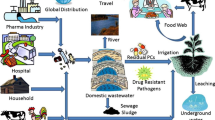
Abstract
The increase in the world population and the use of nonrenewable natural resources has made it necessary to find alternatives for more sustainable production and, at the same time, for the removal of emerging contaminants released into water resources, for example, medicines and pharmaceutical components. Adsorption is a promising technique for the decontamination of waters with emerging contaminants, being efficient in removing contaminants even at concentrations in the order of μg/L and ng/L, which is an advantage over other decontamination techniques. Recent studies report the development of adsorbent composites containing biosurfactants, which is a frontier of knowledge regarding adsorption for the removal of drugs in domestic and/or industrial effluents. Biosurfactants are favorable due to their amphiphilic nature, which can interact with different substances. The chapter aims to approach the state-of-the-art use of biosurfactants in the adsorption processes of drugs present in the liquid phase.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abbasi S, Haeri SA, Naghipour A, Sajjadifar S (2020) Enrichment of cardiovascular drugs using rhamnolipid bioaggregates after dispersive solid phase extraction based water compatible magnetic molecularly imprinted biopolymers. Microchem J 157:104874
Açikyildiz M, Gürses A, Günes K, Yalvaç D (2015) A comparative examination of the adsorption mechanism of an anionic textile dye (RBY 3GL) onto the powdered activated carbon (PAC) using various the isotherm models and kinetics equations with linear and non-linear methods. Appl Surf Sci 354:279–284
Afzal MZ, Sun X-F, Liu J, Song C, Wang S-G, Javed A (2018) Enhancement of ciprofloxacin sorption on chitosan/biochar hydrogel beads. Sci Total Environ 639:560–569
Alshabib M, Onaizi SA (2020) Enzymatic remediation of bisphenol a from wastewaters: effects of biosurfactant, anionic, cationic, nonionic, and polymeric additives. Water Air Soil Pollut 231:1–13
Ambaye TG, Vaccari M, Prasad S, Rtimi S (2021) Preparation, characterization and application of biosurfactant in various industries: a critical review on progress, challenges and perspectives. Environ Technol Innov 24:102090
Andrade CJ, Andrade LM, Rocco SA, Sforça ML, Pastore GM, Jauregi P (2017) a novel approach for the production and purification of mannosylerythritol lipids (MEL) by Pseudozyma tsukubaensis using cassava wastewater as substrate. Sep Purif Technol 180:157–167
Atkins P, Paula J (2006) Chemical equilibrium. In: Atkins’ physical chemistry. W. H. Freeman and Company, pp 200–240
Augustyn AR, Pott RWM, Tadie M (2021) The interactions of the biosurfactant surfactin in coal flotation. Colloids Surf A Physicochem Eng Asp 627:127122
Avrami M (1939) Kinetics of phase change. J Chem Phys 7:1103–1112
Bhosale SS, Rohiwai SS, Chaudhary LS, Pawar KD, Patil PS, Tiwari AP (2019) Photocatalytic decolorization of methyl violet dye using rhamnolipid biosurfactant modified iron oxide nanoparticles for wastewater treatment. J Mater Sci Mater Electron 30:4590–4598
Bonnefille B, Gomez E, Courant F, Escande A, Fenet H (2018) Diclofenac in the marine environment: a review of its occurrence and effects. Mar Pollut Bull 131:496–506
Carolin FC, Kumar PS, NgueagniI PT (2021) A review on new aspects of lipopeptide biosurfactant: types, production, properties and its application in the bioremediation process. J Hazard Mater 407:124827
Champion JT, Gilkey JC, Lamparski H, Retterer J, Miller RM (1995) Electron microscopy of rhamnolipid (biosurfactant) morphology: effects of pH, cadmium, and octadecane. J Colloid Interface Sci 170:569–574
Chandler D (2005) Interfaces and the driving force of hydrophobic assembly. Nature 437:640–647
Chen Q, Li Y, Liu M, Zhu B, Mu J, Chen Z (2021) Removal of Pb and Hg from marine intertidal sediment by using rhamnolipid biosurfactant produced by a Pseudomonas aeruginosa strain. Environ Technol Innov 22:101456
