaneous immunization with an attenuated Burkholderia pseudomallei vaccine. Infect Immun. 2013;81(12):4626–34. https://pubmed.ncbi.nlm.nih.gov/24101688/.
Harding SV, Sarkar-Tyson M, Smither SJ, Atkins TP, Oyston PCF, Brown KA, et al. The identification of surface proteins of Burkholderia pseudomallei. Vaccine. 2007;25(14):2664–72. https://pubmed.ncbi.nlm.nih.gov/17289218/.
Suwannasaen D, Mahawantung J, Chaowagul W, Limmathurotsakul D, Felgner PL, Davies H, et al. Human immune responses to Burkholderia pseudomallei characterized by protein microarray analysis. J Infect Dis. 2011;203(7):1002. /labs/pmc/articles/PMC3068035/.
Tippayawat P, Saenwongsa W, Mahawantung J, Suwannasaen D, Chetchotisakd P, Limmathurotsakul D, et al. Phenotypic and functional characterization of human memory T cell responses to Burkholderia pseudomallei. PLoS Negl Trop Dis. 2009;3(4):e407. https://pubmed.ncbi.nlm.nih.gov/19352426/.
Tippayawat P, Pinsiri M, Rinchai D, Riyapa D, Romphruk A, Gan YH, et al. Burkholderia pseudomallei proteins presented by monocyte-derived dendritic cells stimulate human memory T cells in vitro. Infect Immun. 2011;79(1):305–13. https://pubmed.ncbi.nlm.nih.gov/21041491/.
Reynolds C, Goudet A, Jenjaroen K, Sumonwiriya M, Rinchai D, Musson J, et al. T Cell Immunity to the alkyl hydroperoxide reductase of Burkholderia pseudomallei: a correlate of disease outcome in acute melioidosis. J Immunol Author Choice. 2015;194(10):4814. /labs/pmc/articles/PMC4416739/.
Koosakulnirand S, Phokrai P, Jenjaroen K, Roberts RA, Utaisincharoen P, Dunachie SJ, et al. Immune response to recombinant Burkholderia pseudomallei FliC. PLoS One. 2018;13(6):e0198906. /labs/pmc/articles/PMC6002054/.
Lafontaine ER, Chen Z, Huertas-Diaz MC, Dyke JS, Jelesijevic TP, Michel F, et al. The autotransporter protein BatA is a protective antigen against lethal aerosol infection with Burkholderia mallei and Burkholderia pseudomallei. Vaccine X. 2019;1:100002.
Grund ME, Kramarska E, Choi SJ, McNitt DH, Klimko CP, Rill NO, et al. Predictive and experimental immunogenicity of Burkholderia collagen-like protein 8-derived antigens. Vaccine. 2021;9(11):1219. https://pubmed.ncbi.nlm.nih.gov/34835150/.
Petersen H, Nieves W, Russell-Lodrigue K, Roy CJ, Morici LA. Evaluation of a Burkholderia pseudomallei outer membrane vesicle vaccine in nonhuman primates. Proc Vaccinol. 2014;8:38–42. https://pubmed.ncbi.nlm.nih.gov/25165491/.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Galeas-Pena, M., Morici, L.A. (2023). Vaccine Development Against Melioidosis. In: Christodoulides, M. (eds) Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges . Springer, Cham. https://doi.org/10.1007/978-3-031-24355-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-24355-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24354-7
Online ISBN: 978-3-031-24355-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)