Abstract
Controlled human infection models (CHIMs) are increasingly recognised as having an important role in the early development of vaccines for important human diseases, including those prevalent in low and middle-income countries. The leishmaniases are a group of clinically disparate parasitic diseases caused by multiple species of Leishmania. Widely heralded as potentially vaccine-preventable, progress in vaccine development for different forms of leishmaniasis has over past decades been slow, hampered by lack of funds, good experimental models and the challenges of progression through the normal clinical trial pathway. However, with a new generation of leishmaniasis vaccine candidates now progressing in clinical development, the value of a robust CHIM able to accelerate early-phase evaluation of new vaccine candidates has become increasingly apparent. Here, we briefly review the historic context of human infection studies in leishmaniasis and outline issues pertinent to the development of a new CHIM of sand fly-transmitted Leishmania major infection. Given the diversity and wide geographic distribution of the leishmaniases, we conclude with a discussion of future needs and challenges in the development of CHIMs for these important neglected diseases.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The leishmaniases are poverty-related neglected diseases with a major impact on health worldwide [1,2,3]. Caused by infection with one of several species of Leishmania parasite, disease manifestations can be broadly classified as tegumentary (affecting the skin and mucosa) or systemic (visceral leishmaniasis, VL, affecting the internal organs). With approximately 1 M reported cases of tegumentary leishmaniasis each year and 50,000–90,000 reported cases and up to 20,000 deaths from VL, the leishmaniases rank amongst the most important of the WHO’s Neglected Tropical Diseases [4]. No vaccines are currently licensed for any form of human leishmaniasis, despite licensure of four vaccines for canine leishmaniosis [5], and, with a limited drug arsenal increasingly compromised by drug resistance [6], the need for new approaches to disease control, including vaccination, remains pressing.
The tegumentary leishmaniases represent a complex spectrum of diseases, including localised cutaneous leishmaniasis, disseminated cutaneous leishmaniasis, diffuse cutaneous leishmaniasis and mucocutaneous leishmaniasis. Diverse clinical forms and different geographies reflect the distribution of distinct parasite species belonging to two sub genera, L. (Leishmania) in the Old and New World and L. (Viannia) in the New World [2]. In contrast just two species, L. donovani and L. infantum (previously called. L. chagasi in the New World), are primarily responsible for VL, though other species are occasionally implicated [7, 8]. In addition, the variable presence of endosymbiotic Leishmania viruses [9], the increasing recognition of inter-species hybrids [10,11,12] and recent evidence that even single nucleotide polymorphisms within a species can contribute to diverse clinical outcomes [13] all add to the complexity of these diseases and may pose challenges for vaccine development.
Ninety percent of the VL burden lies in five countries (India, Bangladesh, Nepal, Sudan and Brazil). With the reduction in cases in South Asia, associated with a trinational elimination campaign that included use of single-dose AmBisome treatment and indoor residual spraying [14], Sudan may now harbour the greatest burden of VL of any single country, with a case fatality rate of between 1.1% and 4.8% [15], in part due to the ineffectiveness of AmBisome is East Africa and the reliance on sodium stibogluconate/paromomycin combination therapy [16]. HIV co-infection, which worsens the prognosis for VL patients in all regions, brings additional challenges for patient management [17,18,19,20]. Post-kala-azar dermal leishmaniasis (PKDL) is a severe and chronic form of tegumentary leishmaniasis that usually develops after treatment for VL caused by L. donovani but which can occur in the absence of previous VL or concomitant with VL therapy (para-kala-azar dermal leishmaniasis) [21]. PKDL is found in South Asia and East Africa, with several thousand cases estimated to occur each year. Many PKDL patients do not receive treatment and act as reservoirs for the transmission of VL [22, 23]. A lack of tools to prevent and/or treat PKDL is now a well-recognised challenge to VL elimination campaigns [24]. Like other forms of tegumentary leishmaniasis, PKDL significantly affects quality of life and can result in life-long stigmatisation [25], a feature of these diseases that is only recently beginning to be fully appreciated in terms of its impact on measurements of morbidity associated with leishmaniasis [26].
1.1 Leishmaniasis: A Vaccine-Preventable Disease
The leishmaniases are commonly accepted to be potentially vaccine-preventable diseases [27,28,29], and recent modelling highlights the potential benefits of vaccination as an additional tool to support VL elimination efforts in South Asia [30]. A recent and timely call for action to eliminate VL from East Africa expounds the potential benefits of therapeutic vaccination as an additional treatment option [31]. However, it makes no mention of the benefits of a prophylactic vaccine, perhaps reflecting perceptions of the challenge ahead.
Compared to many eukaryotic parasites, the Leishmania life cycle in the mammalian host is relatively simple. Infection is initiated by the introduction of metacyclic promastigotes into the skin during the bite of an infected phlebotomine sand fly. These highly motile parasites are rapidly engulfed by myeloid cells, including neutrophils, monocytes and resident dermal macrophages, the first of these often serving as an intermediate “shuttle” into the latter [32]. Within monocytes and macrophages, metacyclic parasites differentiate into non-motile intracellular amastigotes that reside within a parasitophorous vacuole that bears the hallmarks of a phagolysosome [33]. Intracellular amastigotes replicate and invade new host cells, though the precise mechanism involved in cell-cell spread remains unclear. Uncovering how Leishmania spreads cell to cell within tissues may open new approaches for vaccine design. Infected cells containing amastigotes disseminate locally or to more distant sites, though again how this occurs and whether it is a pathway amenable for therapeutic intervention are not entirely clear. Recent studies have also pointed to the presence of dormant or quiescent amastigotes [34], somewhat akin to “persisters” observed in tuberculosis [35]. Besides this diversity in metabolic activity within amastigotes, there is limited parasite diversity in the mammalian life cycle, with, for example, no formal mechanisms of antigenic variation or morphologically distinguishable transmissible form equivalent to the trypomastigote of Trypanosoma cruzi having been discovered to date. Thus, the amastigote represents a largely invariant target for the immune system. The longevity of these infections is therefore principally attributable to the ability of amastigotes to manipulate host cell function, directly affecting the infected myeloid cell or indirectly, through the release of virulence determinants in exosomes, affecting the function of other immune cells [36].