Chrzanowski L, Owsianiak M, Szulc A, Marecik R, Piotrowska-Cyplik A, Olejnik-Schmidt AK, Staniewski J, Lisiecki P, Ciesielczyk F, Jesionowski T, Heipieper HJ (2011) Interactions between rhamnolipid biosurfactants and toxic chlorinated phenols enhance biodegradation of a model hydrocarbon-rich effluent. Int Biodeterior Biodegradation 65:605–611
Cohen R, Ozdemir G, Exerowa D (2003) Free thin liquid films (foam films) from rhamnolipids: type of the film and stability. Colloids Surf B: Biointerfaces 29:197–204
Cooney DO (1998) Adsorption design for wastewater treatment. CRC Press
Cooper A, Vance SJ, Smith BO, Kennedy MW (2017) Frog foams and natural protein surfactants. Colloids Surf A Physicochem Eng Asp 534:120–129
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447
Crini G, Lichtfouse E, Wilson LD, Momrin-Crini N (2018) Adsorption-oriented processes using conventional and non-conventional adsorbents for wastewater treatment. In: Green adsorbents for pollutant removal. Springer, pp 23–71
Cunha SC, Pena A, Fernandes JO (2017) Mussels as bioindicators of diclofenac contamination in coastal environments. Environ Pollut 225:354–360
Decesaro A, Machado TS, Cappellaro AC, Rempel A, Margarites AC, Reinehr CO, Eberlin MN, Zampieri D, Thomé A, Colla LM (2020) Biosurfactants production using permeate from whey ultrafiltration and bioproduct recovery by membrane separation process. J Surfactant Deterg 23:539–551
Decesaro A, Rempel A, Machado TS, Cappellaro AC, Machado BS, Cechin I, Thomé A, Colla LM (2021) Bacterial biosurfactant increases ex situ biodiesel bioremediation in clayey soil. Biodegradation 32:389–401
Dotto GL, Salau NPG, Piccin JS, Cadaval TRS Jr, Pinto LAA (2017) Adsorption kinetics in liquid phase: modeling for discontinuous and continuous systems. In: Adsorption processes for water treatment and purification. Springer
Drakontis CE, Amin S (2020) Biosurfactants: formulations, properties, and applications. Curr Opin Colloid Interface Sci 48:77–90
Dubinin MM, Radushkevich LV (1947) The equation of the characteristic curve of the activated charcoal. Proc Acad Sci USSR Phys Chem Sect 55:331–337
Elovich SY, Larinov OG (1962) Theory of adsorption from nonelectrolyte solutions on solid adsorbents. Russ Chem Bull 11:191–197
Freundlich HMF (1906) Over the adsorption in solution. Chem A Eur J 57:385–471
Giles CH, Macewan TH, Nakhwa SN, Smith D (1960) Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc 14:3973–3993
Gudiña EJ, Rangarajan V, Sen R, Rodrigues LR (2013) Potential therapeutic applications of biosurfactants. Trends Pharmacol Sci 34:667–675
Guo Q, Yan J, Wen J, Hu Y, Chen Y, Wu W (2016) Rhamnolipid-enhanced aerobic biodegradation of triclosan (TCS) by indigenous microorganisms in water-sediment systems. Sci Total Environ 571:1304–1311
Haidar CN, Pereira MM, Lima ÁS, Nerli BB, Malpiedi LP (2020) Biosurfactants produced by pseudomonas syringae pv tabaci: a versatile mixture with interesting emulsifying properties. Process Biochem 97:121–129
Hill TL (1946) Statistical mechanics of multimolecular adsorption. J Chem Phys 14:263–267
Hirata H, Ohira A, Iimura N (1996) Measurements of the Krafft point of surfactant molecular complexes: insights into the intricacies of “Solubilization”. Langmuir 12:6044–6052
Ho YS, Mckay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Huber C, Preis M, Harvey PJ, Grosse S, Letzel T, Schröder P (2016) Emerging pollutants and plants - metabolic activation of diclofenac by peroxidases. Chemosphere 146:435–441
Humelnicu I, Baiceanu A, Ignat ME, Dulman V (2017) The removal of basic blue 41 textile dye from aqueous solution by adsorption onto natural zeolitic tuff: kinetics and thermodynamics. Process Saf Environ Prot 105:274–287
Jałowiecki Ł, Zur J, Płaza GA (2017) Norfloxacin degradation by Bacillus subtilis strains able to produce biosurfactants on a bioreactor scale. E3S Web Conferences 17:1–8
