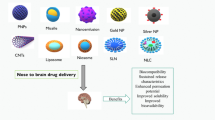
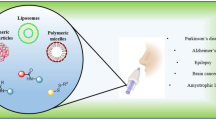
Abstract
Nasal delivery has come up as a promising approach to deliver diverse therapeutic agents including small drug molecules to biomacromolecules like peptides, proteins and genes to treat various disorders of central nervous system including depression, epilepsy, migraine, schizophrenia, Parkinson’s disease, Alzheimer’s disease, and brain tumor. Nasal route unveils the possibility for delivering the drug directly from nose to brain, circumventing the challenging blood-brain barrier enabling their effective brain delivery. The past couple of decades have witnessed tremendous enthusiasm about exploiting nanotechnology-based approaches in the drug delivery area, specifically due to the exponential growth in research efforts employing the nanocarriers’ delivery via different administration routes including the nasal route. Nanocarrier-based nasal delivery of drugs has progressed over the years with an assumption that the use of nanocarriers would enable the drug to access tissues and organs that could otherwise not be accessed effectively by conventional nasal delivery. The present chapter sets out to discuss applications of different nanocarriers in nasal delivery along with their transportation mechanisms and toxicity concerns.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Global Intranasal Drug Delivery Market to Reach $59.2 Billion by 2027. Available from https://pharmprom.ru/en/global-intranasal-drug-delivery-market-to-reach-59-2-billion-by-2027/.
Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013;3(1):42–62.
Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–70. https://doi.org/10.1016/j.brainresbull.2018.10.009. Epub 2018 Oct 25. PMID: 30449731.
Lavelle EC, Ward RW. Mucosal vaccines - fortifying the frontiers. Nat Rev Immunol. 2021:1–15. https://doi.org/10.1038/s41577-021-00583-2. Epub ahead of print. Erratum in: Nat Rev Immunol. 2021;: PMID: 34312520; PMCID: PMC8312369.
Kumar A, Pandey AN, Jain SK. Nasal-nanotechnology: revolution for efficient therapeutics delivery. Drug Deliv. 2016;23(3):681–93. https://doi.org/10.3109/10717544.2014.920431. Epub 2014 Jun 5. PMID: 24901207.
Alshweiat A, Ambrus R, Csoka I. Intranasal nanoparticulate systems as alternative route of drug delivery. Curr Med Chem. 2019;26(35):6459–92. https://doi.org/10.2174/0929867326666190827151741. PMID: 31453778.
Sharma S, Mukkur TK, Benson HA, Chen Y. Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. J Pharm Sci. 2009;98(3):812–43. https://doi.org/10.1002/jps.21493. PMID: 18661544.
Fortuna A, Alves G, Serralheiro A, Sousa J, Falcão A. Intranasal delivery of systemic-acting drugs: small-molecules and biomacromolecules. Eur J Pharm Biopharm. 2014;88(1):8–27. https://doi.org/10.1016/j.ejpb.2014.03.004. Epub 2014 Mar 28. PMID: 24681294.
Illum L. Nasal drug delivery – recent developments and future prospects. J Control Release. 2012;161(2):254–63. https://doi.org/10.1016/j.jconrel.2012.01.024. Epub 2012 Jan 24. PMID: 22300620.
Sobiesk JL, Munakomi S. Anatomy, head and neck, nasal cavity. [Updated 2021 Jul 26]. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK544232/.
Keller LA, Merkel O, Popp A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Deliv Transl Res. 2021;12:1–23. https://doi.org/10.1007/s13346-020-00891-5. Epub ahead of print. PMID: 33491126; PMCID: PMC7829061.
Trevino JT, Quispe RC, Khan F, Novak V. Non-invasive strategies for nose-to-brain drug delivery. J Clin Trials. 2020;10(7):439. Epub 2020 Dec 10. PMID: 33505777; PMCID: PMC7836101.
Türker S, Onur E, Ozer Y. Nasal route and drug delivery systems. Pharm World Sci. 2004;26(3):137–42. https://doi.org/10.1023/b:phar.0000026823.82950.ff. PMID: 15230360.
Maaz A, Blagbrough IS, De Bank PA. In vitro evaluation of nasal aerosol depositions: an insight for direct nose to brain drug delivery. Pharmaceutics. 2021;13(7):1079. https://doi.org/10.3390/pharmaceutics13071079. PMID: 34371770; PMCID: PMC8309016.
