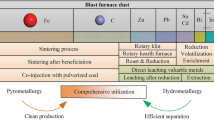
Abstract
The following conclusions can be drawn from a comparison of several waste iron dust (WID) and waste aluminum dust (WAD) characterization results: The materials are made of fine particles, measuring approximately ˂5.00 μm for WID and 1.34–20.00 μm for WAD. They are frequently spherical in shape and typically exist as oxides. Most notably, the characterization reports highlight substantial metal concentrations, which classify this waste as both a profitable by-product and a potentially harmful pollutant. However, due to the lack of mineral resources, all of these data suggest that direct stabilization/solidification strategies for disposal or recirculation were not the most efficient approaches to manage these waste metal dusts (WID, WAD). Therefore, from both an economic and environmental point of view, resource recovery and recycling from these waste metal dusts is a sustainable strategy.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
F. Habashi, Principles of Extractive Metallurgy: Pyrometallurgy (Routledge, Abingdon, 2017)
L. Lu, J. Pan, D. Zhu, Quality requirements of iron ore for iron production, in Iron Ore, (Woodhead Publishing, Oxford, 2015), pp. 475–504
L. Brückner, J. Frank, T. Elwert, Industrial recycling of lithium-ion batteries—A critical review of metallurgical process routes. Metals 10(8), 1107 (2020)
J. Li, B. Ban, Y. Li, X. Bai, T. Zhang, J. Chen, Removal of impurities from metallurgical grade silicon during Ga-Si solvent refining. Silicon 9(1), 77–83 (2017)
S. Esfahani, M. Barati, Purification of metallurgical silicon using iron as an impurity getter part I: Growth and separation of Si. Met. Mater. Int. 17(5), 823–829 (2011)
F. Huang, Q. Lu, M. Wu, L. Zhao, Purification of metallurgical-grade silicon by Sn-Si solvent refining with different tin content. Silicon, 14, 1–11 (2022)
M. Cavallini, Thermodynamics applied to iron smelting techniques. Appl. Phys. A 113(4), 1049–1053 (2013)
K. Murari, R. Siddique, K.K. Jain, Use of waste copper slag, a sustainable material. J. Mater. Cycles Waste Manag. 17(1), 13–26 (2015)
G.A. Flores, C. Risopatron, J. Pease, Processing of complex materials in the copper industry: Challenges and opportunities ahead. JOM 72(10), 3447–3461 (2020)
E.E. Okafor, Early Iron Smelting in Nsukka-Nigeria: Information from Slags and Residues (Doctoral dissertation, University of Sheffield, 1992)
C.R. Borra, B. Blanpain, Y. Pontikes, K. Binnemans, T. Van Gerven, Recovery of rare earths and major metals from bauxite residue (red mud) by alkali roasting, smelting, and leaching. J. Sustain. Metall. 3(2), 393–404 (2017)
M. Shamsuddin, H.Y. Sohn, Constitutive topics in physical chemistry of high-temperature nonferrous metallurgy—A review: Part 1. Sulfide roasting and smelting. JOM 71(9), 3253–3265 (2019)
X.H. Chen, X. Tang, Z.D. Wang, X.D. Hui, M. Li, Y.W. Wang, Manufacturing process and microstructure of copper-coated aluminum wires. Int. J. Miner. Metall. Mater. 22(2), 190–196 (2015)
J.P. Tavener, Development of a standard platinum resistance thermometer for use up to the copper point. Int. J. Thermophys. 36(8), 2027–2035 (2015)
S.A. Oglezneva, V.Y. Bulanov, Y.V. Kontsevoi, I.E. Ignat’ev, Production of nickel and iron nanopowders by hydrogen reduction from salts. Russ. Metall. (Met.) 2012(7), 654–658 (2012)
K.T. Jacob, C.B. Alcock, The oxygen potential of the systems Fe+ FeCr2O4+ Cr2O3 and Fe+ FeV2O4+ V2O3 in the temperature range 750–1600 C. Metall. Trans. B 6(2), 215–221 (1975)
S. Hasani, M. Panjepour, M. Shamanian, The oxidation mechanism of pure aluminum powder particles. Oxid. Met. 78(3), 179–195 (2012)
V.P. Itkin, C.B. Alcock, The Ca-Pb (calcium-lead) system. J. Phase Equilib. 13(2), 162–169 (1992)
C. Guminski, The melting and boiling points of mercury (I-Ig). J. Chem. Thermodyn. 4, 603 (1972)
Z. Han, Z. Guo, Y. Zhang, X. Xiao, Z. Xu, Y. Sun, Pyrolysis characteristics of biomass impregnated with cadmium, copper and lead: Influence and distribution. Waste Biomass Valoriz. 9(7), 1223–1230 (2018)
C. Wang, B. Lei, P. Jiang, X. Xu, G. Mi, Numerical and experimental investigation of vacuum-assisted laser welding for DP590 galvanized steel lap joint without prescribed gap. Int. J. Adv. Manuf. Technol. 94(9), 4177–4185 (2018)