Jayalatha NA, Devatha CP (2019) Degradation of Triclosan from domestic wastewater by biosurfactant produced from bacillus licheniformis. Mol Biotechnol 61:674–680
Kaskatepe B, Yildiz S (2016) Rhamnolipid biosurfactants produced by Pseudomonas Species. Braz Arch Biol Technol 59:e16160786
Kheradmand A, Ghiasinejad H, Javanshir S, Khadir A, Jamshidi E (2021) Efficient removal of ibuprofen via novel core – shell magnetic bio-surfactant rhamnolipid – layered double hydroxide nanocomposite. J Environ Chem Eng 9:1061580
Kinniburgh DG (1986) General purpose adsorption isotherms. Environ Sci Technol 20:895–904
Krafft F (1899) Ueber die Krystallisationsbedingungen colloïdaler Salzlösungen. Ber Dtsch Chem Ges 32:1596–1608
Kreling NE, Zaparoli M, Margarites AC, Friedrich MT, Thomé A, Colla LM (2020) Extracellular biosurfactants from yeast and soil–biodiesel interactions during bioremediation. Int J Environ Sci Technol 17:395–408
Kumar PSM, Ganesan S, Al-Muhtaseb AH, Al-Ha L, Elancheziyan M, Shobana S, Kumar G (2021) Tropical fruit waste-derived mesoporous rock-like Fe2O3/C composite fabricated with amphiphilic surfactant-templating approach showing massive potential for high-tech applications. Int J Energy Res 45:17417–17430
Lagergreen S (1907) Zur Theorie der sogenannten Gelöster de adsorption Stoffe. Kungliga Svenska Vetenskapsakademiens 2:174–175
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lebrón-Paler A, Pemberton JE, Becker BA, Otto WH, Larive CK, Maier RM (2006) Determination of the acid dissociation constant of the biosurfactant monorhamnolipid in aqueous solution by potentiometric and spectroscopic methods. Anal Chem 78:7649–4658
Li JL, Chen BH (2009) Surfactant-mediated Biodegradation of Polycyclic Aromatic Hydrocarbons. Materials 2:76–94
Li Y, Bi H-Y, Li H, Jin Y-S (2016) Adsorption of cu (II) on rhamnolipid-layered double hydroxide nanocomposite. Clay Miner 64:560–570
Liu C-X, Xu Q-M, Yu S-C, Cheng J-S, Yuan Y-J (2020) Bio-removal of tetracycline antibiotics under the consortium with probiotics Bacillus clausii T and bacillus amyloliquefaciens producing biosurfactants. Sci Total Environ 710:136329
Liu Y, Xu H, Yang S-F, Tay J-H (2003) A general model for biosorption of Cd2+, Cu2+ and Zn2+ by aerobic granules. J Biotechnol 102:233–239
Maccabe WL, Smith JC, Harriott P (1993) Unit operations of chemical engineering. McGraw-hill
Machado TS, Crestani L, Marchezi G, Melara F, Mello JR, Dotto GL, Piccin JS (2022) Synthesis of glutaraldehyde-modified silica/chitosan composites for the removal of water-soluble diclofenac sodium. Carbohydr Polym 277:118868
Machado TS, Decesaro A, Cappellaro AC, Machado BS, Reginato KVS, Reinehr CO, Thomé A, Colla LM (2020) Effects of homemade biosurfactant from bacillus methylotrophicus on bioremediation efficiency of a clay soil contaminated with diesel oil. Ecotoxicol Environ Saf 201:110798
Malkapuram ST, Sharma V, Gumfekar SP, Sonawane S, Sonawane S, Boczkaj G, Seepana MM (2021) A review on recent advances in the application of biosurfactants in wastewater treatment. Sustain Energy Technol Assess 48:101576
Markande AR, Acharya SR, Nerurkar AS (2013) Physicochemical characterization of a thermostable glycoprotein bioemulsifier from Solibacillus silvestris AM1. Process Biochem 48:1800–1808
Markande AR, Patel D, Varjani S (2021) A review on biosurfactants: properties, applications and current developments. Bioresour Technol 330:124963
Melara F, Machado TS, Alessandretti I, Manera C, Perondi D, Godinho M, Piccin JS (2021) Synergistic effect of the activated carbon addition from leather wastes in chitosan/alginate-based composites. Environ Sci Pollut Res 28:48666–48680
Mishra S, Linz Z, Pang S, Zhang Y, Bhatt P, Chen S (2021) Biosurfactant is a powerful tool for the bioremediation of heavy metals from contaminated soils. J Hazard Mater 418:126253