Bruinsmann FA, Richter Vaz G, de Cristo Soares Alves A, Aguirre T, Raffin Pohlmann A, Stanisçuaski Guterres S, Sonvico F. Nasal drug delivery of anticancer drugs for the treatment of glioblastoma: preclinical and clinical trials. Molecules. 2019;24(23):4312. https://doi.org/10.3390/molecules24234312. PMID: 31779126; PMCID: PMC6930669.
Kendall JM, Latter VS. Intranasal diamorphine as an alternative to intramuscular morphine: pharmacokinetic and pharmacodynamic aspects. Clin Pharmacokinet. 2003;42(6):501–13. https://doi.org/10.2165/00003088-200342060-00001. PMID: 12793836.
Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–72. https://doi.org/10.1038/jcbfm.2012.126. Epub 2012 Aug 29. PMID: 22929442; PMCID: PMC3494002.
Giunchedi P, Gavini E, Bonferoni MC. Nose-to-brain delivery. Pharmaceutics. 2020;12(2):138. https://doi.org/10.3390/pharmaceutics12020138. PMID: 32041344; PMCID: PMC7076467.
Ghadiri M, Young PM, Traini D. Strategies to enhance drug absorption via nasal and pulmonary routes. Pharmaceutics. 2019;11(3):113. https://doi.org/10.3390/pharmaceutics11030113. PMID: 30861990; PMCID: PMC6470976.
Hanson LR, Frey WH 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008;9 Suppl 3(Suppl 3):S5. https://doi.org/10.1186/1471-2202-9-S3-S5. PMID: 19091002; PMCID: PMC2604883.
Ehrick JD, Shah SA, Shaw C, Kulkarni VS, Coowanitwong I, De S, Suman JD. Considerations for the development of nasal dosage forms. Sterile Product Develop. 2013;6:99–144. https://doi.org/10.1007/978-1-4614-7978-9_5. PMCID: PMC7120012.
Meredith ME, Salameh TS, Banks WA. Intranasal delivery of proteins and peptides in the treatment of neurodegenerative diseases. AAPS J. 2015;17(4):780–7. https://doi.org/10.1208/s12248-015-9719-7. Epub 2015 Mar 24. PMID: 25801717; PMCID: PMC4476983.
Gizurarson S. Anatomical and histological factors affecting intranasal drug and vaccine delivery. Curr Drug Deliv. 2012;9(6):566–82. https://doi.org/10.2174/156720112803529828. PMID: 22788696; PMCID: PMC3480721.
Li A, Yuen VM, Goulay-Dufaÿ S, Sheng Y, Standing JF, Kwok PCL, Leung MKM, Leung AS, Wong ICK, Irwin MG. Pharmacokinetic and pharmacodynamic study of intranasal and intravenous dexmedetomidine. Br J Anaesth. 2018;120(5):960–8. https://doi.org/10.1016/j.bja.2017.11.100. Epub 2018 Feb 2. Erratum in: Br J Anaesth. 2018;121(3):687. PMID: 29661413.
Ozsoy Y, Gungor S, Cevher E. Nasal delivery of high molecular weight drugs. Molecules. 2009;14(9):3754–79. https://doi.org/10.3390/molecules14093754. PMID: 19783956; PMCID: PMC6254717.
Harkema JR, Nikula KJ, Haschek WM. Chapter 14. Respiratory system. In: Wallig MA, Haschek WM, Rousseaux CG, Bolon B, editors. Fundamentals of toxicologic pathology. 3rd ed. Academic Press; 2018. p. 351–93.
Rhee JH. Current and new approaches for mucosal vaccine delivery. Mucosal Vaccines. 2020:325–56. https://doi.org/10.1016/B978-0-12-811924-2.00019-5. Epub 2019 Oct 25. PMCID: PMC7149853.
Thakur A, Foged C. Nanoparticles for mucosal vaccine delivery. In: Nanoengineered biomaterials for advanced drug delivery. 2020. pp. 603–46. https://doi.org/10.1016/B978-0-08-102985-5.00025-5. Epub 2020 Jun 26. PMCID: PMC7329122.
Zhu M, Wang R, Nie G. Applications of nanomaterials as vaccine adjuvants. Hum Vaccin Immunother. 2014;10(9):2761–74. https://doi.org/10.4161/hv.29589. Epub 2014 Nov 17. PMID: 25483497; PMCID: PMC4977448.
Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60(8):915–28. https://doi.org/10.1016/j.addr.2007.05.017. Epub 2008 Feb 7. PMID: 18325628; PMCID: PMC7103321.
Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: current challenges and future approaches. J Pharm Sci. 2009;98(4):1278–316. https://doi.org/10.1002/jps.21523. PMID: 18704954; PMCID: PMC8092333.
Petkar KC, Patil SM, Chavhan SS, Kaneko K, Sawant KK, Kunda NK, Saleem IY. An overview of nanocarrier-based adjuvants for vaccine delivery. Pharmaceutics. 2021;13(4):455. https://doi.org/10.3390/pharmaceutics13040455. PMID: 33801614; PMCID: PMC8066039.
Zolnik BS, González-Fernández A, Sadrieh N, Dobrovolskaia MA. Nanoparticles and the immune system. Endocrinology. 2010;151(2):458–65. https://doi.org/10.1210/en.2009-1082. Epub 2009 Dec 16. PMID: 20016026; PMCID: PMC2817614.
Chatzikleanthous D, O’Hagan DT, Adamo R. Lipid-based nanoparticles for delivery of vaccine adjuvants and antigens: toward multicomponent vaccines. Mol Pharm. 2021;18(8):2867–88. https://doi.org/10.1021/acs.molpharmaceut.1c00447. Epub 2021 Jul 15. PMID: 34264684.
Han J, Zhao D, Li D, Wang X, Jin Z, Zhao K. Polymer-based nanomaterials and applications for vaccines and drugs. Polymers (Basel). 2018;10(1):31. https://doi.org/10.3390/polym10010031. PMID: 30966075; PMCID: PMC6415012.
Köping-Höggård M, Sánchez A, Alonso MJ. Nanoparticles as carriers for nasal vaccine delivery. Expert Rev Vaccines. 2005;4(2):185–96. https://doi.org/10.1586/14760584.4.2.185. PMID: 15889992.
Lobaina Mato Y. Nasal route for vaccine and drug delivery: features and current opportunities. Int J Pharm. 2019;572:118813. https://doi.org/10.1016/j.ijpharm.2019.118813. Epub 2019 Oct 31. PMID: 31678521.
Yusuf H, Kett V. Current prospects and future challenges for nasal vaccine delivery. Hum Vaccin Immunother. 2017;13(1):34–45. https://doi.org/10.1080/21645515.2016.1239668. Epub 2016 Dec 9. PMID: 27936348; PMCID: PMC5287317.
Kelly SH, Shores LS, Votaw NL, Collier JH. Biomaterial strategies for generating therapeutic immune responses. Adv Drug Deliv Rev. 2017;114:3–18. https://doi.org/10.1016/j.addr.2017.04.009. Epub 2017 Apr 25. PMID: 28455189; PMCID: PMC5606982.
Tran NP, Vickery J, Blaiss MS. Management of rhinitis: allergic and non-allergic. Allergy, Asthma Immunol Res. 2011;3(3):148–56. https://doi.org/10.4168/aair.2011.3.3.148. Epub 2011 May 20. PMID: 21738880; PMCID: PMC3121056.
Massoth L, Anderson C, McKinney KA. Asthma and chronic rhinosinusitis: diagnosis and medical management. Med Sci (Basel). 2019;7(4):53. https://doi.org/10.3390/medsci7040053. PMID: 30934800; PMCID: PMC6524348.
Jiang L, Gao L, Wang X, Tang L, Ma J. The application of mucoadhesive polymers in nasal drug delivery. Drug Dev Ind Pharm. 2010;36(3):323–36. https://doi.org/10.1080/03639040903170750. PMID: 19735210.
Sallam MA, Helal HM, Mortada SM. Rationally designed nanocarriers for intranasaltherapy of allergic rhinitis: influence of carrier type on in vivo nasal deposition. Int J Nanomedicine. 2016;11:2345–57. https://doi.org/10.2147/IJN.S98547. PMID: 27307734; PMCID: PMC4887068.
Bajracharya R, Song JG, Back SY, Han HK. Recent advancements in non-invasive formulations for protein drug delivery. Comput Struct Biotechnol J. 2019;17:1290–308. https://doi.org/10.1016/j.csbj.2019.09.004. PMID: 31921395; PMCID: PMC6944732.
Upadhyay RK. Drug delivery systems, CNS protection, and the blood brain barrier. Biomed Res Int. 2014;2014:869269. https://doi.org/10.1155/2014/869269. Epub 2014 Jul 20. PMID: 25136634; PMCID: PMC4127280.
Bourganis V, Kammona O, Alexopoulos A, Kiparissides C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur J Pharm Biopharm. 2018;128:337–62. https://doi.org/10.1016/j.ejpb.2018.05.009. Epub 2018 May 4. PMID: 29733950.
