Abstract
The intranasal (IN) route of drug administration has emerged as an alternative route over the systemic (oral and parenteral) drug delivery to the brain.
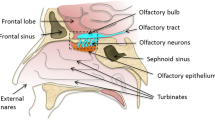
The intranasal route of drug administration exhibits as a non-invasive technique to bypass the BBB for the delivery of drugs inside the brain and the CNS. This method is helpful for those drugs that are unable to invade the BBB to show its action in the CNS and thus erase the demand of systemic delivery and shrink systemic side effects. Drug delivery from the nose to the brain/CNS takes very less time through both olfactory and trigeminal nerves. Intranasal delivery does not require the involvement of any receptor as it occurs through an extracellular route. The delivery of the drug via an IN route offers various advantages over a systemic drug delivery system as it directly delivers the drug into the brain via the olfactory route. The presence of drugs in the olfactory bulb, in turn, increases the drug bioavailability in the brain and reduces degradation as well as wastage of the drug through systemic clearance. However, there are some limitations associated with IN like poor drug permeation through the nasal mucosa and mucociliary clearance. There are many novel drug delivery strategies (nano-drug carrier system, colloidal carriers, mucoadhesive devices, controlled delivery system, pro-drug, etc.) are adapted to overcome the above-stated limitations. Nose-to-brain delivery also involves nasal-associated lymphatic tissues (NALT) and deep cervical lymph nodes. This review focuses on different strategies for nose-to-brain delivery of small molecules.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Yang H. Nanoparticle-mediated brain-specific drug delivery, imaging, and diagnosis. Pharm Res. 2010;27:1759–71.
Wong HL, Chattopadhyay N, Wu XY, Bendayan R. Nanotechnology applications for improved delivery of antiretroviral drugs to the brain. Adv Drug Deliv Rev. 2010;62:503–17.
Biddlestone-Thorpe L, Marchi N, Guo K, Ghosh C, Janigro D, Valerie K, Yang H. Nanomaterial-mediated CNS delivery of diagnostic and therapeutic agents. Adv Drug Deliv Rev. 2012;64:605–13.
Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev. 2012;64:686–700.
Patel T, Zhou J, Piepmeier JM, Saltzman WM. Polymeric nanoparticles for drug delivery to the central nervous system. Adv Drug Deliv Rev. 2012;64:701–5.
Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6:268–86.
Agrawal M, Ajazuddin, Tripathi DK, Saraf S, Antimisiaris SG, Mourtas S, Hammarlund-Udenaes M, Alexander A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J Control Release. 2017;260:61–77.
Invernici G, Cristini S, Alessandri G, Navone SE, Canzi L, Tavian D, Redaelli C, Acerbi F, Parati EA. Nanotechnology advances in brain tumors: the state of the art. Recent Pat Anticancer Drug Discov. 2011;6:58–69.
Chaturvedi M, Kumar M, Pathak K. A review on mucoadhesive polymer used in nasal drug delivery system. J Adv Pharm Technol Res. 2011;2:215–22.
Baltzley S, Mohammad A, Malkawi AH, Al-Ghananeem AM. Intranasal drug delivery of olanzapine-loaded chitosan nanoparticles. AAPS PharmSciTech. 2014;15:1598–602.
Al-Ghananeem AM, Saeed H, Florence R, Yokel RA, Malkawi AH. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by aids viruses. J Drug Target. 2009;18:381–8.
Elnaggar YSR, Etman SM, Abdelmonsif DA, Abdallah OY. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: optimization, biological efficacy, and potential toxicity. J Pharm Sci. 2015;104:3544–56.
Haque S, Md S, Sahni JK, Ali J, Baboota S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J Psychiatr Res. 2014;48:1–12.
Lu CT, Jin RR, Jiang YN, Lin Q, Yu WZ, Mao KL, Tian FR, Zhao YP, Zhao YZ. Gelatin nanoparticle-mediated intranasal delivery of substance P protects against 6-hydroxydopamine-induced apoptosis: an in vitro and in vivo study. Drug Des Devel Ther. 2015;9:1955.
Van Woensel M, Wauthoz N, Rosière R, Mathieu V, Kiss R, Lefranc F, Steelant B, Dilissen E, Van Gool SW, Mathivet T, Gerhardt H, Amighi K, De Vleeschouwer S. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J Control Release. 2016;227:71–81.
Gao X, Tao W, Lu W, Zhang Q, Zhang Y, Jiang X, Fu S. Lectin-conjugated PEG–PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006;27:3482–90.
Gao X, Wu B, Zhang Q, Chen J, Zhu J, Zhang W, Rong Z, Chen H, Jiang X. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Control Release. 2007;121:156–67.
