Abstract
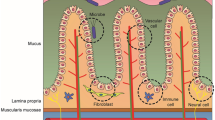
Nasal drug administration is attractive noninvasive approach to rapidly achieve local or systemic effects of small molecular drugs, proteins, and peptides, as well as for nasal immunization and nose-to-brain targeted delivery. However, conventional nasal dosage forms such as nasal drops, sprays, and nasal powders, are often suboptimal to provide bioavailability higher than 10% of drug molecules with high lipophilicity, hydrophilicity, or molecular mass, due to the nasal mucociliary clearance mechanism. The most extensively investigated formulation strategy for improving nasal bioavailability is the development of mucoadhesive drug delivery systems. Novel nasal liquid preparations with in situ gelling polymers have been proposed as a promising concept suitable for convinient instillation, precise dosing, good spreadability, enhanced nasal retention, and achievement of controlled drug release. Furthermore, mucoadhesive polymeric nanoparticles (up to 300 nm) have been evaluated as carriers for increased nose-to-brain bioavailability. This chapter reviews widely used mucoadhesive polymers and their derivatives considered so far as components of nasal drug delivery systems, including: natural polymers (chitosan, cellulose, starch, xanthan gum, gellan gum, pectins, alginates, gelatin) and their derivatives, synthetic polymers (polyacrylates, polycarbophils), copolymers and polymer blends (physical mixtures, polyelectrolyte complexes, cross-linked polymers). Many of novel polymers enable enhanced permeation enhancement capacity, in situ gelling and drug delivery control in response to nasal temperature, pH, ions, or enzymes, thus providing significant improvements in bioavailability of both small molecules and macromolecules. The versatility of the concept grows continuously by combining such responsive (smart) polymers or by functionalization of polymers by targeting ligands. Future prospects in development of mucoadhesive polymers as pharmaceutical excipients for nasal application are also briefly overviewed.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Tai J, Lee K, Kim TH. Current perspective on nasal delivery systems for chronic rhinosinusitis. Pharmaceutics. 2021;13(2):246.
Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2002;7(23):1184–9.
Illum L. Nasal drug delivery-possibilities, problems and solutions. J Control Release. 2003;87(1–3):187–98.
Bitter C, Suter-Zimmermann K, Surber C. Nasal drug delivery in humans. Curr Probl Dermatol. 2011;40:20–35.
Nave R, Schmitt H, Popper L. Faster absorption and higher systemic bioavailability of intranasal fentanyl spray compared to oral transmucosal fentanyl citrate in healthy subjects. Drug Deliv. 2012;20(5):216–23.
Ozsoy Y, Gungor S, Cevher E. Nasal delivery of high molecular weight drugs. Molecules. 2009;14:3754–79.
Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Discov Today. 2002;7(18):967–75.
Mato YL. Nasal route for vaccine and drug delivery: features and current opportunities. Int J Pharm. 2019;572:118813.
van den Berg AIS, Yun C-O, Schiffelers RM, Hennink WE. Polymeric delivery systems for nucleic acid therapeutics: approaching the clinic. J Control Release. 2021;331:121–41.
Rhea EM, Salameh TS, Banks WA. Intranasal delivery of proteins and peptides in the treatment of neurodegenerative diseases. AAPS J. 2015;17(4):780–7.
Charlton S, Jones NS, Davis SS, Illum L. Distribution and clearance of bioadhesive formulations from the olfactory region in man: effect of polymer type and nasal delivery device. Eur J Pharma Sci. 2007;30(3–4):295–302.
Agrawal M, Saraf S, Saraf S, et al. Nose-to-brain drug delivery: an update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J Control Release. 2018;281:139–77.
Alexander A, Agrawal M, Chougule MB, Saraf S, Saraf S. Nose-to-brain drug delivery: an alternative approach for effective brain drug targeting. In: Shegokar R, editor. Nanopharmaceuticals, volume 1: expectations and realities of multifunctional drug delivery systems. Amsterdam/New York: Elsevier; 2020. p. 175–200.
Giunchedi P, Gavini E, Bonferoni MC. Nose-to-brain delivery. Pharmaceutics. 2020;12(2):138.
Wang Z, Xiong G, Tsang WC, Schätzlein AG, Uchegbu IF. Nose-to-brain delivery. J Pharmacol Exp Ther. 2019;370(3):593–601.
Khan AR, Liu M, Khan MW, Zhai G. Progress in brain targeting drug delivery system by nasal route. J Control Release. 2017;268:364–89.
Kozlovskaya L, Abou-Kaoud M, Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189:133–40.
Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harb Perspect Biol. 2017;9(4):a028241.
Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17.
Pathak K. Mucoadhesion; A prerequisite or a constraint in nasal drug delivery? Int J Pharm Investig. 2011;1(2):62–3.
Chaturvedi M, Kumar M, Pathak K. A review on mucoadhesive polymer used in nasal drug delivery system. J Adv Pharm Technol Res. 2011;2(4):215–22.
Bertram U, Bodmeier R. In situ gelling, bioadhesive nasal inserts for extended drug delivery: in vitro characterization of a new nasal dosage form. Eur J Pharm Sci. 2006;27:62–71.
Jelkmann M, Leichner C, Zaichik S, Laffleur F, Bernkop-Schnürch A. A gellan gum derivative as in-situ gelling cationic polymer for nasal drug delivery. Int J Biol Macromol. 2020;158:1037–46.
Olafsson DR, Gizurarson S. Access to the olfactory region. Proc Control Release Bioact Mater. 2000;27:6318.