Despite this apparent simplicity in lifestyle, leishmaniasis has proved challenging from the perspective of vaccine development. First-generation prophylactic Leishmania vaccines composed of whole killed (autoclaved) promastigotes often adjuvanted with Bacillus Calmette-Guérin (BCG) were not efficacious [37]. Second-generation recombinant polyprotein vaccines adjuvanted with a variety of lipid-based adjuvants have entered early-phase clinical trials (often as therapeutic vaccines) but have not been progressed [38]. A third-generation adenovirus-vectored vaccine, ChAd63-KH, has progressed to Phase II as a therapeutic in PKDL patients [39, 40] and a genetically modified attenuated live vaccine (L. major Cen−/−) is approaching Good Manufacturing Practice (GMP) and likely to enter Phase I in 2023 [28, 41]. ChAd63-KH is based on a well-characterised simian adenovirus backbone (ChAd63), extensively tested in human volunteers and shown to have an excellent safety record [42]. ChAd-vectored vaccines induce potent CD8+ and CD4+ T-cell responses and antibodies in humans and are amenable to scalable manufacture at GMP, as evidenced by their use in the control of the COVID-19 pandemic [43]. ChAd63-KH encodes two Leishmania antigens, kinetoplastid membrane protein-11, a highly conserved protein in all Leishmania spp., and hydrophilic acylated surface protein B, engineered to increase strain coverage [44]. ChAd63-KH therefore fulfils many of the criteria for a pan-leishmaniasis vaccine. Likewise, the L. major Cen−/− vaccine has been shown to provide protection against both cutaneous and visceral leishmaniasis in rodent models, including after sand fly challenge [45]. Current approaches to Leishmania vaccine antigen discovery and vaccine development are further discussed elsewhere in this book (Chaps. 11, 13 and 14).
1.2 The Case for Controlled Human Infection
Controlled human infection models provide the unique opportunity of monitoring the course of infection from a defined starting point to a defined endpoint (be that clinical, microbiological or immunological) [46,47,48,49]. They uniquely offer delivery of important insights into the pathogenesis of disease (including characterisation of the incubation period), the identification of early correlates of protection or disease progression and a clearer understanding of the relationship between pathogen load, immunity and transmission. For the vaccine developer, they can play a central role in increasing efficiency and robustness of candidate selection and provide for important time and cost reductions. These attributes are of relevance where target disease incidence is low, and hence there is a need for large-scale efficacy trials under conditions of natural exposure. For example, a recent feasibility study on the development of a Nipah vaccine indicated that even in an epidemic setting in Bangladesh, Phase III trials might require decades and millions of doses for completion [50]. Similarly, in the case of VL, low incidence in even the most highly endemic regions would make conventional Phase III trials almost impossible [51].
The relative ease with which CHIMs can be conducted and their small scale also favours the application of newer more efficient adaptive clinical trials, particularly when multiple candidate vaccines are available for comparison, when a range of dosing schedules or formulations need to be evaluated or when strain/species-specific efficacy needs to be demonstrated. In the context of pandemic viral infections, the use of CHIMs can also extend to the rapid evaluation of new variant-specific vaccine candidates and for the understanding of the value of public health measures [52]. This may be increasingly applicable in the face of our changing understanding of Leishmania genetic diversity. CHIMs may also pose risks, however, including clinical risks associated with the nature of the infectious challenge and risks related to the ethical perception of experimentation on humans. These ethical and societal concerns are of particular importance in developing CHIMs for endemic countries [53, 54]. The risk of oversimplification in a CHIM is also ever present, supporting a view that CHIMs for neglected diseases should also be established outside centres of excellence in the Global North, where confounding factors may be more matched to those where vaccines would be deployed.
2 Historical Perspective of Experimental Human Infections with Leishmania
2.1 The Early Years and Leishmanization: From Community to Mass Usage
In the tradition of tropical medicine at the time, once the parasites causing leishmaniasis had been identified, studies focused on identifying the mode of parasite transmission, leading to the first study involving the deliberate inoculation of parasites into human subjects in the early 1900s. The history of these early human infection studies, and their contribution to our understanding of leishmaniasis is described in detail elsewhere [47, 55, 56]. These scientifically led experimental studies were, however, predated by a local practice in endemic countries that sought to limit the disfigurement associated with leishmaniasis.
“Leishmanization” is the named coined for the deliberate intradermal inoculation of infectious material derived from an active cutaneous leishmaniasis lesion into a healthy person to promote immunity in that individual. The historic record is unclear when the process of leishmanization began, but it had likely been practiced across the Middle East for centuries, akin to variolation for smallpox. These practices, predating Jenner’s adaptation of using a heterologous agent to provide protection, clearly demonstrated the ability of live infection to produce resistance to reinfection and represent a key evidence base supporting the argument that leishmaniasis is vaccine-preventable. Though widely practiced, leishmanization was discontinued as a public health tool due to safety concerns about excessive lesion development in the immunocompromised and poor standardisation, though many millions are likely to have benefited from its use. It provides the practical and theoretical basis for human infection models developed in the modern era.
2.2 Using Leishmanization for Vaccine Evaluation
In 1979, Greenblatt and colleagues reported on the results of inoculating 39 soldiers in a hyperendemic area of Israel with a previously frozen isolate of Leishmania tropica major. All developed lesions, with 39% (12/31 examined) having ulcerated lesions and 61% (19/31) having nodular lesions at 1 month postinoculation. Further trials of this frozen isolate in 257 soldiers (151 males, 106 females) using needle and intradermal injector delivery methods showed more variable take rates, also dependent upon storage conditions and dose, though with no obvious differences due to the sex of the participants [57].