Dwibhashyam VS, Nagappa AN. Strategies for enhanced drug delivery to the central nervous system. Indian J Pharm Sci. 2008;70(2):145–53. https://doi.org/10.4103/0250-474X.41446. PMID: 20046703; PMCID: PMC2792490.
Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. https://doi.org/10.1602/neurorx.2.1.3. PMID: 15717053; PMCID: PMC539316.
Tuomanen E. Breaching the blood-brain barrier. Sci Am. 1993;268(2):80–4. https://doi.org/10.1038/scientificamerican0293-80. PMID: 8430297.
Costa CP, Moreira JN, Sousa Lobo JM, Silva AC. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: a current overview of in vivo studies. Acta Pharm Sin B. 2021;11(4):925–40. https://doi.org/10.1016/j.apsb.2021.02.012. Epub 2021 Mar 13. PMID: 33996407; PMCID: PMC8105874.
Pardeshi CV, Belgamwar VS. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: an excellent platform for brain targeting. Expert Opin Drug Deliv. 2013;10(7):957–72. https://doi.org/10.1517/17425247.2013.790887. Epub 2013 Apr 16. PMID: 23586809.
Laksitorini M, Prasasty VD, Kiptoo PK, Siahaan TJ. Pathways and progress in improving drug delivery through the intestinal mucosa and blood-brain barriers. Ther Deliv. 2014;5(10):1143–63. https://doi.org/10.4155/tde.14.67. PMID: 25418271; PMCID: PMC4445828.
Hajal C, Campisi M, Mattu C, Chiono V, Kamm RD. In vitro models of molecular and nano-particle transport across the blood-brain barrier. Biomicrofluidics. 2018;12(4):042213. https://doi.org/10.1063/1.5027118. PMID: 29887937; PMCID: PMC5980570.
Johnson NJ, Hanson LR, Frey WH. Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm. 2010;7(3):884–93. https://doi.org/10.1021/mp100029t. PMID: 20420446; PMCID: PMC2892271.
Gänger S, Schindowski K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics. 2018;10(3):116. https://doi.org/10.3390/pharmaceutics10030116. PMID: 30081536; PMCID: PMC6161189.
Agu UR. Challenges in nasal drug absorption: how far have we come ? Ther Deliv. 2016;7(7):495–510. https://doi.org/10.4155/tde-2016-0022. © 2016 Future Science Ltd
Lee R. Addressing the challenges of nasal biotherapeutic drug delivery. ONdrugDelivery. 2021;(127):16–8.
Dhuria SV, Hanson LR, Frey WH 2nd. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–73. https://doi.org/10.1002/jps.21924. PMID: 19877171.
Xing H, Hwang K, Lu Y. Recent developments of liposomes as nanocarriers for theranostic applications. Theranostics. 2016;6(9):1336–52. https://doi.org/10.7150/thno.15464. PMID: 27375783; PMCID: PMC4924503.
Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J Control Release. 2019;303:130–50. https://doi.org/10.1016/j.jconrel.2019.04.025. Epub 2019 May 3. PMID: 31022431; PMCID: PMC7111479.
Lee MK. Liposomes for enhanced bioavailability of water-insoluble drugs: in vivo evidence and recent approaches. Pharmaceutics. 2020;12(3):264. https://doi.org/10.3390/pharmaceutics12030264. PMID: 32183185; PMCID: PMC7151102.
Upadhyay P, Trivedi J, Pundarikakshudu K, Sheth N. Direct and enhanced delivery of nanoliposomes of anti schizophrenic agent to the brain through nasal route. Saudi Pharm J. 2017;25(3):346–58. https://doi.org/10.1016/j.jsps.2016.07.003. Epub 2016 Aug 5. PMID: 28344488; PMCID: PMC5357100.
Narayan R, Singh M, Ranjan O, Nayak Y, Garg S, Shavi GV, Nayak UY. Development of risperidone liposomes for brain targeting through intranasal route. Life Sci. 2016;163:38–45. https://doi.org/10.1016/j.lfs.2016.08.033. Epub 2016 Sep 1. PMID: 27593571.
Al Asmari AK, Ullah Z, Tariq M, Fatani A. Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des Devel Ther. 2016;10:205–15. https://doi.org/10.2147/DDDT.S93937. PMID: 26834457; PMCID: PMC4716722.
Alsarra IA, Hamed AY, Alanazi FK. Acyclovir liposomes for intranasal systemic delivery: development and pharmacokinetics evaluation. Drug Deliv. 2008;15(5):313–21. https://doi.org/10.1080/10717540802035251. PMID: 18763162.