Manniello MD, Hosseini S, Alfaifi A, et al. In vitro evaluation of regional nasal drug delivery using multiple anatomical nasal replicas of adult human subjects and two nasal sprays. Int J Pharm. 2021;593:120103.
Abd El-Hameed MD, Kellaway IW. Preparation and in vitro characterisation of mucoadhesive polymeric microspheres as intra-nasal delivery systems. Eur J Pharm Biopharm. 1997;44:53–60.
Trows S, Scherließ R. Carrier-based dry powder formulation for nasal delivery of vaccines utilizing BSA as model drug. Powder Technol. 2016;292:223–31.
Javia A, Kore G, Misra A. Polymers in nasal drug delivery: an overview. In: Misra A, Shahiwala A, editors. Applications of polymers in drug delivery. 2nd ed. Amsterdam/New York: Elsevier; 2021. p. 305–32.
Dong J, Shang Y, Inthavong K, Chan H-K, Tu J. Partitioning of dispersed nanoparticles in a realistic nasal passage for targeted drug delivery. Int J Pharm. 2018;543:83–95.
Fasiolo LT, Manniello MD, Tratta E, Buttini F, Rossi A, Sonvico F, Bortolotti F, Russo P, Colombo G. Opportunity and challenges of nasal powders: drug formulation and delivery. Eur J Pharm Sci. 2018;113:2–1.
Feczko T. Polymeric nanotherapeutics acting at special regions of body. J Drug Deliv Sci Technol. 2021;64:102597.
Inthavonga K, Tiana ZF, Jy T, Yang W, Xue C. Optimising nasal spray parameters for efficient drug delivery using computational fluid dynamics. Comput Biol Med. 2008;38:713–26.
Prajapati SK, Jain A, Jain A, Jain S. Biodegradable polymers and constructs: a novel approach in drug delivery. Eur Polym J. 2019;120:109191.
Ritthidej GC. Nasal delivery of peptides and proteins with chitosan and related mucoadhesive polymers. In: Van Der Walle C, editor. Peptide and protein delivery. Elsevier: Academic Press; 2011. p. 47–68.
Djekic L, Martinovic M. In vitro, ex vivo and in vivo methods for characterization of bioadhesiveness of drug delivery systems. In: Mittal KL, Bakshi IS, Narang JK, editors. Bioadhesives in drug delivery. Wiley/Scrivener Publishing; 2020. p. 57–98.
Kumar K, Dhawan N, Sharma H, Vaidya S, Vaidya B. Bioadhesive polymers: novel tool for drug delivery. Artif Cells, Nanomed Biotechnol. 2014;42:274–83.
Casettari L, Illum L. Chitosan in nasal delivery systems for therapeutic drugs. J Control Release. 2014;190:189–200.
Khan S, Patil K, Bobade N, Yeole P, Gaikwad R. Formulation of intranasal mucoadhesive temperature-mediated in situ gel containing ropinirole and evaluation of brain targeting efficiency in rats. J Drug Target. 2010;18:223–34.
Patil S, Sawant K. Chitosan microspheres as a delivery system for nasal insufflation. Colloids Surf B Biointerfaces. 2011;84(2):384–9.
Sun M, Yu X, Wang T, Bi S, Liu Y, Chen X. Nasal adaptive chitosan-based nano-vehicles for anti-allergic drug delivery. Int J Biol Macromol. 2019;135:1182–92.
Chonkar A, Nayak U, Udupa N. Smart polymers in nasal drug delivery. Indian J Pharm Sci. 2015;77(4):367–75.
Ćirić A, Krajišnik D, Čalija B, Đekić L. Biocompatible non-covalent complexes of chitosan and different polymers: characteristics and application in drug delivery. Arch Pharm. 2020;70(4):173–97.
Ćirić A, Medarević Đ, Čalija B, Dobričić V, Mitrić M, Djekic L. Study of chitosan/xanthan gum polyelectrolyte complexes formation, solid state and influence on ibuprofen release kinetics. Int J Biol Macromol. 2020;148:942–955.r.
Abdel Mouez M, Zaki NM, Mansour S, Geneidi AS. Bioavailability enhancement of verapamil HCl via intranasal chitosan microspheres. Eur J Pharm Sci. 2014;51:59–66.
Patil S, Babbar A, Mathur R, Mishra A, Sawant K. Mucoadhesive chitosan microspheres of carvedilol for nasal administration. J Drug Target. 2010;18:321–31.
Pavis H, Wilcock A, Edgecombe J, et al. Pilot study of nasal morphine–chitosan for the relief of breakthrough pain in patients with cancer. J Pain Symptom Manag. 2002;24:598–602.
Stoker DG, Reber KR, Waltzman LS, et al. Analgesic efficacy and safety of morphine–chitosan nasal solution in patients with moderate to severe pain following orthopedic surgery. Pain Med. 2008;9:3–12.
Roon KI, Soons PA, Uitendaal MP, De Beukelaar F, Ferrari MD. Pharmacokinetic profile of alniditan nasal spray during and outside migraine attacks. Br J Clin Pharmacol. 1999;47:285–90.
Illum L, Davis SS. Chitosan as a delivery system for the transmucosal administration of drugs. In: Dumitriu S, editor. Polysaccharides. New York: Marcel Dekker; 2005. p. 643–60.
Gavini E, Hegge AB, Rassu G, et al. Nasal administration of carbamazepine using chitosan microspheres: in vitro/in vivo studies. Int J Pharm. 2006;307:9–15.
Wang X, Chi N, Tang X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur J Pharm Biopharm. 2008;70:735–40.
Varshosaz J, Sadrai H, Alinagari R. Nasal delivery of insulin using chitosan microspheres. J Microencapsul. 2004;21:761–74.