Khamesipour and colleagues updated this approach and further expounded the use of leishmanization as a means to evaluate vaccines [58]. In a key study conducted in Iran, 23 participants were first inoculated intradermally with 5 × 105 stationary phase L. major (MRHO/IR/75/ER), produced under GMP from a seed bank first used for leishmanization during the Iran-Iraq war. Overall take rate was 83% (19/23) with induration around the inoculation site of 17.4 ± 10.7 mm (mean, SD; n = 18 measured). This excellent take rate was achieved despite only 6% of the parasite inoculum being judged as viable based on motility. Ulceration was observed in 17 participants (7.97 ± 4.4 mm diameter; n = 18 measured) with a mean time to onset of 66 ± 23 days (n = 19). The remaining two participants developed ulcerated lesions on day 105 and 150, respectively. Self-healing occurred between day 75 and day 285 with a mean duration from onset to scar of 166 ± 67.7 days (n = 19). Two participants had a scar of 3 cm diameter, though, in general, scars were small (8.4 ± 6.2 mm; n = 18). Healing was associated with leishmanin skin test (LST) conversion in 100% (11/11) of participants tested, whereas 100% (3/3) of non-takes tested remained LST negative. After 18 months, 14 participants from the original study (11 takes and 3 non-takes) were subsequently rechallenged along with 5 new participants not previously exposed. Apart from one outlier that developed a very late ulcer (day 330), four of the five new participants developed lesions of similar size and magnitude as in the first study, whereas none of the 11 previous “takes” developed a lesion after rechallenge. Only one out of three “non-takes” developed a lesion on secondary challenge. These results are in line with an expectation that first exposure conferred protective immunity and the observation that not all exposed individuals develop disease. The authors concluded that this approach may be of value for rapid vaccine evaluation, given that a clinical readout could be obtained in approximately 2 months for most individuals. In addition, the immunity induced by the process of leishmanization itself would provide enhanced protection even in vaccine-naive individuals (i.e. those receiving a placebo), providing benefit to all participants in a clinical trial.
3 Ethical, Regulatory and Scientific Advances to Incorporate in a Modern-Day CHIM
The ethical and regulatory framework governing CHIMs is under constant review and subject to regional differences in interpretation and practice. However, some common guidelines are emerging regarding manufacturing principles and the conduct of such studies. There is also the recognition that CHIM studies may support regulatory approval of vaccines [59]. For leishmaniasis, further evolution of the model described by Khamesipour et al. has considered many of these new recommendation and guidelines, resulting in a CHIM that places more emphasis on the importance of vector transmission, greater quality control in terms of the challenge agent and more restrictive clinical endpoints (see below).
3.1 The Importance of Vector Transmission
Virulence of Leishmania is now recognised to be attributable to a combination of both parasite and vector (including vector microbiota-related) factors [60]. Precisely how these various factors collectively ensure high infectivity of metacyclic promastigotes and, equally surprisingly, how they may even contribute to late stages of disease remain to be unravelled. Critically, it has been observed that when tested in experimental models, at least one candidate vaccine, a killed vaccine comprised of autoclaved L. major antigen (ALM) + CpG oligodeoxynucleotides that showed protection against needle challenge failed to show similar protection after sand fly challenge [61]. Whilst the door remains open for development of CHIMs that use needle challenge (with potentially greater ease of use and standardisation of infectious dose), the scientific case for employing vector transmission as part of a CHIM, as for malaria [62], is at present compelling.
3.2 Regulatory Standards and Other Risk Mitigation
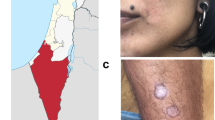
Increased awareness of the potential for human adventitious agents to contaminate challenge agents during manufacture, notably those associated with spongiform encephalopathies and HIV, has led to an increasing emphasis on understanding the provenance of challenge agents used in CHIM studies. To circumvent deficiencies in the record related to most stocks of Leishmania available from repositories, the identification and characterisation of new challenge strains were deemed essential for a modern-day CHIM. As such, a new strain of L. major (L. major MHOM/IL/2019/MRC-02) was sourced directly from a patient who developed cutaneous leishmaniasis after exposure during a hiking trip in an endemic region of Israel. The adult male patient was screened as negative for HIV, HTLV-1, HBV and HCV and had presented with two lesions of approximately 1.5 cm diameter on the shin and a smaller lesion on the neck. He refused treatment, and the lesions all spontaneously resolved 3–4 months later leaving a scar. The patient has been followed up for over 2 years with no recurrence of leishmaniasis or any other unexpected clinical events of note. Parasites were isolated and frozen at passage one as a seed bank, after brief culture in media certified as free of agents causing spongiform encephalopathies. This seed stock was used directly to prepare pre-GMP research banks for quality control and further analysis and the final GMP clinical bank. Minimising the number of passages in vitro was deemed to be an important factor to help maintain virulence, although to some extent any loss of virulence may also be mitigated by vector transmission.
Further risk mitigation was achieved by confirmation of parasite species identity by next-generation sequencing, confirmation of the absence of Leishmania viruses (LRV1), evaluation for infectivity and drug sensitivity in pre-clinical models and effective development in sand fly vectors. Sand fly colonies used for these studies (and subsequently for the CHIM) were screened to eliminate any risks associated with sand fly transmitted viral diseases [63]. Prior to exposing participants to infective sand fly bites, volunteers were also evaluated to ensure the safety and reproducibility of the sand fly biting procedure, independently of infection (the FLYBITE study; ClinicalTrials.gov Identifier NCT03999970). Included within this study was an evaluation of the public perception of the proposed CHIM protocol [64, 65], emphasising the role of public participation and involvement in the design of studies of this nature.
To establish optimum conditions for a CHIM that could be beneficial for vaccine evaluation, participants from the UK were sought. Altruism was a major factor behind volunteers agreeing to participate in these studies. Unlike the previous studies of Khamesipour et al. [58], participants were unlikely to derive any direct benefit from the procedure itself, though arguably some protection might be afforded to them if they were to travel to a leishmaniasis-endemic country. Hence, alongside development of the model itself, risk mitigation for CHIMs might also include research that seeks to ensure that vaccines evaluated using CHIMs have both a route to market and can deliver public health good within the constraints of endemic country health service. Recent “vaccine agnostic” appraisals incorporating the ability of countries to pay for leishmaniasis vaccines and the manufacturing demand are encouraging that this principle can be met [66, 67]. Benefit directly accrued for the individual will, of course, increase, if CHIMs are conducted directly in endemic areas, where the “leishmanization” effect of the challenge would be more evident.
4 A Leishmania major CHIM Initiated by Sand Fly Bite
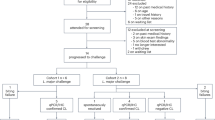
The LEISH_CHALLENGE study (ClinicalTrials.gov Identifier NCT04512742) was approved on 8 December 2020 by the UK Health Research Authority (IRAS ID 286420) with a positive ethical opinion provided by the South Central—Hampshire a Research Ethics Committee (REC reference 20/SC/0348). Key attributes of the CHIM are shown in Fig. 12.1.