Dahiya S, Dahiya R. Chapter 37. Organic nanocarriers for targeted delivery of anticancer agents. In: Dua K, Mehta M, de Jesus Andreoli Pinto T, Pont LG, Williams KA, Rathbone MJ, editors. Advanced drug delivery systems in the management of cancer. Netherlands: Academic Press, Elsevier; 2021. p. 467–97.
Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76. https://doi.org/10.1208/s12249-010-9563-0. Epub 2010 Dec 21. PMID: 21174180; PMCID: PMC3066374.
Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305–13. https://doi.org/10.15171/apb.2015.043. Epub 2015 Sep 19. PMID: 26504751; PMCID: PMC4616893.
Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598–613.
Fatouh AM, Elshafeey AH, Abdelbary A. Intranasal agomelatine solid lipid nanoparticles to enhance brain delivery: formulation, optimization and in vivo pharmacokinetics. Drug Des Devel Ther. 2017;11:1815–25. https://doi.org/10.2147/DDDT.S102500. PMID: 28684900; PMCID: PMC5484509.
Joshi AS, Patel HS, Belgamwar VS, Agrawal A, Tekade AR. Solid lipid nanoparticles of ondansetron HCl for intranasal delivery: development, optimization and evaluation. J Mater Sci Mater Med. 2012;23(9):2163–75. https://doi.org/10.1007/s10856-012-4702-7. Epub 2012 Jul 17. PMID: 22802103.
Aboud HM, El Komy MH, Ali AA, El Menshawe SF, Abd Elbary A. Development, optimization, and evaluation of carvedilol-loaded solid lipid nanoparticles for intranasal drug delivery. AAPS PharmSciTech. 2016;17(6):1353–65. https://doi.org/10.1208/s12249-015-0440-8. Epub 2016 Jan 7. PMID: 26743643.
Patel S, Chavhan S, Soni H, Babbar AK, Mathur R, Mishra AK, Sawant K. Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J Drug Target. 2011;19(6):468–74. https://doi.org/10.3109/1061186X.2010.523787. Epub 2010 Oct 19. PMID: 20958095.
Yasir M, Sara UV. Solid lipid nanoparticles for nose to brain delivery of haloperidol: in vitro drug release and pharmacokinetics evaluation. Acta Pharm Sin B. 2014;4(6):454–63. https://doi.org/10.1016/j.apsb.2014.10.005. Epub 2014 Nov 21. PMID: 26579417; PMCID: PMC4629108.
Uppuluri CT, Ravi PR, Dalvi AV. Design, optimization and pharmacokinetic evaluation of Piribedil loaded solid lipid nanoparticles dispersed in nasal in situ gelling system for effective management of Parkinson’s disease. Int J Pharm. 2021;606:120881. https://doi.org/10.1016/j.ijpharm.2021.120881. Epub 2021 Jul 14. PMID: 34273426.
Sun Y, Li L, Xie H, Wang Y, Gao S, Zhang L, Bo F, Yang S, Feng A. Primary studies on construction and evaluation of ion-sensitive in situ gel loaded with paeonol-solid lipid nanoparticles for intranasal drug delivery. Int J Nanomedicine. 2020;15:3137–60. https://doi.org/10.2147/IJN.S247935. PMID: 32440115; PMCID: PMC7210040.
Hasan N, Imran M, Kesharwani P, Khanna K, Karwasra R, Sharma N, Rawat S, Sharma D, Ahmad FJ, Jain GK, Bhatnagar A, Talegaonkar S. Intranasal delivery of naloxone-loaded solid lipid nanoparticles as a promising simple and non-invasive approach for the management of opioid overdose. Int J Pharm. 2021;599:120428. https://doi.org/10.1016/j.ijpharm.2021.120428. Epub 2021 Mar 1. PMID: 33662465.
Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–7. https://doi.org/10.1007/s13205-014-0214-0. Epub 2014 Apr 8. PMID: 28324579; PMCID: PMC4362737.
Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–41. https://doi.org/10.1039/c5sm02958a. PMID: 26924445.
Barkat MA, Harshita RM, Pottoo FH, Beg S, Akhter S, Ahmad FJ. Therapeutic nanoemulsion: concept to delivery. Curr Pharm Des. 2020;26(11):1145–66. https://doi.org/10.2174/1381612826666200317140600. PMID: 32183664.