Illum L, Watts P, Fisher AN, Jabbal Gill I, Davis SS. Novel chitosan-based delivery systems for the nasal administration of a LHRH-analogue. STP Pharm Sci. 2000;10:89–94.
Snegovskikh DV. Intranasal morphine. In: Sinatra SR, Jahr JS, Watkins-Pitchford JM, editors. The essence of analgesia and analgesics. Cambridge: Cambridge University Press; 2010. p. 437–9.
Borchard G, Lueβen HL, de Boer AG, Verhoef JC, Lehr CM, Junginger HE. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. III: effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J Control Release. 1996;39(2–3):131–8.
Chenite A, Chaput C, Wang D, et al. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials. 2000;21:2155–61.
Sofi HS, Abdal-hay A, Ivanovski S, Zhang YS, Sheikh FA. Electrospun nanofibers for the delivery of active drugs through nasal, oral and vaginal mucosa: current status and future perspectives. Mater Sci Eng C. 2020;111:110756.
England RJA, Homer JJ, Knight LC, Ell SR. Nasal pH measurement: a reliable and repeatable parameter. Clin Otolaryngol Allied Sci. 1999;24:67–8.
Nazar H, Fatouros DG, van der Merwe SM, et al. Thermosensitive hydrogels for nasal drug delivery: the formulation and characterisation of systems based on N-trimethyl chitosan chloride. Eur J Pharm Biopharm. 2011;77:225–32.
Chung T, Liu D, Yang J. Effects of interpenetration of thermosensitive gels by crosslinking of chitosan on nasal delivery of insulin: in vitro characterization and in vivo study. Carbohydr Polym. 2010;82(2):316–22.
Al-Ghananeem AM, Saeed H, Florence R, Yokel RA, Malkawi AH. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by aids viruses. J Drug Target. 2010;18:381–8.
Haque S, Md S, Fazil M, Kumar M, Sahni JK, Ali J, Baboota S. Venlafaxine loaded chitosan NPs for brain targeting: pharmacokinetic and pharmacodynamic evaluation. Carbohydr Polym. 2021;89:72–9.
Md S, Khan RA, Mustafa G, et al. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur J Pharm Sci. 2013;48:393–405.
Fazil M, Md S, Haque S, et al. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur J Pharm Sci. 2012;47:6–15.
Vaka SRK, Sammeta SM, Day LB, Murthy SN. Delivery of nerve growth factor to brain via intranasal administration and enhancement of brain uptake. J Pharm Sci. 2009;98:3640–6.
Alhalaweh A, Andersson S, Velaga SP. Preparation of zolmitriptan–chitosan microparticles by spray drying for nasal delivery. Eur J Pharm Sci. 2009;38:206–14.
Gungor S, Okyar A, Erturk-Toker S, Baktir G, Ozsoy Y. Ondansetron-loaded chitosan microspheres for nasal antiemetic drug delivery: an alternative approach to oral and parenteral routes. Drug Dev Ind Pharm. 2010;36:806–13.
Akel H, Ismail R, Katona G, Sabir F, Ambrus R, Csóka. A comparison study of lipid and polymeric nanoparticles in the nasal delivery of meloxicam: formulation, characterization, and in vitro evaluation. Int J Pharm. 2021;604:120724.
Soane RJ, Hinchcliffe M, Davis SS, Illum L. Clearance characteristics of chitosan based formulations in the sheep nasal cavity. Int J Pharm. 2001;217:183–91.
Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51:81–96.
Kang ML, Cho CS, Yoo HS. Application of chitosan microspheres for nasal delivery of vaccines. Biotechnol Adv. 2009;27:857–65.
Vila A, Sánchez A, Janes K, et al. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur J Pharm Biopharm. 2004;57:123–31.
van der Lubben IM, Kersten G, Fretz MM, Beuvery C, Verhoef JC, Junginger HE. Chitosan microparticles for mucosal vaccination against diphtheria: oral and nasal efficacy studies in mice. Vaccine. 2003;21(13–14):1400–8.
Bacon A, Makin J, Sizer PJ, et al. Carbohydrate biopolymers enhance antibody responses to mucosally delivered vaccine antigens. Infect Immun. 2000;68:5764–70.
Amidi M, Romeijn SG, Borchard G, Junginger HE, Hennink WE, Jiskoot W. Preparation and characterization of protein-loaded N-trimethyl chitosan nanoparticles as nasal delivery system. J Control Release. 2006;111:107–16.
Bernkop-Schnürch A, Hornof M, Zoidl T. Thiolated polymers-thiomers: synthesis and in vitro evaluation of chitosan–2-iminothiolane conjugates. Int J Pharm. 2003;260(2):229–37.
Krauland AH, Guggi D, Bernkop-Schnurch A. Thiolated chitosan microparticles: a vehicle for nasal peptide drug delivery. Int J Pharm. 2006;307:270–7.
Vetter A, Bernkop-Schnürch A. Nasal delivery of antisense oligonucleotides: in vitro evaluation of a thiomer/glutathione microparticulate delivery system. J Drug Target. 2010;18:303–12.
Palmbereger TF, Augustijns P, Vetter A, Bernkop-Schnürch A. Safety assessment of thiolated polymers: effect on ciliary beat frequency in human nasal epithelial cells. Drug Dev Ind Pharm. 2011;37(12):1455–62.
Hornof MD, Kast CE, Bernkop-Schnurch A. In vitro evaluation of the viscoelastic properties of chitosanethioglycolic acid conjugates. Eur J Pharm Biopharm. 2003;55:185–90.