Key components of the Leishmania CHIM. The figure summarises the key components of a recently developed CHIM for sand fly transmitted Leishmania major infection. For further details, see text. (Created with BioRender.com)
Following an adaptive study design, up to 18 participants are scheduled to be enrolled and exposed (in cohorts of 6) to the bite of five L. major MRC-02-infected Phlebotomus dubosqi. Sand flies are placed in a watch-like biting chamber which participants wear for 30 min (details of the sand fly biting procedure are provided elsewhere [64]). Clinical and immunological follow-up is expected to be at least 28 days and until a lesion diameter of approximately 6 mm is achieved. At this time, the lesion will be excised with a margin of normal looking skin under local anaesthesia, removing parasites and providing some therapeutic benefit. The biopsy site will be sutured and allowed to heal spontaneously. Recovered tissue will be used for parasitological confirmation of leishmaniasis and for immunological evaluation using conventional techniques to evaluate T-cell and antibody responses, as well as state of the art approaches in spatial and single-cell transcriptomics. If a secondary lesion develops, cryotherapy or other treatment options may be used. The first cohort of volunteers were enrolled and challenged in January 2022. Results from this study will be reported elsewhere.
One feature of this new CHIM that clearly sets it apart from the original model used by Khamesipour et al. [58] is the use of natural sand fly transmission. Apart from introducing elements from the sand fly that facilitate infectivity, the forced differentiation of the GMP parasite stock through the natural vector may serve to compensate for any loss of virulence that might occur during long-term frozen storage of parasites [57]. Another distinction is the early termination of the lesion by excision biopsy. This approach was adopted to minimise potential treatment-associated discomfort to participants whilst retaining a clinical window that should provide sufficient opportunity to evaluate the protective value of a vaccine in terms of (1) attack rate (i.e. the proportion of participants developing lesions), (2) rate of lesion development and (3) parasite load. Focus group studies of potential participants provided strong support for lesion excision as treatment [62]. A possible limitation of this approach may be that de-bulking parasites in this way may somehow limit the naturally acquired immunity induced by “leishmanization” [58]. However, this seems unlikely given that studies in the L. major-BALB/c mouse model suggest that low numbers of parasites may in fact have a greater ability to induce cell-mediated immune protection than that which occurs following full-blown infection [68] and the finding that two of three non-take participants in the Khamesipour study appeared to be protected against secondary challenge [58].
5 Accommodating Diversity: Leishmania CHIMs in Endemic Country Settings
5.1 CHIMs Using Other Leishmania Strains (Including Genetically Modified Strains)
L. major represents arguably the safest parasite species with which to conduct CHIM studies, given the focal nature of lesions and lack of reported disease reactivation following immune suppression. We have an incomplete understanding of the patterns of cross protection between species of Leishmania derived from rodent models and human epidemiology [27] and no evidence for or against cross protection based on human vaccine trials. In rodent models, L. major and L. donovani have been shown to induce cross species protection [69], and L. major Cen−/− cross protects against sand fly-transmitted visceral leishmaniasis due to L. donovani [45]. At least in the Old World, therefore, there is good reason to suspect that results from the use of a L. major CHIM might inform on the likely value of vaccines targeting VL or cutaneous leishmaniasis caused by L. tropica, as well as directly informing on vaccine protection against L. major.
In the New World and in the case of L. aethiopica in the Old World [70], patterns of disease are more challenging and diverse. At the present time, with our lack of complete understanding of the determinants or predictors of these complex manifestations and a paucity of effective treatments, it seems unlikely that the use of wild-type parasites of these species in a CHIM would be acceptable. Studies on cross protection in rodent models have been somewhat hampered by their failure to faithfully recapitulate these diverse clinical syndromes seen in humans. Nevertheless, a L. donovani fucose-mannose ligand vaccine cross-protected against L. mexicana (as well as L. infantum) [71] and a DNA vaccine encoding L. amazonensis P4 nuclease protected against both L. amazonensis and L. major, albeit with the need for different adjuvants [72]. This latter study suggests that if specific correlates of protection were available for different forms of leishmaniasis, a single-species CHIM could prove valuable in assessing how a vaccine containing conserved antigens might be tailored using different doses, routes or co-administered immune modulators to generate such species-selective responses.
CRSIPR-Cas9 allows rapid generation of unmarked strains defective in putative virulence factors or disabled in terms of replication competence [73,74,75]. One such strain, L. major Cen−/− [45] has been extensively assessed for its potential as a live attenuated vaccine for leishmaniasis, with impressive performance in animal models of cutaneous and visceral leishmaniasis, including those using sand fly challenge. There is clearly the potential to use this or other strains as future challenge agents, including a recently described L. braziliensis Cen−/− strain [76], though some hurdles remain. GMP manufacture and first-in-human studies of these genetically modified strains will need to be conducted to assess safety and growth characteristics in healthy subjects. Whilst there is sufficient replication in animals to promote long-lived immunity akin to leishmanization (at least in the case of the L. major Cen−/− strain), lesion development in animals is muted or absent, and if this was recapitulated in humans, alternate clinical or parasitological endpoints would also be required for such strains to be used as challenge agents.
5.2 Sand Fly Diversity and Other Confounders
The intimate relationship between Leishmania and its vector is well-known, but only limited studies have directly addressed vector competence using transmission experiments and compared molecular mechanisms associated with vector-enhanced parasite infectivity. Laboratory-reared sand flies may also have their own specific and distinct microbiota, and how this may contribute to immune modulation is not known. Such considerations might be seen to argue for the development of CHIMs that are region-specific, each using naturally associated parasite-vector pairs. However, whilst this may provide the best scientific match, it is unclear at this time whether the effort would be justified. Few centres have current expertise to manage sand fly colonies suitable for the use in CHIMs. In the VL endemic state of Bihar, India, the Bill and Melinda Gates Foundation recently invested significantly to establish a suitably accredited facility for xenodiagnosis. It is unlikely and probably unnecessary for CHIMs to be established in every country with a diverse form of leishmaniasis, particularly when evidence of need is absent, and the flow of candidate vaccines limited. However, investment in regional centres, for example, covering South Asia, East Africa, the Middle East and South America, linked to sites where vaccine manufacture might take place, could make a major contribution to capacity building and the decolonisation of science.