Bonferoni MC, Rossi S, Sandri G, Ferrari F, Gavini E, Rassu G, Giunchedi P. Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics. 2019;11(2):84. https://doi.org/10.3390/pharmaceutics11020084. PMID: 30781585; PMCID: PMC6409749.
Tedesco AC, Silva EPO, Jayme CC, Piva HL, Franchi LP. Cholesterol-rich nanoemulsion (LDE) as a novel drug delivery system to diagnose, delineate, and treat human glioblastoma. Mater Sci Eng C Mater Biol Appl. 2021;123:111984. https://doi.org/10.1016/j.msec.2021.111984. Epub 2021 Feb 23. PMID: 33812612.
Kotta S, Aldawsari HM, Badr-Eldin SM, Alhakamy NA, Md S. Coconut oil-based resveratrol nanoemulsion: optimization using response surface methodology, stability assessment and pharmacokinetic evaluation. Food Chem. 2021;357:129721.
Hao XY, Zhang YD, Hou YY, Sun X. [Applying chitosan-modified nanoemulsion in nasal vaccine delivery]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2021;52(4):592–597. Chinese. https://doi.org/10.12182/20210760104. PMID: 34323036.
Aderibigbe BA, Naki T. Design and efficacy of nanogels formulations for intranasal administration. Molecules. 2018;23(6):1241. https://doi.org/10.3390/molecules23061241. PMID: 29789506; PMCID: PMC6100477.
Hajebi S, Rabiee N, Bagherzadeh M, Ahmadi S, Rabiee M, Roghani-Mamaqani H, Tahriri M, Tayebi L, Hamblin MR. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019;92:1–18. https://doi.org/10.1016/j.actbio.2019.05.018. Epub 2019 May 13. PMID: 31096042; PMCID: PMC6661071.
Soni KS, Desale SS, Bronich TK. Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J Control Release. 2016;240:109–26. https://doi.org/10.1016/j.jconrel.2015.11.009. Epub 2015 Nov 10. PMID: 26571000; PMCID: PMC4862943.
Saindane NS, Pagar KP, Vavia PR. Nanosuspension based in situ gelling nasal spray of carvedilol: development, in vitro and in vivo characterization. AAPS PharmSciTech. 2013;14(1):189–99. https://doi.org/10.1208/s12249-012-9896-y. Epub 2012 Dec 20. PMID: 23255198; PMCID: PMC3581647.
Vashist A, Kaushik A, Vashist A, Bala J, Nikkhah-Moshaie R, Sagar V, Nair M. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov Today. 2018;23(7):1436–43. https://doi.org/10.1016/j.drudis.2018.05.018. Epub 2018 May 20. PMID: 29775669; PMCID: PMC6598698.
Picone P, Sabatino MA, Ditta LA, Amato A, San Biagio PL, Mulè F, Giacomazza D, Dispenza C, Di Carlo M. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J Control Release. 2018;270:23–36. https://doi.org/10.1016/j.jconrel.2017.11.040. Epub 2017 Dec 2. PMID: 29196041.
Gadhave D, Rasal N, Sonawane R, Sekar M, Kokare C. Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: pharmacological and in vitro cytotoxicity studies. Int J Biol Macromol. 2021;167:906–20. https://doi.org/10.1016/j.ijbiomac.2020.11.047. Epub 2020 Nov 10. PMID: 33186648.
Nochi T, Yuki Y, Takahashi H, Sawada S, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, Tokuhara D, Kurokawa S, Takahashi Y, Tsukada H, Kozaki S, Akiyoshi K, Kiyono H. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater. 2010;9(7):572–8. https://doi.org/10.1038/nmat2784. Epub 2010 Jun 20. Erratum in: Nat Mater. 2010 Aug;9(8):685. PMID: 20562880.
Azegami T, Yuki Y, Sawada S, et al. Nanogel-based nasal ghrelin vaccine prevents obesity. Mucosal Immunol. 2017;10:1351–60. https://doi.org/10.1038/mi.2016.137.
Azegami T, Yuki Y, Nakahashi R, Itoh H, Kiyono H. Nanogel-based nasal vaccines for infectious and lifestyle-related diseases. Mol Immunol. 2018;98:19–24. https://doi.org/10.1016/j.molimm.2017.10.022. Epub 2017 Oct 31. PMID: 29096936.
Dahiya S, Dahiya R, Hernández E. Nanocarriers for anticancer drug targeting: recent trends and challenges. Crit Rev Ther Drug Carrier Syst. 2021;38(6):49–103. https://doi.org/10.1615/CritRevTherDrugCarrierSyst.2021035650.