Liu Q, Shao X, Chen J, Shen Y, Feng C, Gao X, Zhao Y, Li J, Zhang Q, Jiang X. In vivo toxicity and immunogenicity of wheat germ agglutinin conjugated poly (ethylene glycol)-poly (lactic acid) nanoparticles for intranasal delivery to the brain. Toxicol Appl Pharmacol. 2011;251:79–84.
Lavelle EC, Grant G, Pusztai A, Pfüller U, O’Hagan DT. Mucosal immunogenicity of plant lectins in mice. Immunology. 2000;99:30–7.
Lavelle EC, Grant G, Pfuller U, O’Hagan DT. Immunological implications of the use of plant lectins for drug and vaccine targeting to the gastrointestinal tract. J Drug Target. 2004;12:89–95.
Li J, Wu H, Hong J, Xu X, Yang H, Wu B, Wang Y, Zhu J, Lai R, Jiang X, Lin D, Prescott MC, Rees HH. Odorranalectin is a small peptide lectin with potential for drug delivery and targeting. PLoS One. 2008;3:e2381.
Lundh B, Brockstedt U, Kristensson K. Lectin-binding pattern of neuroepithelial and respiratory epithelial cells in the mouse nasal cavity. Histochem J. 1989;21:33–43.
Abuirmeileh A, Harkavyi A, Kingsbury A, Lever R, Whitton PS. The CRF-like peptide urocortin produces a long-lasting recovery in rats made hemiparkinsonian by 6-hydroxydopamine or lipopolysaccharide. J Neurol Sci. 2008;271:131–6.
Abuirmeileh A, Lever R, Kingsbury AE, Lees AJ, Locke IC, Knight RA, Chowdrey HS, Biggs CS, Whitton PS. The corticotrophin-releasing factor-like peptide urocortin reverses key deficits in two rodent models of Parkinson’s disease. Eur J Neurosci. 2007;26:417–23.
Wen Z, Yan Z, Hu K, Pang Z, Cheng X, Guo L, Zhang Q, Jiang X, Fang L, Lai R. Odorranalectin-conjugated nanoparticles: preparation brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J Control Release. 2011;151:131–8.
Wu H, Li J, Zhang Q, Yan X, Guo L, Gao X, Qiu M, Jiang X, Lai R, Chen H. A novel small Odorranalectin-bearing cubosomes: preparation, brain delivery and pharmacodynamic study on amyloid- β25–35-treated rats following intranasal administration. Eur J Pharm Biopharm. 2012;80:368–78.
Fillebeen C, Descamps L, Dehouck MP, Fenart L, Benaïssa M, Spik G, Cecchelli R, Pierce A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J Biol Chem. 1999;274:7011–7.
Huang RQ, Ke WL, Qu YH, Zhu JH, Pei YY, Jiang C. Characterization of lactoferrin receptor in brain endothelial capillary cells and mouse brain. J Biomed Sci. 2007;14:121–8.
Suzuki YA, Lopez V, Lönnerdal B. Mammalian lactoferrin receptors: structure and function. Cell Mol Life Sci. 2005;62:2560–75.
Bi C, Wang A, Chu Y, Liu S, Mu H, Liu W, Wu Z, Sun K, Li Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int J Nanomedicine. 2016;11:6547–59.
Devalia JL, Sapsford RJ, Wells CW, Richman P, Davies RJ. Culture and comparison of human bronchial and nasal epithelial cells in vitro. Respir Med. 1990;84:303–12.
Liu Z, Jiang M, Kang T, Miao D, Gu G, Song Q, Yao L, Hu Q, Tu Y, Pang Z, Chen H, Jiang X, Gao X, Chen J. Lactoferrin-modified PEG-o-PCL nanoparticles for enhanced brain delivery of NAPde following intranasal administration. Biomaterials. 2013;34:3870–81.
Mussbach F, Franke M, Zoch A, Schaefer B, Reissmann S. Transduction of peptides and proteins into live cells by cell penetrating peptides. J Cell Biochem. 2011;112:3824–33.
Mussbach F, Franke M, Zoch A, Schaefer B, Reissmann S. Transduction of peptides. Proteins and nucleotides into live cells by cell penetrating peptides. ChemPlusChem. 2015;13:90–5.
Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv Drug Deliv Rev. 2007;59:134–40.
Meade BR, Dowdy SF. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv Drug Deliv Rev. 2008;60:530–6.
Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18:385–93.
Lehto T, Kurrikoff K, Langel Ü. Cell-penetrating peptides for the delivery of nucleic acids. Expert Opin Drug Deliv. 2012;9:823–36.
Margus H, Padari K, Pooga M. Cell-penetrating peptides as versatile vehicles for oligonucleotide delivery. Mol Ther. 2012;20:525–33.