Patel D, Naik S, Misra A. Improved transnasal transport and brain uptake of tizanidine HCl-loaded thiolated chitosan nanoparticles for alleviation of pain. J Pharm Sci. 2012;101:690–706.
Shahnaz G, Vetter A, Barthelmes J, et al. Thiolated chitosan nanoparticles for the nasal administration of leuprolide: bioavailability and pharmacokinetic characterization. Int J Pharm. 2012;428:164–70.
Nazar H, Caliceti P, Carpenter B, et al. A once-a-day dosage form for the delivery of insulin through the nasal route: in vitro assessment and in vivo evaluation. Biomater Sci. 2013;1:306–14.
Wang X, Zheng C, Wu Z, et al. Chitosan-NAC nanoparticles as a vehicle for nasal absorption enhancement of insulin. J Biomed Mater Res B Appl Biomater. 2009;88(1):150–61.
Thanou M, Verhoef JC, Verheijden JH, Junginger HE. Intestinal absorption of octeriotide: N-trimethyl chitosan chloride (TMC) ameliorates the permeability and absorption properties of the somatostatin analogue in vitro and in vivo. J Pharm Sci. 2000;89:951–7.
Hamman JH, Stander M, Kotze AF. Effect of the degree of quaternisation of N-trimethyl chitosan chloride on absorption enhancement: in vivo evaluation in rat nasal epithelia. Int J Pharm. 2002;232:235–42.
du Plessis LH, Kotzé AF, Junginger HE. Nasal and rectal delivery of insulin with chitosan and N-trimethyl chitosan chloride. Drug Deliv. 2010;17:399–407.
Domard A, Rinaudo M, Terrassin C. New method for the quaternization of chitosan. Int J Biol Macromol. 1986;8:105–7.
Kotze AF, Thanou MM, Luessen HL, De Boer ABG, Verhoef JC, Junginger HE. Effect of the degree of quaternization of N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). Eur J Pharm Biopharm. 1999;47:269–74.
Kumar M, Pandey RS, Patra KC, et al. Evaluation of neuropeptide loaded trimethyl chitosan nanoparticles for nose to brain delivery. Int J Biol Macromol. 2013;61:189–95.
Chen KH, Di Sabatino M, Albertini B, Passerini N, Kett VL. The effect of polymer coatings on physicochemical properties of spray-dried liposomes for nasal delivery of BSA. Eur J Pharm Sci. 2013;50:312–22.
Mei D, Mao S, Sun W, Wang Y, Kissel T. Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats. Eur J Pharm Biopharm. 2008;70:874–81.
Rassu G, Gavini E, Jonassen H, et al. New chitosan derivatives for the preparation of rokitamycin loaded microspheres designed for ocular or nasal administration. J Pharm Sci. 2009;98(12):4852–65.
Gavini E, Rassu G, Muzzarelli C, Cossu M, Giunchedi P. Spray-dried microspheres based on methylpyrrolidinone chitosan as new carrier for nasal administration of metoclopramide. Eur J Pharm Biopharm. 2008;68(2):245–52.
Na L, Mao S, Wang J, Sun W. Comparison of different absorption enhancers on the intranasal absorption of isosorbide dinitrate in rats. Int J Pharm. 2010;397(1–2):59–66.
Elliot JH, Ganz AJ. Some rheological properties of sodium carboxymethylcellulose solutions and gels. Rheol Acta. 1974;13:670–4.
El-Gizawy SA, Osman MA, El-Hagaar SM, Hisham DM. Nasal drug delivery of a mucoadhesive oxybutynin chloride gel: in vitro evaluation and in vivo in situ study in experimental rats. Drug Del Sci Tech. 2013;23(6):569–75. Zhou N, Donovan MD. Intranasal mucociliary clearance of putative bioadhesive polymer gels. Int J Pharm. 1996;135:115–25.
Zaki NM, Awada GA, Mortadaa ND, Abd ElHadyb SS. Enhanced bioavailability of metoclopramide HCl by intranasal administration of mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur J Pharm Sci. 2007;32:296–307.
Suzuki Y, Makino Y. Mucosal drug delivery using cellulose derivatives as a functional polymer. J Control Release. 1999;62(1–2):101–7.
Ugwoke MI, Agu RU, Vanbilloen H, et al. Scintigraphic evaluation in rabbits of nasal drug delivery systems based on carbopol 971p and carboxymethylcellulose. J Control Release. 2000;68:207–14.
Quadir M, Zia H, Needham TE. Development and evaluation of nasal formulations of ketorolac. Drug Deliv. 2000;7:223–9.
Illum L, Jorgensen H, Bisgaard H, Krogsgaard O, Rossing N. Bioadhesive microspheres as a potential nasal drug delivery system. Int J Pharm. 1987;39:189–99.
Torikai Y, Sasaki Y, Sasaki K, Kyuno A, Haruta S, Tanimoto A. Evaluation of systemic and mucosal immune responses induced by a nasal powder delivery system in conjunction with an OVA antigen in cynomolgus monkeys. J Pharm Sci. 2021;110:2038–46.
Pearson RG, Masud T, Blackshaw E, et al. Nasal administration and plasma pharmacokinetics of parathyroid hormone peptide PTH 1-34 for the treatment of osteoporosis. Pharmaceutics. 2019;11(6):265.
Duan X, Mao S. New strategies to improve the intranasal absorption of insulin. Drug Discov Today. 2010;15(11–12):416–27.
D'Souza SA, Ray J, Pandey S, Udupa N. Absorption of ciprofloxacin and norfloxacin when administered as niosome-encapsulated inclusion complexes. J Pharm Pharmacol. 1997;49(2):145–9.
