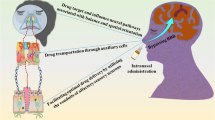
Abstract
Over the recent years, many strategies have improved the delivery of bioactive agents to the brain, improving the treatment of several pathologies. Nonetheless, the design of new formulations is highly dependent on the capacity of the drugs to permeate the blood-brain barrier (BBB) and reach a significant effect on the neurological disorders. Therefore, different approaches have been studied in order to facilitate the delivery of different drugs into the brain; for instance, intranasal administration has gained special interest. The nose-to-brain delivery provides a direct pathway of drug delivery to the brain without the need to permeate the BBB, potentially avoiding adverse effects that could occur when the drug is systemically absorbed. Although it may overcome BBB, there are alternative barriers that have to be circumvented for nose-to-brain route and different strategies have been massively studied. This chapter provides a comprehensive overview of factors related to the physiology of nasal cavity, the drug, and the formulation that can affect the development of formulations for nose-to-brain drug delivery.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Illum L. Bioadhesive formulations for nasal peptide delivery. In: Mathiowitz E, Chickering III DE, Lehr C-M, editors. Bioadhesive drug delivery systems: fundamentals, novel approaches, and development. New York: Marcel Dekker; 1999. p. 507–39.
Touitou E, Illum L. Nasal drug delivery. Drug Deliv Transl Res. 2013;3:1–3.
Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2003;7(23):118–1189.
Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov Today [Internet] 2002;7(18):967–75. Available from: https://doi.org/10.1016/S1359-6446(02)02452-2.
Illum L. Nasal drug delivery — possibilities, problems and solutions. J Control Release. 2003;87:187–98.
Bahadur S, Pathak K. Physicochemical and physiological considerations for efficient nose-to-brain targeting Physicochemical and physiological considerations for efficient nose-to-brain targeting. Expert Opin Drug Deliv. 2012;9:19–31.
Wu H, Hu K, Jiang X. From nose to brain: understanding transport capacity and transport rate of drugs. Expert Opin Drug Deliv. 2008;5(10):1159–68.
Grassin-Delyle S, Buenestado A, Naline E, Faisy C, Blouquit-Laye S, Couderc LJ, et al. Intranasal drug delivery: an efficient and non-invasive route for systemic administration - focus on opioids. Pharmacol Ther [Internet]. 2012;134(3):366–79. Available from: https://doi.org/10.1016/j.pharmthera.2012.03.003.
Behl CR, Pimplaskar HK, Sileno AP, DeMeireles J, Romeo VD. Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv Drug Deliv Rev. 1998;29(1–2):89–116.
Laffleur F, Bauer B. Progress in nasal drug delivery systems. Int J Pharm [Internet]. 2021;607(July):120994. Available from: https://doi.org/10.1016/j.ijpharm.2021.120994.
Vyas TK, Tiwari SB, Amiji MM. Formulation and physiological factors influencing cns delivery upon intranasal administration. Crit Rev Therap Drug Carrier Syst. 2006;23(4):319–47.
Journal AI, Kaur P, Garg T, Rath G, Goyal AK, Kaur P, et al. In situ nasal gel drug delivery: a novel approach for brain targeting through the mucosal membrane. Artif Cells Nanomed Biotechnol. 2016;44:1167–76.
Jadhav KR, Gambhire MN, Shaikh IM, Kadam VJ, Pisal SS. Nasal drug delivery system-factors affecting and applications. Curr Drug Ther. 2007;2:27–38.
Singla DR, Kohrt BA, Murray LK, Anand A, Chorpita BF, Patel V. Psychological treatments for the world: lessons from low- and middle-income countries. Annu Rev Clin Psychol. 2017;13:149–81.
Martins PP, Smyth HDC, Cui Z. Strategies to facilitate or block nose-to-brain drug delivery. Int J Pharm. 2019;570(May):118635.
Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1–18.
Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci. 2003;6(2):252–73.
Quintana DS, Guastella AJ, Westlye LT, Andreassen OA. The promise and pitfalls of intranasally administering psychopharmacological agents for the treatment of psychiatric disorders. Mol Psychiatry. 2016;21(1):29–38.
Illum L. Nasal drug delivery - recent developments and future prospects. J Control Release [Internet]. 2012;161(2):254–63. Available from: https://doi.org/10.1016/j.jconrel.2012.01.024.
Xi J, Zhang Z, Si XA. Improving intranasal delivery of neurological nanomedicine to the olfactory region using magnetophoretic guidance of microsphere carriers. Int J Nanomedicine. 2015;10:1211–22.
Sakane T, Akizuki M, Taki Y, Yamashita S, Sezaki H, Nadai T. Direct drug transport from the rat nasal cavity to the cerebrospinal fluid: the relation to the molecular weight of drugs. J Pharm Pharmacol. 1995;47(5):379–81.