P. Kumar, A. Kumar, R. Kumar, Phytoremediation and Nanoremediation, in New Frontiers of Nanomaterials in Environmental Science, (Springer, Singapore, 2021), pp. 281–297
R.H. Hanewald, W.A. Munson, D.L. Schweyer, Processing EAF dusts and other nickel-chromium waste materials pyrometallurgically at INMETCO. Min. Metall. Explor. 9(4), 169–173 (1992)
J. Banhart, Manufacturing routes for metallic foams. JOM 52(12), 22–27 (2000)
Z. Wang, Z. Cui, L. Liu, Q. Ma, X. Xu, Toxicological and biochemical responses of the earthworm Eisenia fetida exposed to contaminated soil: Effects of arsenic species. Chemosphere 154, 161–170 (2016)
G. Kim, I. Sohn, Selective metal cation concentration during the solidification of stainless steel EAF dust and slag mixtures from high temperatures for increased Cr recovery. J. Hazard. Mater. 359, 174–185 (2018)
I.F. Kurunov, The direct production of iron and alternatives to the blast furnace in iron metallurgy for the 21st century. Metallurgist 54(5), 335–342 (2010)
J. Rieger, J. Schenk, Residual processing in the European steel industry: A technological overview. J. Sustain. Metall. 5(3), 295–309 (2019)
T. Sofilić, A. Rastovčan-Mioč, Š. Cerjan-Stefanović, V. Novosel-Radović, M. Jenko, Characterization of steel mill electric-arc furnace dust. J. Hazard. Mater. 109(1–3), 59–70 (2004)
C.L. Li, M.S. Tsai, Mechanism of spinel ferrite dust formation in electric arc furnace steelmaking. ISIJ Int. 33(2), 284–290 (1993)
P.J. Nolasco-Sobrinho, D.C.R. Espinosa, J.A.S. Tenório, Characterisation of dusts and sludges generated during stainless steel production in Brazilian industries. Ironmak. Steelmak. 30(1), 11–17 (2003)
G. Laforest, J. Duchesne, Characterization and leachability of electric arc furnace dust made from remelting of stainless steel. J. Hazard. Mater. 135(1–3), 156–164 (2006)
M.T. Tang, J. Peng, B. Peng, D. Yu, C.B. Tang, Thermal solidification of stainless steelmaking dust. Trans. Nonferrous Metals Soc. China 18(1), 202–206 (2008)
G. Ma, A.M. Garbers-Craig, Stabilisation of Cr (VI) in stainless steel plant dust through sintering using silica-rich clay. J. Hazard. Mater. 169(1–3), 210–216 (2009)
L. Wu, N.J. Themelis, The flash reduction of electric arc furnace dusts. JOM 44(1), 35–39 (1992)
C. Takano, F.L. Cavallante, D.M. dos Santos, M.B. Mourão, Recovery of Cr, Ni and Fe from dust generated in stainless steelmaking. Miner. Process. Extr. Metall. 114(4), 201–206 (2005)
Z. Huaiwei, H. Xin, An overview for the utilization of wastes from stainless steel industries. Resour. Conserv. Recycl. 55(8), 745–754 (2011)
F. Škvára, F. Kaštánek, I. Pavelková, O. Šolcová, Y. Maléterová, P. Schneider, Solidification of waste steel foundry dust with Portland cement. J. Hazard. Mater. 89(1), 67–81 (2002)
P. Rocabois, E. Lectard, J.C. Huber, F. Patisson, Thermodynamic assessment of the oxide phase in the Fe–Zn–O system-application to dust formation in electric arc furnace. In Proceedings of the 10th International IUPAC Conference on High Temperature Materials Chemistry, Julich, Germany, 10–14 April 2000; pp. 1–12.
H. Zhang, J. Dong, H. Xiong, Z. Wang, Y. Lu, Investigation on cooperative desulfurization efficiency for bearing carbon stainless steel dust briquettes chromium and nickel recovery process. J. Alloys Compd. 699, 408–414 (2017)
S. Ri, M. Chu, Separation of metal nugget from self-reduced product of coal composite stainless steel dust briquette. ISIJ Int. 55(8), 1565–1572 (2015)
S.S. Jung, G.B. Kim, I. Sohn, Understanding the solidification of stainless steel slag and dust mixtures. J. Am. Ceram. Soc. 100(8), 3771–3783 (2017)
A.J.B. Dutra, P.R.P. Paiva, L.M. Tavares, Alkaline leaching of zinc from electric arc furnace steel dust. Miner. Eng. 19(5), 478–485 (2006)
B. Lindblom, C. Samuelsson, G. Ye, Fine-particle characterization—An important recycling tool. JOM 54(12), 35–38 (2002)
N. Menad, J.N. Ayala, F. Garcia-Carcedo, E. Ruiz-Ayúcar, A. Hernandez, Study of the presence of fluorine in the recycled fractions during carbothermal treatment of EAF dust. Waste Manag. 23(6), 483–491 (2003)
S. Kelebek, S. Yörük, B. Davis, Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Miner. Eng. 17(2), 285–291 (2004)
J. Geldenhuis, A.W. Home, in 85th Steelmaking Conference Proceedings, Iron and Steel Society, Nashville TN, 661–668 (2002).