Ikeda K, Murata K, Kobayashi M, Noda K. Enhancement of bioavailability of dopamine via nasal route in beagle dogs. Chem Pharm Bull. 1992;40:2155–8.
Tester RF, Karkalas J, Qi X. Starch – composition, fine structure and architecture. J Cereal Sci. 2004;39:151–65.
Callens C, Pringels E, Remon JP. Influence of multiple nasal administrations of bioadhesive powders on the insulin bioavailability. Int J Pharm. 2003;250:415–22.
Yadav AV, Mote HH. Development of biodegradable starch microspheres for intranasal delivery. Indian J Pharm Sci. 2008;70(2):170–4.
Jain AK, Khar RK, Ahmed FJ, Diwan PV. Effective insulin delivery using starch nanoparticles as a potential trans-nasal mucoadhesive carrier. Eur J Pharm Biopharm. 2008;69:426–35.
Illum L, Fisher AN, Jabbal-Gill I, Davis SS. Bioadhesive starch microspheres and absorption enhancing agents act synergistically to enhance the nasal absorption of polypeptides. Int J Pharm. 2001;222:109–19.
Abu Elella MH, Sabaa MW, Hanna DH, Abdel-Aziz MM, Mohamed RR. Antimicrobial pH-sensitive protein carrier based on modified xanthan gum. J Drug Deliv Sci Technol. 2020;57:101673.
El-Sawy NM, Raafat AI, Badawy NA, Mohamed AM. Radiation development of pH-responsive (xanthan-acrylic acid)/MgO nanocomposite hydrogels for controlled delivery of methotrexate anticancer drug. Int J Biol Macromol. 2020;142:254–64.
Kennedy JRM, Kent KE, Brown JR. Rheology of dispersions of xanthan gum, locust bean gum and mixed biopolymer gel with silicon dioxide nanoparticles. Mater Sci Eng C. 2015;48:347–53.
Kumar A, Rao KM, Han SS. Application of xanthan gum as polysaccharide in tissue engineering: a review. Carbohydr Polym. 2018;180:128–44.
Morariu S, Bercea M, Brunchi CE. Phase separation in xanthan solutions. Cellul Chem Technol. 2018;52:569–76.
Gils PS, Ray D, Sahoo PK. Characteristics of xanthan gum-based biodegradable superporous hydrogel. Int J Biol Macromol. 2009;45:364–71.
Kang M, Oderinde O, Liu S, et al. Characterization of Xanthan gum-based hydrogel with Fe3+ ions coordination and its reversible sol-gel conversion. Carbohydr Polym. 2019;203:139–47.
Petri DFS. Xanthan gum: a versatile biopolymer for biomedical and technological applications. J Appl Polym Sci. 2015;132(23):42035.
Huang J, Deng Y, Ren J, et al. Novel in situ forming hydrogel based on xanthan and chitosan re-gelifying in liquids for local drug delivery. Carbohydr Polym. 2018;186:54–63.
Bernkop-Schnürch A, Obermair K, Greimel A, et al. In vitro evaluation of the potential of thiomers for the nasal administration of Leu-enkephalin. Amino Acids. 2006;30:417–23.
Peppas NA, Carr DA. Impact of absorption and transport on intelligent therapeutics and nanoscale delivery of protein therapeutic agents. Chem Eng Sci. 2009;64(22):4553–65.
Hintzen F, Hauptstein S, Perera G, Bernkop-Schnürch A. Synthesis and in vitro characterization of entirely S-protected thiolated pectin for drug delivery. Eur J Pharma Biopharm. 2013;85(3):1266–73.
Menzel C, Jelkmann M, Laffleur F, Bernkop-Schnürch A. Nasal drug delivery: design of a novel mucoadhesive and in situ gelling polymer. Int J Pharm. 2017;517:196–202.
Yuguchi Y, Urakawa H, Kitamura S, Wataoka I, Kajiwara K. The sol-gel transition of gellan gum aqueous solutions in the presence of various metal salts. Progr Colloid Polym Sci. 1999;114:41–7.
Mahdi MH, Conway BR, Smith AM. Evaluation of gellan gum fluid gels as modified release oral liquids. Int J Pharm. 2014;475(1–2):335–43.
Cao SL, Zhang QZ, Jiang XG. Preparation of ion-activated in situ gel systems of scopolamine hydrobromide and evaluation of its antimotion sickness efficacy. Acta Pharmacol Sin. 2007;28:584–90.
Picone CSF, Cunha RL. Influence of pH on formation and properties of gellan gels. Carbohydr Polym. 2011;84(1):662–8.
Salunke SR, Patil SB. Ion activated in situ gel of gellan gum containing salbutamol sulphate for nasal administration. Int J Biol Macromol. 2016;87:41–7.
Krauland AH, Leitner VM, Bernkop-Schnürch A. Improvement in the in situ gelling properties of deacetylated gellan gum by the immobilization of thiol groups. J Pharm Sci. 2003;92:1234–41.
Hao J, Zhao J, Zhang S, Tong T, Zhuang Q, Jin K, Chen W, Tang H. Fabrication of an ionic-sensitive in situ gel loaded with resveratrol nanosuspensions intended for direct nose-to-brain delivery. Colloids Surf B Biointerfaces. 2016;147:376–86.
Liu L, Fishman ML, Hicks KB. Pectin in controlled drug delivery – a review. Cellulose. 2007;14:15–24.
Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18.
Watts P, Smith A. PecSys: in situ gelling system for optimised nasal drug delivery. Expert Opin Drug Deliv. 2009;6(5):543–52.