Horvát S, Fehér A, Wolburg H, Sipos P, Veszelka S, Tóth A, et al. Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue. Eur J Pharm Biopharm. 2009;72(1):252–9.
Li Y, Li J, Zhang X, Ding J, Mao S. Non-ionic surfactants as novel intranasal absorption enhancers: in vitro and in vivo characterization. Drug Deliv. 2016;23(7):2272–9.
Rassu G, Soddu E, Cossu M, Gavini E, Giunchedi P, Dalpiaz A. Particulate formulations based on chitosan for nose-to-brain delivery of drugs. A review. J Drug Deliv Sci Technol [Internet]. 2016;32:77–87. Available from: https://doi.org/10.1016/j.jddst.2015.05.002.
Shi NQ, Qi XR, Xiang B, Zhang Y. A survey on “trojan Horse” peptides: opportunities, issues and controlled entry to “troy.” J Control Release [Internet]. 2014;194:53–70. Available from: https://doi.org/10.1016/j.jconrel.2014.08.014.
Kamei N, Takeda-Morishita M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J Control Release [Internet]. 2015;197:105–10. Available from: https://doi.org/10.1016/j.jconrel.2014.11.004.
Kanazawa T, Akiyama F, Kakizaki S, Takashima Y, Seta Y. Delivery of siRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micelles. Biomaterials [Internet]. 2013;34(36):9220–6. Available from: https://doi.org/10.1016/j.biomaterials.2013.08.036.
Khan T, Ranjan R, Dogra Y, Pandya SM, Shafi H, Singh SK, et al. Intranasal eutectic powder of zolmitriptan with enhanced bioavailability in the rat brain. Mol Pharm. 2016;13(9):3234–40.
Li F, Feng J, Cheng Q, Zhu W, Jin Y. Delivery of 125 I-cobrotoxin after intranasal administration to the brain: a microdialysis study in freely moving rats. Int J Pharm. 2007;328:161–7.
Hussain MA, Shenvi AB, Rowe SM, Shefter E. The use of α-aminoboronic acid derivatives to stabilize peptide drugs during their intranasal absorption. Pharm Res. 1989;6:186–9.
Dhamankar V, Donovan MD. Modulating nasal mucosal permeation using metabolic saturation and enzyme inhibition techniques. J Pharm Pharmacol. 2017;69:1075–83.
Shingaki T, Hidalgo IJ, Furubayashi T, Sakane T, Katsumi H, Yamamoto A, et al. Nasal delivery of P-gp substrates to the brain through the nosebrain pathway. Drug Metab Pharmacokinet. 2011;26(3):248–55.
Graff CL, Zhao R, Pollack GM. Pharmacokinetics of substrate uptake and distribution in murine brain after nasal instillation. Pharm Res. 2005;22(2):235–44.
Hada N, Netzer WJ, Belhassan F, Wennogle LP, Gizurarson S. Nose-to-brain transport of imatinib mesylate: a pharmacokinetic evaluation. Eur J Pharm Sci. 2017;102:46–54.
Gao X, Tao W, Lu W, Zhang Q, Zhang Y, Jiang X, et al. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006;27(18):3482–90.
Mistry A, Stolnik S, Illum L. Nanoparticles for direct nose-to-brain delivery of drugs. Int J Pharm. 2009;379(1–2):146–57.
Singh A, Ubrane R, Prasad P, Ramteke S. Preparation and characterization of rizatriptan benzoate loaded solid lipid nanoparticles for brain targeting. Mater Today Proc [Internet]. 2015;2(9):4521–43. Available from: https://doi.org/10.1016/j.matpr.2015.10.067.
Yasir M, Sara UVS. Solid lipid nanoparticles for nose to brain delivery of haloperidol: in vitro drug release and pharmacokinetics evaluation. Acta Pharm Sin B [Internet]. 2014;4(6):454–63. Available from: https://doi.org/10.1016/j.apsb.2014.10.005.
Al Asmari AK, Ullah Z, Tariq M, Fatani A. Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des Devel Ther. 2016;10:205–15.
Agrawal M, Saraf S, Saraf S, Dubey SK, Puri A, Gupta U, et al. Stimuli-responsive in situ gelling system for nose-to-brain drug delivery. J Control Release [Internet]. 2020;327:235–65. Available from: https://doi.org/10.1016/j.jconrel.2020.07.044.
Emad NA, Ahmed B, Alhalmi A, Alzobaidi N, Al-Kubati SS. Recent progress in nanocarriers for direct nose to brain drug delivery. J Drug Deliv Sci Technol [Internet]. 2021;64(June):102642. Available from: https://doi.org/10.1016/j.jddst.2021.102642.
Pokharkar V, Suryawanshi S, Dhapte-Pawar V. Exploring micellar-based polymeric systems for effective nose-to-brain drug delivery as potential neurotherapeutics. Drug Deliv Transl Res. 2020;10(4):1019–31.