Dahiya S, Dahiya R. Chapter 11. Recent nanotechnological advancements in delivery of peptide and protein macromolecules. In: Rauta PR, Mohanta YK, Nayak D, editors. Nanotechnology in biology and medicine: research advancements and future perspectives. BocaRaton: CRC Press, Taylor & Francis Group; 2019. p. 143–57.
de Oliveira Junior ER, Santos LCR, Salomão MA, Nascimento TL, de Almeida Ribeiro Oliveira G, Lião LM, Lima EM. Nose-to-brain drug delivery mediated by polymeric nanoparticles: influence of PEG surface coating. Drug Deliv Transl Res. 2020;10(6):1688–99. https://doi.org/10.1007/s13346-020-00816-2. PMID: 32613550.
Islam SU, Shehzad A, Ahmed MB, Lee YS. Intranasal delivery of nanoformulations: a potential way of treatment for neurological disorders. Molecules. 2020;25(8):1929. https://doi.org/10.3390/molecules25081929. PMID: 32326318; PMCID: PMC7221820.
Musumeci T, Bonaccorso A, Puglisi G. Epilepsy disease and nose-to-brain delivery of polymeric nanoparticles: an overview. Pharmaceutics. 2019;11(3):118. https://doi.org/10.3390/pharmaceutics11030118. PMID: 30871237; PMCID: PMC6471219.
Merkus FW, van den Berg MP. Can nasal drug delivery bypass the blood-brain barrier?: questioning the direct transport theory. Drugs R D. 2007;8(3):133–44. https://doi.org/10.2165/00126839-200708030-00001. PMID: 17472409.
Jani P, Vanza J, Pandya N, Tandel H. Formulation of polymeric nanoparticles of antidepressant drug for intranasal delivery. Ther Deliv. 2019;10(11):683–96. https://doi.org/10.4155/tde-2019-0060. Epub 2019 Nov 20. PMID: 31744396.
Yu S, Xu X, Feng J, Liu M, Hu K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int J Pharm. 2019;560:282–93. https://doi.org/10.1016/j.ijpharm.2019.02.012. Epub 2019 Feb 14. PMID: 30772458.
Cortés H, Alcalá-Alcalá S, Caballero-Florán IH, Bernal-Chávez SA, Ávalos-Fuentes A, González-Torres M, González-Del Carmen M, Figueroa-González G, Reyes-Hernández OD, Floran B, Del Prado-Audelo ML, Leyva-Gómez G. A reevaluation of chitosan-decorated nanoparticles to cross the blood-brain barrier. Membranes (Basel). 2020;10(9):212. https://doi.org/10.3390/membranes10090212. PMID: 32872576; PMCID: PMC7559907.
Dahiya S. Block copolymeric micelles: basic concept and preparation techniques. Bull Pharma Res. 2019;9(1–3):166. https://doi.org/10.21276/bpr.2019.9.7.
Dahiya R, Dahiya S. Chapter 13. Advanced drug delivery applications of self-assembled nanostructures and polymeric nanoparticles. In: Anand K, Saravanan M, Chandrasekaran B, Kanchi S, Panchu SJ, Chen Q-S, editors. Handbook on nano-biomaterials for therapeutics and diagnostic applications. Netherlands: Elsevier; 2021. p. 297–339.
Sipos B, Csóka I, Budai-Szűcs M, Kozma G, Berkesi D, Kónya Z, Balogh GT, Katona G. Development of dexamethasone-loaded mixed polymeric micelles for nasal delivery. Eur J Pharm Sci. 2021;166:105960. https://doi.org/10.1016/j.ejps.2021.105960. Epub 2021 Jul 31. PMID: 34339828.
Cerqueira SR, Ayad NG, Lee JK. Neuroinflammation treatment via targeted delivery of nanoparticles. Front Cell Neurosci. 2020;14:576037. https://doi.org/10.3389/fncel.2020.576037. PMID: 33192321; PMCID: PMC7555434.
Sipos B, Szabó-Révész P, Csóka I, Pallagi E, Dobó DG, Bélteky P, Kónya Z, Deák Á, Janovák L, Katona G. Quality by design based formulation study of meloxicam-loaded polymeric micelles for intranasal administration. Pharmaceutics. 2020;12(8):697. https://doi.org/10.3390/pharmaceutics12080697. PMID: 32722099; PMCID: PMC7464185.