Fisher T, Knight A, Watling M, Smith A. Fentanyl pectin nasal spray (FPNS) with PecSys® provides most favorable pharmacokinetic/tolerability profile compared with nasal chitosan-based fentanyl and oral transmucosal fentanyl citrate (OTFC). J Pain. 2009;10(Suppl):S45.
Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P. Development of a new drug carrier made from alginate. J Pharm Sci. 1993;82(9):912–7.
Kesavan K, Nath G, Pandit JK. Sodium alginate based mucoadhesive system for gatifloxacin and its in vitro antibacterial activity. Sci Pharm. 2010;78(4):941–57.
Wee S, Gombotz WR. Protein release from alginate matrices. Adv Drug Deliv Rev. 1998;31(3):267–85.
Bernkop-Schnurch A. Mucoadhesive polymers. In: Dumitriu S, editor. Polymer biomaterial. 2nd ed. New York: Marcel Dekker; 2002. p. 147–65.
Braccini I, Pérez S. Molecular basis of Ca2+-induced gelation in alginates and pectins: the egg-box model revisited. Biomacromolecules. 2001;2(4):1089–96.
Morimoto K, Katsumata H, Yabuta T, et al. Evaluation of gelatin microspheres for nasal and intramuscular administrations of salmon calcitonin. Eur J Pharm Sci. 2001;13:179e185.
Wang J, Sakai S, Deguchi Y, Bi D, Tabata Y, Morimoto K. Aminated gelatin as a nasal absorption enhancer for peptide drugs: evaluation of absorption enhancing effect and nasal mucosa perturbation in rats. J Pharm Pharmacol. 2002;54:181e188.
Wang J, Tabata Y, Morimoto K. Aminated gelatin microspheres as a nasal delivery system for peptide drugs: evaluation of in vitro release and in vivo insulin absorption in rats. J Control Release. 2006;113(1):31–7.
Seki T, Kanbayashi H, Chono S, Tabata Y, Morimoto K. Effects of a sperminated gelatin on the nasal absorption of insulin. Int J Pharm. 2007;338:213–8.
Hanif M, Zaman M, Qureshi S. Thiomers: a blessing to evaluating era of pharmaceuticals. Int J Polym Sci. 2015;146329
Kun N, Bae YH. pH sensitive polymers for drug delivery. In: Kwon GS, editor. Polymeric drug delivery systems. 1st ed. Talor and Francis Group: Florida; 2005. p. 129–94.
Rathnam G, Narayanan N, Ilavarasan R. Carbopol-based gels for nasal delivery of progesterone. AAPS PharmSciTech. 2008;9(4):1078–82.
Najafabadi AR, Moslemi P, Tajerzadeh H. Intranasal bioavailability of insulin from carbopol-based gel spray in rabbits. Drug Deliv. 2004;11(5):295–300.
Ugwoke MI, Sam E, Van Den Mooter G, Verbeke N, Kinget R. Bioavailability of apomorphine following intranasal administration of mucoadhesive drug delivery systems in rabbits. Eur J Pharm Sci. 1999;9:213–9.
Nandgude T, Thube R, Jaiswal N, Deshmukh P, Chatap V, Hire N. Formulation and evaluation pH induced in-situ nasal gel of salbutamol sulphate. Int J Pharm Sci Nanotechnol. 2008;1:177–83.
Ravi V, Mahendra C, Datta MV, Gowda DV, Shivakumar HG, Bhargav E. Thiomers fresh drift of polymers & their prospective in pharmaceuticals: a review. World J Pharm Pharm Sci. 2013;3(1):204–20.
Kafedjiiski K, Franzens L. Multifunctional polymeric excipients in no-invasive delivery of hydrophilic macromolecular drugs: the thiomer-technology. The Drug Delivery Companies Report Autumn/Winter 47, The Drug Delivery Companies. 2004. https://www.semanticscholar.org/paper/Multifunctional-Polymeric-Excipients-in-Delivery-of-Kafedjiiski/c3f0d72d433dc58d12bfca74853fc0df37785b2e.
Greimel A, Del Curto MD, D’Antonio M, Palmberger T, Sprinzl GM, Bernkop-Schnürch A. In vitro evaluation of thiomer microparticles for nasal drug delivery. J Drug Del Sci Tech. 2006;16:103–8.
Aikawa K, Matsumoto K, Uda H, et al. Hydrogel formation of the pH response polymer polyvinylacetal diethylaminoacetate (AEA). Int J Pharm. 1998;167(1–2):97–104.
Migniani S, Shi X, Karpus A, Majoral J-P. Non-invasive intranasal administration route directly to the brain using dendrimer nanoplatforms: an opportunity to develop new CNS drugs. Eur J Med Chem. 2021;209:112905.
Xie H, Li L, Sun Y, et al. An available strategy for nasal brain transport of nanocomposite based on PAMAM dendrimers via in situ gel. Nanomaterials (Basel). 2019;9(2):147.
Janaszewska A, Studzian M, Petersen JF, et al. Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups-its uptake, efflux, and location in a cell. Colloids Surf B Biointerfaces. 2017;159:211–6.
Esfand R, Tomalia DA. Poly (amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6(8):427–36.
Otto DP, de Villiers MM. Poly (amidoamine) dendrimers as a pharmaceutical excipient. Are we there yet? J Pharm Sci. 2018;107(1):75–83.
Xia H, Gao X, Gu G, et al. Low molecular weight protamine functionalized nanoparticles for drug delivery to the brain after intranasal administration. Biomaterials. 2011;32:9888–98.
Kamei N, Takeda-Morishita M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J Control Release. 2015;197:105–10.