Khutoryanskiy VV. Advances in mucoadhesion and mucoadhesive polymers. Macromol Biosci. 2011;11(6):748–64.
Bassi da Silva J, de Ferreira SBS, de Freitas O, Bruschi ML. A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Dev Ind Pharm [Internet]. 2017;9045(March):1–67. Available from: https://www.tandfonline.com/doi/full/10.1080/03639045.2017.1294600.
Charlton ST, Davis SS, Illum L. Evaluation of effect of ephedrine on the transport of drugs from the nasal cavity to the systemic circulation and the central nervous system. J Drug Target. 2007;15(5):370–7.
Jansson B, Hägerström H, Fransén N, Edsman K, Björk E. The influence of gellan gum on the transfer of fluorescein dextran across rat nasal epithelium in vivo. Eur J Pharm Biopharm. 2005;59(3):557–64.
Mura P, Mennini N, Nativi C, Richichi B. In situ mucoadhesive-thermosensitive liposomal gel as a novel vehicle for nasal extended delivery of opiorphin. Eur J Pharm Biopharm [Internet]. 2018;122(June 2017):54–61. Available from: https://doi.org/10.1016/j.ejpb.2017.10.008.
Cook MT, Brown MB. Polymeric gels for intravaginal drug delivery. J Control Release [Internet]. 2018;270(December 2017):145–57. Available from: https://doi.org/10.1016/j.jconrel.2017.12.004.
Porfiryeva NN, Semina II, Salakhov IA, Moustafine RI, Khutoryanskiy VV. Mucoadhesive and mucus-penetrating interpolyelectrolyte complexes for nose-to-brain drug delivery. Nanomedicine Nanotechnol Biol Med [Internet]. 2021;37:102432. Available from: https://doi.org/10.1016/j.nano.2021.102432.
da Silva JB, Cook MT, Bruschi ML. Thermoresponsive systems composed of poloxamer 407 and HPMC or NaCMC: mechanical, rheological and sol-gel transition analysis. Carbohydr Polym [Internet]. 2020;240(March):116268. Available from: https://doi.org/10.1016/j.carbpol.2020.116268.
Bruschi ML, de Souza Ferreira SB, Bassi da Silva J. Mucoadhesive and mucus-penetrating polymers for drug delivery. Nanotechnol Oral Drug Deliv. 2020:77–141.
Li L, Shan H, Yue CY, Lam YC, Tam KC, Hu X. Thermally induced association and dissociation of methylcellulose in aqueous solutions. Langmuir. 2002;18(20):7291–8.
Date AA, Halpert G, Babu T, Ortiz J, Kanvinde P, Dimitrion P, et al. Mucus-penetrating budesonide nanosuspension enema for local treatment of inflammatory bowel disease. Biomaterials [Internet]. 2018;185(June):97–105. Available from: https://doi.org/10.1016/j.biomaterials.2018.09.005.
Dzieciuch M, Rissanen S, Szydłowska N, Bunker A, Kumorek M, Jamróz D, et al. Pegylated liposomes as carriers of hydrophobic porphyrins. J Phys Chem B. 2015;119(22):6646–57.
Dhuria SV, Hanson LR, Frey WH. Intranasal drug targeting of hypocretin-1 (orexin-A) to the central nervous system. J Pharm Sci. 2009;98(7):2501–15.
Bruschi ML, de Toledo LAS. Pharmaceutical applications of iron-oxide magnetic nanoparticles. In: Katz E, editor. Magnetic Nanoparticles. Bassel: MDPI; 2020. p. 408.
Chen H, Yang GZX, Getachew H, Acosta C, Sierra Sánchez C, Konofagou EE. Focused ultrasound-enhanced intranasal brain delivery of brain-derived neurotrophic factor. Sci Rep. 2016;6(June):1–8.
Bruschi ML. Strategies to modify the drug release from pharmaceutical systems. 1st ed. Bruschi ML, editor. Elsevier; 2015. 208 p.
Jassim ZE, Al-Akkam E. A review on strategies for improving nasal drug delivery systems. Drug Innov Today. 2018;10(1):2857–64.
Yu L. Pharmaceutical quality by design: product and process development, understanding, and control. Pharm Res. 2008;25(4):781–91.
ICH Expert Working Group. Pharmaceutical development Q8. ICH Harmon Tripart Guide. 2009;8(August):1–28.
Bruns RE., Scarmínio IS, Barros Neto B. Statistical design - Chemometrics. Amsterdam: Elsevier; 2006. 412 p.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
da Silva, J.B., Botan, M.V.G., Bruschi, M.L. (2023). Factors Affecting the Design of Nasal Drug Delivery System. In: Pathak, Y.V., Yadav, H.K.S. (eds) Nasal Drug Delivery. Springer, Cham. https://doi.org/10.1007/978-3-031-23112-4_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-23112-4_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23111-7
Online ISBN: 978-3-031-23112-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)