Perhaps the more significant confounder in translating results from a CHIM conducted in the Global North is going to be in the target population. The presence of concurrent infections and differences in nutrition will have collective impact on immune health, either direct or indirect via resulting changes in the microbiota, that are not possible to mimic in populations in the Global North. Whilst baseline differences in skin structure due to ethnicity [77], age or gender may be evaluated by suitably-targeted recruitment into CHIM studies, the effects of, for example, long-term ultraviolet exposure on skin function would be harder to evaluate.
6 Prospects
Diversity is what makes leishmaniasis fascinating, yet challenging, from the perspective of vaccine development and the use of CHIM studies. Extending the argument that diversity matters, we would be faced with establishing CHIMs in every endemic setting where a potential vaccine might be used. More pragmatically, the view could be taken that efficacy of a vaccine or vaccines in a single-species CHIM model in the Global North would already dramatically reduce uncertainty about vaccine potential and encourage early-phase clinical trials in different disease endemic countries linked to established correlates of protection (e.g. LST conversion or new biomarkers identified through CHIM studies). There is also precedent for CHIM studies sufficiently demonstrating efficacy to allow licensure of vaccines, and thus a larger-scale CHIM-based vaccination study could potentially result in vaccine approval [78]. Should endemic country CHIMs be required in the future, early appreciation of the challenges is essential, with an important case study from Malawi [79] paving the way for identifying the many issues needing to be considered.
Finally, in addition to vaccine development, many of the arguments for and against the use of CHIMs could also be extended to their use for the early-stage evaluation of new chemical entities or the re-purposing of immunotherapies for the treatment of leishmaniasis. Notably, the use of CHIMs could provide initial, more precise determination of pharmacokinetics and pharmacodynamics without the confounding factors associated with conducting such studies in patient populations. The development of CHIMs under such a “dual-purpose” banner may provide a greater incentive for funders than an argument based solely upon vaccine development.
References
GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. https://doi.org/10.1016/S0140-6736(18)32203-7.
Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. https://doi.org/10.1371/journal.pone.0035671.
GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–22. https://doi.org/10.1016/S0140-6736(20)30925-9.
Organisation WH. 2022. https://www.who.int/health-topics/leishmaniasis#tab=tab_1. Accessed 18 Feb 2022.
Velez R, Gallego M. Commercially approved vaccines for canine leishmaniosis: a review of available data on their safety and efficacy. Trop Med Int Health. 2020;25(5):540–57. https://doi.org/10.1111/tmi.13382.
Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, Lopez-Velez R, Garcia-Hernandez R, et al. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis. 2017;11(12):e0006052. https://doi.org/10.1371/journal.pntd.0006052.
Magill AJ, Grogl M, Gasser RA Jr, Sun W, Oster CN. Visceral infection caused by Leishmania tropica in veterans of operation desert storm. N Engl J Med. 1993;328(19):1383–7. https://doi.org/10.1056/NEJM199305133281904.
Barral A, Badaro R, Barral-Netto M, Grimaldi G Jr, Momem H, Carvalho EM. Isolation of Leishmania mexicana amazonensis from the bone marrow in a case of American visceral leishmaniasis. Am J Trop Med Hyg. 1986;35(4):732–4. https://doi.org/10.4269/ajtmh.1986.35.732.
Rossi M, Fasel N. The criminal association of Leishmania parasites and viruses. Curr Opin Microbiol. 2018;46:65–72. https://doi.org/10.1016/j.mib.2018.07.005.
Cotton JA, Durrant C, Franssen SU, Gelanew T, Hailu A, Mateus D, et al. Genomic analysis of natural intra-specific hybrids among Ethiopian isolates of Leishmania donovani. PLoS Negl Trop Dis. 2020;14(4):e0007143. https://doi.org/10.1371/journal.pntd.0007143.
Inbar E, Shaik J, Iantorno SA, Romano A, Nzelu CO, Owens K, et al. Whole genome sequencing of experimental hybrids supports meiosis-like sexual recombination in Leishmania. PLoS Genet. 2019;15(5):e1008042. https://doi.org/10.1371/journal.pgen.1008042.
Kato H, Caceres AG, Gomez EA, Tabbabi A, Mizushima D, Yamamoto DS, et al. Prevalence of genetically complex Leishmania strains with hybrid and mito-nuclear discordance. Front Cell Infect Microbiol. 2021;11:625001. https://doi.org/10.3389/fcimb.2021.625001.
Grace CA, Sousa KS, Sousa LMI, Vladimir CS, Luís RCJ, Brune MJ, Forrester SJ, Pedrozo de Silva de Azevedo C d M, Costa DL, Speed D, Mottram JC, Jeffares DC, Costa CHN. Leishmania infantum genotype is a major factor in visceral leishmaniasis mortality in Brazil. MBio. 2022;13(6):e0206822. https://doi.org/10.2139/ssrn.4023210.
Thakur CP, Thakur M. Accelerating kala-azar elimination in India. Indian J Med Res. 2020;152(6):538–40. https://doi.org/10.4103/ijmr.IJMR_841_19.
Anon. 2021. https://apps.who.int/gho/data/node.main.NTDLEISHVNUM?lang=en.
Kimutai R, Musa AM, Njoroge S, Omollo R, Alves F, Hailu A, et al. Safety and effectiveness of sodium stibogluconate and paromomycin combination for the treatment of visceral Leishmaniasis in eastern Africa: results from a pharmacovigilance programme. Clin Drug Investig. 2017;37(3):259–72. https://doi.org/10.1007/s40261-016-0481-0.
Adriaensen W, Dorlo TPC, Vanham G, Kestens L, Kaye PM, van Griensven J. Immunomodulatory therapy of visceral leishmaniasis in human immunodeficiency virus-coinfected patients. Front Immunol. 2017;8:1943. https://doi.org/10.3389/fimmu.2017.01943.