Zhang L, Yang S, Huang L, Ho PC. Poly (ethylene glycol)-block-poly (D, L-lactide) (PEG-PLA) micelles for brain delivery of baicalein through nasal route for potential treatment of neurodegenerative diseases due to oxidative stress and inflammation: an in vitro and in vivo study. Int J Pharm. 2020;591:119981. https://doi.org/10.1016/j.ijpharm.2020.119981. Epub 2020 Oct 15. PMID: 33069896.
Kanazawa T. Brain delivery of small interfering ribonucleic acid and drugs through intranasal administration with nano-sized polymer micelles. Med Devices (Auckl). 2015;8:57–64. https://doi.org/10.2147/MDER.S70856. PMID: 25610007; PMCID: PMC4294762.
Torres-Martinez EJ, Cornejo Bravo JM, Serrano Medina A, Pérez González GL, Villarreal Gómez LJ. A summary of electrospun nanofibers as drug delivery system: drugs loaded and biopolymers used as matrices. Curr Drug Deliv. 2018;15(10):1360–74. https://doi.org/10.2174/1567201815666180723114326. PMID: 30033869; PMCID: PMC6376322.
Shahriar SMS, Mondal J, Hasan MN, Revuri V, Lee DY, Lee YK. Electrospinning nanofibers for therapeutics delivery. Nanomaterials (Basel). 2019;9(4):532. https://doi.org/10.3390/nano9040532. PMID: 30987129; PMCID: PMC6523943.
Si Y, Wen Y, Kelly SH, Chong AS, Collier JH. Intranasal delivery of adjuvant-free peptide nanofibers elicits resident CD8+ T cell responses. J Control Release. 2018;282:120–30. https://doi.org/10.1016/j.jconrel.2018.04.031. Epub 2018 Apr 17. PMID: 29673645; PMCID: PMC6309200.
Abbasi E, Aval SF, Akbarzadeh A, Milani M, Nasrabadi HT, Joo SW, Hanifehpour Y, Nejati-Koshki K, Pashaei-Asl R. Dendrimers: synthesis, applications, and properties. Nanoscale Res Lett. 2014;9(1):247. https://doi.org/10.1186/1556-276X-9-247. PMID: 24994950; PMCID: PMC4074873.
Xie H, Li L, Sun Y, Wang Y, Gao S, Tian Y, Ma X, Guo C, Bo F, Zhang L. An available strategy for nasal brain transport of nanocomposite based on PAMAM dendrimers via in situ gel. Nanomaterials (Basel). 2019;9(2):147. https://doi.org/10.3390/nano9020147. PMID: 30682799; PMCID: PMC6409925.
De Jong WH, Borm PJ. Drug delivery and nanoparticles:applications and hazards. Int J Nanomedicine. 2008;3(2):133–49. https://doi.org/10.2147/ijn.s596. PMID: 18686775; PMCID: PMC2527668.
Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, Qoronfleh MW. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23:20. https://doi.org/10.1186/s40824-019-0166-x. PMID: 31832232; PMCID: PMC6869321.
Ahmed TA, Aljaeid BM. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des Devel Ther. 2016;10:483–507. https://doi.org/10.2147/DDDT.S99651. PMID: 26869768; PMCID: PMC4734734.
Ahmad N, Ahmad R, Ahmad FJ, Ahmad W, Alam MA, Amir M, Ali A. Poloxamer-chitosan-based Naringenin nanoformulation used in brain targeting for the treatment of cerebral ischemia. Saudi J Biol Sci. 2020;27(1):500–17. https://doi.org/10.1016/j.sjbs.2019.11.008. Epub 2019 Nov 22. PMID: 31889876; PMCID: PMC6933235.
Li Y, Li J, Zhang X, Ding J, Mao S. Non-ionic surfactants as novel intranasal absorption enhancers: in vitro and in vivo characterization. Drug Deliv. 2016;23(7):2272–9. https://doi.org/10.3109/10717544.2014.971196. Epub 2014 Oct 27. PMID: 25347689.
Anand U, Feridooni T, Remigius U. Novel mucoadhesive polymers for nasal drug delivery. 2012. Intechopen Book Series. Open access peer-reviewed chapter. https://doi.org/10.5772/52560. Available from https://www.intechopen.com/chapters/40266
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dahiya, S., Dahiya, R. (2023). An Overview on Nanocarriers for Nasal Delivery. In: Pathak, Y.V., Yadav, H.K.S. (eds) Nasal Drug Delivery. Springer, Cham. https://doi.org/10.1007/978-3-031-23112-4_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-23112-4_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23111-7
Online ISBN: 978-3-031-23112-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)