E. Ordoñez, H.A. Colorado, Additive manufacturing via the direct ink writing technique of kaolinite-based clay with electric arc furnace steel dust (EAF dust), in Energy Technology 2020: Recycling, Carbon Dioxide Management, and Other Technologies, (Springer, Cham, 2020), pp. 307–315
M.F. Gándara, Aluminium: The metal of choice. Mater. Tehnol. 47(3), 261–265 (2013)
B. Wang, K. Xu, Y. Wang, Using sodium D-gluconate to suppress hydrogen production in wet aluminium waste dust collection systems. J. Hazard. Mater. 397, 122780 (2020)
M. Bertram, S. Ramkumar, H. Rechberger, G. Rombach, C. Bayliss, K.J. Martchek, D.B. Müller, G. Liu, A regionally-linked, dynamic material flow modelling tool for rolled, extruded and cast aluminium products. Resour. Conserv. Recycl. 125, 48–69 (2017)
R. Galindo, I. Padilla, O. Rodríguez, R. Sánchez-Hernández, S. López-Andrés, A. López-Delgado, Characterization of solid wastes from aluminum tertiary sector: The current state of spanish industry. J. Miner. Mater. Charact. Eng. 3(2), 55–64 (2015)
C. Directive, 96/61/EC of 24 September 1996 concerning integrated pollution prevention and control. Off. J. L 257(10), 10 (1996)
M. Nifuku, S. Koyanaka, H. Ohya, C. Barre, M. Hatori, S. Fujiwara, S. Horiguchi, I. Sochet, Ignitability characteristics of aluminium and magnesium dusts that are generated during the shredding of post-consumer wastes. J. Loss Prev. Process Ind. 20(4–6), 322–329 (2007)
Y. Liu, B.S. Leong, Z.T. Hu, E.H. Yang, Autoclaved aerated concrete incorporating waste aluminum dust as foaming agent. Constr. Build. Mater. 148, 140–147 (2017)
D. Eliche-Quesada, S. Ruiz-Molina, L. Pérez-Villarejo, E. Castro, P.J. Sánchez-Soto, Dust filter of secondary aluminium industry as raw material of geopolymer foams. J. Build. Eng. 32, 101656 (2020)
L. Marmo, D. Cavallero, M.L. Debernardi, Aluminium dust explosion risk analysis in metal workings. J. Loss Prev. Process Ind. 17(6), 449–465 (2004)
R. Malviya, R. Chaudhary, Factors affecting hazardous waste solidification/stabilization: A review. J. Hazard. Mater. 137(1), 267–276 (2006)
R.B. Moussa, C. Proust, M. Guessasma, K. Saleh, J. Fortin, Physical mechanisms involved into the flame propagation process through aluminum dust-air clouds: A review. J. Loss Prev. Process Ind. 45, 9–28 (2017)
E. David, J. Kopac, Aluminum recovery as a product with high added value using aluminum hazardous waste. J. Hazard. Mater. 261, 316–324 (2013)
N. Kongkajun, B. Cherdhirunkorn, W. Borwornkiatkaew, P. Chakartnarodom, Utilization of aluminium buffing dust as a raw material for the production of mullite. J. Met. Mater. Miner. 29(3), 71–75 (2019)
Acknowledgments
The author will want to appreciate the Council for scientific and industrial research, Pretoria, South Africa and Tshwane University of Technology, Pretoria, South Africa, for the financial support. The author additionally acknowledges the facilities provided by Gravity concentrator Africa (PTY), Randburg, South Africa; Vaal University of Technology, Vanderbijlpark, South Africa; and University of Pretoria, Pretoria, South Africa.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Okanigbe, D.O. (2023). Resource Recovery and Recycling from Waste Metal Dust (I): Waste Iron Dust and Waste Aluminum Dust. In: Ogochukwu Okanigbe, D., Popoola, A.P. (eds) Resource Recovery and Recycling from Waste Metal Dust. Springer, Cham. https://doi.org/10.1007/978-3-031-22492-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-22492-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22491-1
Online ISBN: 978-3-031-22492-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)