Burza S, Mahajan R, Kazmi S, Alexander N, Kumar D, Kumar V, et al. AmBisome monotherapy and combination AmBisome—miltefosine therapy for the treatment of visceral leishmaniasis in patients co-infected with HIV in India: a randomised open label, parallel arm, phase 3 trial. Clin Infect Dis. 2022;75:1423. https://doi.org/10.1093/cid/ciac127.
Dos Reis ES, Ribeiro CJN, Dos Santos AD, da Conceicao AD, Bezerra-Santos M, da Silva ER, et al. Magnitude of visceral leishmaniasis and HIV coinfection and association with social determinants of health in the northeast region of Brazil: a retrospective, spatiotemporal model (2010-2018). Parasitol Res. 2022;121:1021. https://doi.org/10.1007/s00436-022-07450-6.
Takele Y, Mulaw T, Adem E, Shaw CJ, Franssen SU, Womersley R, et al. Immunological factors, but not clinical features, predict visceral leishmaniasis relapse in patients co-infected with HIV. Cell Rep Med. 2022;3(1):100487. https://doi.org/10.1016/j.xcrm.2021.100487.
Le Rutte EA, Zijlstra EE, de Vlas SJ. Post-kala-azar dermal leishmaniasis as a reservoir for visceral leishmaniasis transmission. Trends Parasitol. 2019;35(8):590–2. https://doi.org/10.1016/j.pt.2019.06.007.
Mondal D, Bern C, Ghosh D, Rashid M, Molina R, Chowdhury R, et al. Quantifying the infectiousness of post-kala-azar dermal leishmaniasis toward sand flies. Clin Infect Dis. 2019;69(2):251–8. https://doi.org/10.1093/cid/ciy891.
Singh OP, Tiwary P, Kushwaha AK, Singh SK, Singh DK, Lawyer P, et al. Xenodiagnosis to evaluate the infectiousness of humans to sandflies in an area endemic for visceral leishmaniasis in Bihar, India: a transmission-dynamics study. Lancet Microbe. 2021;2(1):e23–31. https://doi.org/10.1016/S2666-5247(20)30166-X.
Gedda MR, Singh B, Kumar D, Singh AK, Madhukar P, Upadhyay S, et al. Post kala-azar dermal leishmaniasis: a threat to elimination program. PLoS Negl Trop Dis. 2020;14(7):e0008221. https://doi.org/10.1371/journal.pntd.0008221.
Pires M, Wright B, Kaye PM, da Conceicao V, Churchill RC. The impact of leishmaniasis on mental health and psychosocial well-being: a systematic review. PLoS One. 2019;14(10):e0223313. https://doi.org/10.1371/journal.pone.0223313.
Bailey F, Mondragon-Shem K, Haines LR, Olabi A, Alorfi A, Ruiz-Postigo JA, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13(2):e0007092. https://doi.org/10.1371/journal.pntd.0007092.
Kaye PM, Mohan S, Mantel C, Malhame M, Revill P, Le Rutte E, et al. Overcoming roadblocks in the development of vaccines for leishmaniasis. Expert Rev Vaccines. 2021;20(11):1419–30. https://doi.org/10.1080/14760584.2021.1990043.
Volpedo G, Huston RH, Holcomb EA, Pacheco-Fernandez T, Gannavaram S, Bhattacharya P, et al. From infection to vaccination: reviewing the global burden, history of vaccine development, and recurring challenges in global leishmaniasis protection. Expert Rev Vaccines. 2021;20(11):1431–46. https://doi.org/10.1080/14760584.2021.1969231.
Working Group on Research Priorities for Development of Leishmaniasis Vaccines, Costa CH, Peters NC, Maruyama SR, de Brito EC Jr, Santos IK. Vaccines for the leishmaniases: proposals for a research agenda. PLoS Negl Trop Dis. 2011;5(3):e943. https://doi.org/10.1371/journal.pntd.0000943.
Le Rutte EA, Coffeng LE, Malvolti S, Kaye PM, de Vlas SJ. The potential impact of human visceral leishmaniasis vaccines on population incidence. PLoS Negl Trop Dis. 2020;14(7):e0008468. https://doi.org/10.1371/journal.pntd.0008468.
Alvar J, den Boer M, Dagne DA. Towards the elimination of visceral leishmaniasis as a public health problem in East Africa: reflections on an enhanced control strategy and a call for action. Lancet Glob Health. 2021;9(12):e1763–e9. https://doi.org/10.1016/S2214-109X(21)00392-2.
Passelli K, Billion O, Tacchini-Cottier F. The impact of neutrophil recruitment to the skin on the pathology induced by Leishmania infection. Front Immunol. 2021;12:649348. https://doi.org/10.3389/fimmu.2021.649348.
Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nat Rev Microbiol. 2011;9(8):604–15. https://doi.org/10.1038/nrmicro2608.
Kloehn J, Boughton BA, Saunders EC, O’Callaghan S, Binger KJ, McConville MJ. Identification of metabolically quiescent Leishmania mexicana parasites in peripheral and cured dermal granulomas using stable isotope tracing imaging mass spectrometry. MBio. 2021;12(2):e00129–1. https://doi.org/10.1128/mBio.00129-21.
Mandal S, Njikan S, Kumar A, Early JV, Parish T. The relevance of persisters in tuberculosis drug discovery. Microbiology (Reading). 2019;165(5):492–9. https://doi.org/10.1099/mic.0.000760.
Atayde VD, Hassani K, da Silva Lira Filho A, Borges AR, Adhikari A, Martel C, et al. Leishmania exosomes and other virulence factors: impact on innate immune response and macrophage functions. Cell Immunol. 2016;309:7–18. https://doi.org/10.1016/j.cellimm.2016.07.013.
Noazin S, Khamesipour A, Moulton LH, Tanner M, Nasseri K, Modabber F, et al. Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis: a meta-analysis. Vaccine. 2009;27(35):4747–53. https://doi.org/10.1016/j.vaccine.2009.05.084.
Coler RN, Duthie MS, Hofmeyer KA, Guderian J, Jayashankar L, Vergara J, et al. From mouse to man: safety, immunogenicity and efficacy of a candidate leishmaniasis vaccine LEISH-F3+GLA-SE. Clin Transl Immunol. 2015;4(4):e35. https://doi.org/10.1038/cti.2015.6.