Jaafari M, Tafaghodi M, Sa ST. Evaluation of the clearance characteristics of liposomes in the human nose by gamma-scintigraphy. Iran J Pharm Res. 2005;1:3–11.
Andresen TL, Jensen SS, Jørgensen K. Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res. 2005;44:68–97.
Li SD, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145:178–81.
Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65:403–18.
Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm. 2006;322:60–6.
Jain S, Jain P, Umamaheshwari RB, Jain NK. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: development. Characterization and performance evaluation. Drug Dev Ind Pharm. 2003;29:1013–26.
Li W, Zhou Y, Zhao N, Hao B, Wang X, Kong P, et al. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ Toxicol Pharmacol. 2012;34:272–9.
Tenovuo O. Central acetylcholinesterase inhibitors in the treatment of chronic traumatic brain injury - clinical experience in 111 patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:61–7.
Venkatesh K, Bullock R, Akbaş A. Strategies to improve tolerability of rivastigmine: a case series. Curr Med Res Opin. 2007;23:93–5.
Lamb HM, Goa KL. Rivastigmine - A pharmacoeconomic review of its use in Alzheimer’s disease. PharmacoEconomics. 2001;19:303–18.
Spencer CM, Noble S. Rivastigmine - A review of its use in Alzheimer’s disease. Drugs Aging. 1998;13:391–411.
Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: fit to deliver small RNA. Commun Integr Biol. 2010;3:447–50.
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9.
El-Andaloussi S, Lakhal S, Mäger I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391–7.
Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. 2012;3:228.
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–14.
Born J, Lange T, Kern W, et al. Sniffi ng neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–6.
Fehm HL, Perras B, Smolink R, et al. Manipulating neuropeptidergic pathways in expert Opin. Drug Deliv. Downloaded from informahealthcare.com by Dalhousie University on 07/25/12 For personal humans: a novel approach to neuropharmacology. Eur J Pharmacol. 2000;405:43–54.
Sakane T, Akizuki M, Yamashita S, et al. The transport of a drug to the cerebrospinal fluid directly from the nasal cavity: the relation to the lipophilicity of the drug. Chem Pharm Bull. 1991;39:2456–85.
Jiang XG, Lu X, Cui JB, et al. Studies on octanol-water partition coefficient and nasal drug absorption. Acta Pharm Sin. 1997;32:458–60.
Sakane T, Akizuki M, Yamashita S, et al. Direct drug transport from the nasal cavity to the cerebrospinal fluid: the relation to the dissociation of the drug. J Pharm Pharmacol. 1994;46:378.
Alagusundara M, Chengaiah B, Gnanaprakash K, et al. Nasal drug delivery system; an overview. Int J Pharm Sci Res. 2010;1(4):454–65.
Maliheh G, Young PM, Traini D, et al. Strategies to enhance drug absorption via nasal and pulmonary routes; A review. Pharmaceutics. 2019;11:113.
Aurora J. Development of nasal drug delivery system; A review. Drug Deliv Technol. 2002;2(7):1–8.
Chhajed S, Sangale S, Barhate SD. Advantageous nasal drug delivery system: A review. ijpsr. 2011;2(6):1322–36.
Jassim ZE, Al Akkam EJ. A review on strategies for improving nasal drug delivery system. Drug Invention Today. 2018;10:1.
Jadhav K, Manoj NG, Shaikh IM, et al. Nasal drug delivery system- factors affecting and applications. Current Drug Therapy. 2007;2(1):27–38.
Prajapati M, Mandloi R, Pillai S, et al. The review on the nasal drug delivery. Asian J Pharm Res. 2020;10(2):110–6.
Dhakar RC, Maurya SD, Tilak VK, et al. A review on Factor affecting the design of nasal drug delivery system. Int J Drug Deliv. 2011;(3):194–208.
Erdoa F, Borsa LA, Farkasa D, Bajzaa A, Gizurarsonb S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–70, 165.
Jassim ZE, Al-Akkam EJ. A review on strategies for improving nasal drug delivery systems. Drug Invention Today. 2018;10(Special Issue 1):2861.
Savale S, Mahajan H. Nose to brain: a versatile mode of drug delivery system. Asian J Biomater Res. 2017;3(1):16–38, 31.
Djupesland G, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv. 2014;5(6):709–33, 722.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Borkar, S.P., Raizaday, A. (2023). Different Strategies for Nose-to-Brain Delivery of Small Molecules. In: Pathak, Y.V., Yadav, H.K.S. (eds) Nasal Drug Delivery. Springer, Cham. https://doi.org/10.1007/978-3-031-23112-4_17
Download citation
DOI: https://doi.org/10.1007/978-3-031-23112-4_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23111-7
Online ISBN: 978-3-031-23112-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)