Mattiuz E, Franklin R, Gillespie T, et al. Disposition and metabolism of olanzapine in mice, dogs, and rhesus monkeys. Drug Metab Dispos. 1997;25:573–83.
Roy S, Pal K, Anis A, Pramanik K, Prabhakar B. Polymers in mucoadhesive drug-delivery systems: a brief note. Des Monomers Polym. 2009;12:483–95.
Laffleur F, Bauer B. Progress in nasal drug delivery systems. Int J Pharm. 2021;607:120994.
Emanuele M, Balasubramaniam B. Differential effects of commercial-grade and purified poloxamer 188 on renal function. Drugs R D. 2014;14:73–83.
Cho H-J, Balakrishnan P, Park E-K, et al. Poloxamer/cyclodextrin/chitosan-based thermoreversible gel for intranasal delivery of fexofenadine hydrochloride. J Pharm Sci. 2011;100(2):681–91.
Katakam M, Ravis WR, Banga AK. Controlled release of human growth hormone in rats following parenteral administration of poloxamer gels. J Control Release. 1997;49(1):21–6.
Fakhari A, Corcoran M, Schwarz A. Thermogelling properties of purified poloxamer 407. Heliyon. 2017;3(8):e00390.
Russo E, Villa C. Poloxamer hydrogels for biomedical applications. Pharmaceutics. 2019;11:671.
Bromberg LE. Enhanced nasal retention of hydrophobically modified polyelectrolytes. J Pharm Pharmacol. 2001;53:109–14.
Uttarwar S. Formulation and development of in situ gelling system for nasal administration for an antiemetic drug ondansetron hydrochloride by using Pluronics 127P and Pluronics 68. Int J Res Pharm Biomed Sci. 2012;3:1103–18.
Kandimella KK, Donovan MD. Localization and differential activity of P-glycoprotein in the bovine olfactory and nasal respiratory mucosae. Pharm Res. 2005;22:1121–8.
Kabanov AV, Batrakova EV, Miller DW. Pluronic block copolymers as modulators of drug efflux transporter activity in the blood-brain barrier. Adv Drug Deliv Rev. 2003;55(1):151–64.
Bromberg L. Poly(ethylene oxide)-b-poly(propylene oxide)-bpoly(ethylene oxide)-g-poly(acrylic acid) copolymers as in-situ vehicle for nasal delivery. In: Rathbene MJ, Hadgraft J, Roberts MS, editors. Modified release drug technology. 1st ed. New York: Marcel Dekker; 2002. p. 749–58.
Ruel-Gariépy E, Leroux JC. In situ-forming hydrogels – review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–26.
Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37–51.
Rydén L, Edman P. Effect of polymers and microspheres on the nasal absorption of insulin in rats. Int J Pharm. 1982;83:1–10.
Porfiryeva NN, Nasibullin SF, Abdullina SG, et al. Acrylated Eudragit® E PO as a novel polymeric excipient with enhanced mucoadhesive properties for application in nasal drug delivery. Int J Pharm. 2019;562:241–8.
Casettari L, Vllasaliu D, Mantovani G, Howdle SM, Stolnik S, Illum L. Effect of PEGylation on the toxicity and permeability enhancement of chitosan. Biomacromolecules. 2010;11:2854–65.
Casettari L, Vllasaliu D, Castagnino E, Stolnik S, Howdle S, Illum L. PEGylated chitosan derivatives: synthesis, characterizations and pharmaceutical applications. Prog Polym Sci. 2012;37:659–85.
Zhang X, Zhang H, Wu Z, Wang Z, Niu H, Li C. Nasal absorption enhancement of insulin using PEG-grafted chitosan nanoparticles. Eur J Pharm Biopharm. 2008;68:526–34.
Gorshkova MY, Volkova IF, Vanchugova LV, et al. Sodium alginate based mucoadhesive hydrogels. Appl Biochem Microbiol. 2019;55:371–4.
Tafaghodi M, Abolghasem Sajadi Tabassi S, Jaafari M-R, Zakavi SR, Momen-Nejad M. Evaluation of the clearance characteristics of various microspheres in the human nose by gamma-scintigraphy. Int J Pharm. 2004;280:125–35.
Makadia HK, Siegel SJ. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymer. 2011;3:1377–97.
Musumeci T, Pellitteri R, Spatuzza M, Puglisi G. Nose-to-brain delivery: evaluation of polymeric nanoparticles on olfactory ensheathing cells uptake. J Pharm Sci. 2014;103:628–35.
Md S, Ali M, Baboota S, Sahni KJ, Bhatnagar A, Ali J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev Ind Pharm. 2014;40(2):278–87.
Mythri G, Kavitha K, Kumar MR, Singh SJ. Novel mucoadhesive polymers-a review. J App Pharm Sci. 2011;01(08):37–42.
Gao X, Tao W, Lu W, et al. Lectin-conjugated PEG-PLA nanoparticles: preparation and brain delivery after intranasal administration. Biomaterials. 2006;27(18):3482–90.
Chen J, Zhang C, Liu Q, et al. Solanum tuberosum lectin-conjugated PLGA nanoparticles for nose-to-brain delivery: in vivo and in vitro evaluations. J Drug Target. 2012;20(2):174–84.
Smart JD, Nicholls TJ, Green KL, Rogers DJ, Cook JD. Lectins in drug delivery: a study of the acute local irritancy of the lectins from Solanum tuberosum and Helix pomatia. Eur J Pharm Sci. 1999;9(1):93–8.
Andrews G, Laverty T, Jones D. Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm. 2009;71(3):505–18.