Osman M, Mistry A, Keding A, Gabe R, Cook E, Forrester S, et al. A third generation vaccine for human visceral leishmaniasis and post kala azar dermal leishmaniasis: first-in-human trial of ChAd63-KH. PLoS Negl Trop Dis. 2017;11(5):e0005527. https://doi.org/10.1371/journal.pntd.0005527.
Younis BM, Osman M, Khalil EAG, Santoro F, Furini S, Wiggins R, et al. Safety and immunogenicity of ChAd63-KH vaccine in post-kala-azar dermal leishmaniasis patients in Sudan. Mol Ther. 2021;29(7):2366–77. https://doi.org/10.1016/j.ymthe.2021.03.020.
Zhang WW, Karmakar S, Gannavaram S, Dey R, Lypaczewski P, Ismail N, et al. A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing. Nat Commun. 2020;11(1):3461. https://doi.org/10.1038/s41467-020-17154-z.
Bliss CM, Bowyer G, Anagnostou NA, Havelock T, Snudden CM, Davies H, et al. Assessment of novel vaccination regimens using viral vectored liver stage malaria vaccines encoding ME-TRAP. Sci Rep. 2018;8(1):3390. https://doi.org/10.1038/s41598-018-21630-4.
Mendonca SA, Lorincz R, Boucher P, Curiel DT. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines. 2021;6(1):97. https://doi.org/10.1038/s41541-021-00356-x.
Maroof A, Brown N, Smith B, Hodgkinson MR, Maxwell A, Losch FO, et al. Therapeutic vaccination with recombinant adenovirus reduces splenic parasite burden in experimental visceral leishmaniasis. J Infect Dis. 2012;205(5):853–63. https://doi.org/10.1093/infdis/jir842.
Karmakar S, Ismail N, Oliveira F, Oristian J, Zhang WW, Kaviraj S, et al. Preclinical validation of a live attenuated dermotropic Leishmania vaccine against vector transmitted fatal visceral leishmaniasis. Commun Biol. 2021;4(1):929. https://doi.org/10.1038/s42003-021-02446-x.
Langenberg MCC, Hoogerwerf MA, Koopman JPR, Janse JJ, Kos-van Oosterhoud J, Feijt C, et al. A controlled human Schistosoma mansoni infection model to advance novel drugs, vaccines and diagnostics. Nat Med. 2020;26(3):326–32. https://doi.org/10.1038/s41591-020-0759-x.
Melby PC. Experimental leishmaniasis in humans: review. Rev Infect Dis. 1991;13(5):1009–17. https://doi.org/10.1093/clinids/13.5.1009.
Payne RO, Griffin PM, McCarthy JS, Draper SJ. Plasmodium vivax controlled human malaria infection—progress and prospects. Trends Parasitol. 2017;33(2):141–50. https://doi.org/10.1016/j.pt.2016.11.001.
Roestenberg M, Hoogerwerf MA, Ferreira DM, Mordmuller B, Yazdanbakhsh M. Experimental infection of human volunteers. Lancet Infect Dis. 2018;18(10):e312–e22. https://doi.org/10.1016/S1473-3099(18)30177-4.
Nikolay B, Ribeiro Dos Santos G, Lipsitch M, Rahman M, Luby SP, Salje H, et al. Assessing the feasibility of Nipah vaccine efficacy trials based on previous outbreaks in Bangladesh. Vaccine. 2021;39(39):5600–6. https://doi.org/10.1016/j.vaccine.2021.08.027.
Pacheco-Fernandez T, Volpedo G, Gannavaram S, Bhattacharya P, Dey R, Satoskar A, et al. Revival of leishmanization and Leishmanin. Front Cell Infect Microbiol. 2021;11:639801. https://doi.org/10.3389/fcimb.2021.639801.
Killingley B, Mann A, Kalinova M, Boyers A, et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med. 2022;28(5):1031–41. https://doi.org/10.21203/rs.3.rs-1121993/v1.
Elliott AM, Roestenberg M, Wajja A, Opio C, Angumya F, Adriko M, et al. Ethical and scientific considerations on the establishment of a controlled human infection model for schistosomiasis in Uganda: report of a stakeholders’ meeting held in Entebbe, Uganda. AAS Open Res. 2018;1:2. https://doi.org/10.12688/aasopenres.12841.2.
Njue M, Njuguna P, Kapulu MC, Sanga G, Bejon P, Marsh V, et al. Ethical considerations in controlled human malaria infection studies in low resource settings: experiences and perceptions of study participants in a malaria challenge study in Kenya. Wellcome Open Res. 2018;3:39. https://doi.org/10.12688/wellcomeopenres.14439.2.
Mohebali M, Nadim A, Khamesipour A. An overview of leishmanization experience: a successful control measure and a tool to evaluate candidate vaccines. Acta Trop. 2019;200:105173. https://doi.org/10.1016/j.actatropica.2019.105173.
Parkash V, Kaye PM, Layton AM, Lacey CJ. Vaccines against leishmaniasis: using controlled human infection models to accelerate development. Expert Rev Vaccines. 2021;20(11):1407–18. https://doi.org/10.1080/14760584.2021.1991795.
Green MS, Kark JD, Witztum E, Greenblatt CL, Spira DT. Frozen stored Leishmania tropica vaccine: the effects of dose, route of administration and storage on the evolution of the clinical lesion. Two field trials in the Israel defense forces. Trans R Soc Trop Med Hyg. 1983;77(2):152–9. https://doi.org/10.1016/0035-9203(83)90054-8.
Khamesipour A, Dowlati Y, Asilian A, Hashemi-Fesharki R, Javadi A, Noazin S, et al. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine. 2005;23(28):3642–8. https://doi.org/10.1016/j.vaccine.2005.02.015.
Bekeredjian-Ding I, Trouvin JH, Depraetere H, La C, Suvarnapunya AE, Bell A, et al. Controlled human infection studies: proposals for guidance on how to design, develop and produce a challenge strain. Biologicals. 2021;74:16–23. https://doi.org/10.1016/j.biologicals.2021.09.002.
Serafim TD, Coutinho-Abreu IV, Dey R, Kissinger R, Valenzuela JG, Oliveira F, et al. Leishmaniasis: the act of transmission. Trends Parasitol. 2021;37(11):976–87. https://doi.org/10.1016/j.pt.2021.07.003.
Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, Sacks DL. Vector transmission of Leishmania abrogates vaccine-induced protective immunity. PLoS Pathog. 2009;5(6):e1000484. https://doi.org/10.1371/journal.ppat.1000484.
Stanisic DI, McCarthy JS, Good MF. Controlled human malaria infection: applications, advances, and challenges. Infect Immun. 2018;86(1):e00479–17. https://doi.org/10.1128/IAI.00479-17.
Ashwin H, Sadlova J, Vojtkova B, Becvar T, Lypaczewski P, Schwartz E, et al. Characterization of a new Leishmania major strain for use in a controlled human infection model. Nat Commun. 2021;12(1):215. https://doi.org/10.1038/s41467-020-20569-3.
Parkash V, Ashwin H, Sadlova J, Vojtkova B, Jones G, Martin N, et al. A clinical study to optimise a sand fly biting protocol for use in a controlled human infection model of cutaneous leishmaniasis (the FLYBITE study). Wellcome Open Res. 2021;6:168. https://doi.org/10.12688/wellcomeopenres.16870.1.
Parkash V, Jones G, Martin N, Steigmann M, Greensted E, Kaye P, et al. Assessing public perception of a sand fly biting study on the pathway to a controlled human infection model for cutaneous leishmaniasis. Res Involv Engagem. 2021;7(1):33. https://doi.org/10.1186/s40900-021-00277-y.
Malvolti S, Malhame M, Mantel CF, Le Rutte EA, Kaye PM. Human leishmaniasis vaccines: use cases, target population and potential global demand. PLoS Negl Trop Dis. 2021;15(9):e0009742. https://doi.org/10.1371/journal.pntd.0009742.
Mohan S, Revill P, Malvolti S, Malhame M, Sculpher M, Kaye PM. Estimating the global demand curve for a leishmaniasis vaccine: a generalisable approach based on global burden of disease estimates. MedRxiv preprint. 2021. https://doi.org/10.1101/2021.08.26.21262379.
Bretscher PA, Wei G, Menon JN, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257(5069):539–42. https://doi.org/10.1126/science.1636090.
Romano A, Doria NA, Mendez J, Sacks DL, Peters NC. Cutaneous infection with Leishmania major mediates heterologous protection against visceral infection with Leishmania infantum. J Immunol. 2015;195(8):3816–27. https://doi.org/10.4049/jimmunol.1500752.
van Henten S, Adriaensen W, Fikre H, Akuffo H, Diro E, Hailu A, et al. Cutaneous leishmaniasis due to Leishmania aethiopica. EClinicalMedicine. 2018;6:69–81. https://doi.org/10.1016/j.eclinm.2018.12.009.
Aguilar-Be I, da Silva ZR, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun. 2005;73(2):812–9. https://doi.org/10.1128/IAI.73.2.812-819.2005.
Campbell K, Diao H, Ji J, Soong L. DNA immunization with the gene encoding P4 nuclease of Leishmania amazonensis protects mice against cutaneous leishmaniasis. Infect Immun. 2003;71(11):6270–8. https://doi.org/10.1128/IAI.71.11.6270-6278.2003.
Elikaee S, Mohebali M, Rezaei S, Eslami H, Khamesipour A, Keshavarz H, et al. Leishmania major p27 gene knockout as a novel live attenuated vaccine candidate: protective immunity and efficacy evaluation against cutaneous and visceral leishmaniasis in BALB/c mice. Vaccine. 2019;37(24):3221–8. https://doi.org/10.1016/j.vaccine.2019.04.068.
Solana JC, Ramirez L, Corvo L, de Oliveira CI, Barral-Netto M, Requena JM, et al. Vaccination with a Leishmania infantum HSP70-II null mutant confers long-term protective immunity against Leishmania major infection in two mice models. PLoS Negl Trop Dis. 2017;11(5):e0005644. https://doi.org/10.1371/journal.pntd.0005644.
Barazandeh AF, Mou Z, Ikeogu N, Mejia EM, Edechi CA, Zhang WW, et al. The phosphoenolpyruvate carboxykinase is a key metabolic enzyme and critical virulence factor of Leishmania major. J Immunol. 2021;206(5):1013–26. https://doi.org/10.4049/jimmunol.2000517.
Sharma R, al e. Targeted deletion of centrin in Leishmania braziliensis using CRISPR-Cas9-based editing. Front Cell Infect Microbiol. 2022;11:790418. https://doi.org/10.3389/fcimb.2021.790418.
Rawlings AV. Ethnic skin types: are there differences in skin structure and function? Int J Cosmet Sci. 2006;28(2):79–93. https://doi.org/10.1111/j.1467-2494.2006.00302.x.
Levine MM, Chen WH, Kaper JB, Lock M, Danzig L, Gurwith M. PaxVax CVD 103-HgR single-dose live oral cholera vaccine. Expert Rev Vaccines. 2017;16(3):197–213. https://doi.org/10.1080/14760584.2017.1291348.
Gordon SB, Rylance J, Luck A, Jambo K, Ferreira DM, Manda-Taylor L, et al. A framework for controlled human infection model (CHIM) studies in Malawi: report of a wellcome trust workshop on CHIM in low income countries held in Blantyre, Malawi. Wellcome Open Res. 2017;2:70. https://doi.org/10.12688/wellcomeopenres.12256.1.
Acknowledgements
The authors acknowledge the contributions of the LEISH Challenge team (https://leishchallenge.org) to developing the ideas expressed in this chapter, especially Helen Ashwin, Charle Jaffe, Jovana Sadlova, Eli Schwartz, Barbora Vojtkova and Petr Volf. The development of a CHIM for leishmaniasis is funded by the UK Medical Research Council and the Department for International Development (grant No. MR/R014973/1).
PMK and CJNL are co-authors on a patent protecting the gene insert used for the development of the ChAd63-KH leishmaniasis vaccine.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Kaye, P.M., Parkash, V., Layton, A.M., Lacey, C.J.N. (2023). The Utility of a Controlled Human Infection Model for Developing Leishmaniasis Vaccines. In: Christodoulides, M. (eds) Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges . Springer, Cham. https://doi.org/10.1007/978-3-031-24355-4_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-24355-4_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-24354-7
Online ISBN: 978-3-031-24355-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)