Zhang C, Chen J, Feng C, et al. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer's disease. Int J Pharm. 2014;461(1–2):192–202.
Hu K, Shi Y, Jiang W, Han J, Huang S, Jiang X. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: preparation, characterization and efficacy in Parkinson's disease. Int J Pharm. 2011;415(1–2):273–83.
Yan X, Xu L, Bi C, et al. Lactoferrin-modified rotigotine nanoparticles for enhanced nose-to-brain delivery: LESA-MS/MS-based drug biodistribution, pharmacodynamics, and neuroprotective effects. Int J Nanomedicine. 2018;13:273–81.
Jadhav PP, Jadhav NR, Hosmani AH, Patil S. Development and evaluation of in situ thermoresponsive nasal gel system for Nardostachys jatamansi. Pharm Lett. 2013;5:113–25.
Srivastava R, Srivastava S, Singh SP. Thermoreversible in-situ nasal gel formulations and their pharmaceutical evaluation for the treatment of allergic rhinitis containing extracts of Moringa olifera and Embelia ribes. Int J App Pharm. 2017;9(6):16–20.
Shelke S, Shahi S, Jalalpure S, Dhamecha D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan-loaded nanoethosomes: formulation, optimization, evaluation and permeation studies. J Liposome Res. 2016;26(4):313–23.
Perez AP, Mundina-Weilenmann C, Romero EL, Morilla MJ. Increased brain radioactivity by intranasal P-labeled siRNA dendriplexes within in situ-forming mucoadhesive gels. Int J Nanomedicine. 2012;7:1373–85.
Agrawal AK, Gupta PN, Khanna A, et al. Development and characterization of in situ gel system for nasal insulin delivery. Pharmazie. 2010;65:188–93.
Zao J. Chitosan-based gels for the drug delivery system. In: Yao K, Li J, Yao F, Yin Y, editors. Chitosan-based hydrogels: functions and applications. 1st ed. CRC Press; 2011. p. 263–314.
Al-Ghananeem AM, Malkawi AH, Crooks PA. Bioavailability of Δ9-tetrahydrocannabinol following intranasal administration of a mucoadhesive gel spray delivery system in conscious rabbits. Drug Dev Ind Pharm. 2011;37:329–34.
Jose S, Ansa CR, Cinu TA, et al. Thermo-sensitive gels containing lorazepam microspheres for intranasal brain targeting. Int J Pharm. 2013;441:516–26.
Zafar A, Afzal M, Quazi AM, et al. Chitosan-ethyl cellulose microspheres of domperidone for nasal delivery: preparation, in-vitro characterization, in-vivo study for pharmacokinetic evaluation and bioavailability enhancement. J Drug Deliv Sci Technol. 2021;63:102471.
Gavini E, Rassu G, Ferraro L, et al. Influence of polymeric microcarriers on the in vivo intranasal uptake of an anti-migraine drug for brain targeting. Eur J Pharm Biopharm. 2013;83:174–83.
Wu J, Wei W, Wang LY, Su ZG, Ma GH. A thermosensitive hydrogel based on quaternized chitosan and poly(ethylene glycol) for nasal drug delivery system. Biomaterials. 2007;28:2220–32.
Wu J, Su ZG, Ma GH. A thermo- and pH-sensitive hydrogel composed ofquaternized chitosan/glycerophosphate. Int J Pharm. 2006;315:1–11.
Chenite A, Chaput C, Cpmbes C, Selmani A, Jalal F. Temperature-controlled pH-dependant formation of ionic polysaccharide gels. US Patent 6344488. 2002. https://pubchem.ncbi.nlm.nih.gov/patent/US6344488.
Goycoolea FM, Lollo G, Remuñán-López C, Quaglia F, Alonso M.a.J. Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules. 2009;10:1736–43.
Verestiuc L, Ivanov C, Barbu E, Tsibouklis J. Dual-stimuli-responsive hydrogels based on poly(N-isopropylacrylamide)/chitosan semi-interpenetrating networks. Int J Pharm. 2004;269:185–94.
El-Dakrouri WA, Ibrahim HK, Ghorab MK, Ghorab MM. Enhancement of the intranasal delivery of insulin via a novel mucoadhesive Carbopol gel. J Pharm Pharmacol. 2010;62(7):866–72.
Dehghan MHG, Kazi M. Lyophilized chitosan/xanthan polyelectrolyte complex based mucoadhesive inserts for nasal delivery of promethazine hydrochloride. Iran J Pharm Res. 2014;13(3):769–184.
Luppi B, Bigucci F, Mercolini L, et al. Novel mucoadhesive nasal inserts based on chitosan/hyaluronate polyelectrolyte complexes for peptide and protein delivery. J Pharm Pharmacol. 2009;61(2):151–7.
Luppi B, Bigucci F, Abbruzzo A, Corace G, Cerchiara T, Zecchi V. Freeze-dried chitosan/pectin nasal inserts for antipsychotic drug delivery. Eur J Pharm Biopharm. 2010;75(3):381–7.
Deutel B, Laffleur F, Palmberger T, Saxer A, Thaler M, Bernkop-Schnurch A. In vitro characterization of insulin containing thiomeric microparticles as nasal drug delivery system. Eur J Pharm Sci. 2016;81:157–61.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Djekic, L. (2023). Novel Mucoadhesive Polymers for Nasal Drug Delivery. In: Pathak, Y.V., Yadav, H.K.S. (eds) Nasal Drug Delivery. Springer, Cham. https://doi.org/10.1007/978-3-031-23112-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-031-23112-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23111-7
Online ISBN: 978-3-031-23112-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)