Mohajeri E, Noudeh GD (2012) Effect of temperature on the critical micelle concentration and Micellization thermodynamic of nonionic surfactants: Polyoxyethylene Sorbitan fatty acid esters. E-J Chem 9:2268–2274
Molina-Sabio M, Rodriguez-Reinoso F (2004) Role of chemical activation in the development of carbon porosity. Colloids Surf A Physicochem Eng Asp 241(1–3):15–25
Najafi AR, Rahimpour MR, Jahanmiri AH, Roostaazad R, Arabian D, Ghobadi Z (2010) Enhancing biosurfactant production from an indigenous strain of Bacillus mycoides by optimizing the growth conditions using a response surface methodology. Chem Eng J 163:188–194
Nascimento RF, Lima ACA, Vidal CB, Melo DQ, Raulino GSC (2014) Adsorption: theoretical aspects and environmental applications. University Press. In Portuguese
Nascimento RF, Lima ACA, Vidal CB, Melo DQ, Raulino GSC (2020) Adsorption: theoretical aspects and environmental applications. University press of the Federal University of Ceará. In Portuguese
Natarajan R, Kumar MA, Vaidyanathan VK (2022) Synthesis and characterization of rhamnolipid based chitosan magnetic nanosorbents for the removal of acetaminophen from aqueous solution. Chemosphere 288:132532
Newcombe G, Hayes R, Drikas M (1993) Granular activated carbon: importance of surface properties in the adsorption of naturally occurring organics. Colloids Surf A Physicochem Eng Asp 74:275–286
Nguyen TT, Sabatini DA (2011) Characterization and emulsification properties of rhamnolipid and sophorolipid biosurfactants and their applications. Int J Mol Sci 12:1232–1244
Noguera-Oviedo K, Aga DS (2016) Lessons learned from more than two decades of research on emerging contaminants in the environment. J Hazard Mater 316:242–251
Onaizi SA (2018) Dynamic surface tension and adsorption mechanism of surfactin biosurfactant at the air–water interface. Eur Biophys J 47:631–640
Pal P, Pal A (2019) Treatment of real wastewater: kinetic and thermodynamic aspects of cadmium adsorption onto surfactant-modified chitosan beads. Int J Biol Macromol 131:1092–1100
Patel M, Kumar R, Kishor K, Mlsna T, Pittaman CU Jr, Mohan D (2019) Pharmaceuticals of Emerging Concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem Rev 119:3510–3673
Patowary R, Patowary K, Kalita MC, Deka S, Borah JM, Joshi SJ, Zhang M, Peng W, Sharma G, Rinklebe J, Sarma H (2022) Biodegradation of hazardous naphthalene and cleaner production of rhamnolipids - green approaches of pollution mitigation. Environ Res 209:112875
Perez-Ameneiro M, Vecino X, Cruz JM, Moldes AB (2015) Wastewater treatment enhancement by applying a lipopeptide biosurfactant to a lignocellulosic biocomposite. Carbohydr Polym 131:186–196
Pettersson M, Bââth E (2003) Temperature-dependent changes in the soil bacterial community in limed and unlimed soil. FEMS Microbiol Ecol 45:13–21
Piccin JS, Cadaval TRS Jr, Pinto LAA, Dotto GL (2017) Adsorption isotherms in liquid phase: experimental, modeling, and interpretations. In: Adsorption processes for water treatment and purification. Springer
Piccin JS, Vieira MLG, Gonçalves JO, DOTTO, G.L., Pinto, L.A.A. Adsorption of FD&C Red No. (2009) 40 by chitosan: isotherms analysis. J Food Eng 95:16–20
Qiu H, Lv L, Pan B, ZHhang Q, Zhang W, Zhang Q, Critical review in adsorption kinetic models (2009) Journal of Zhejiang University-Science A 10:716–724
Rastogi S, Kumar R (2020) Remediation of heavy metals using non-conventional adsorbents and biosurfactant-producing bacteria. In: Environmental degradation: causes and remediation strategies, pp 133–153
Rastogi S, Kumar R (2021) Statistical optimization of biosurfactant production using waste biomaterial and biosorption of Pb2+ under concomitant submerged fermentation. J Environ Manag 295:113158
Rathi BS, Kumar PS, Show P-L (2021) A review on effective removal of emerging contaminants from aquatic systems: current trends and scope for further research. J Hazard Mater 409:124413
Redlich OJDL, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63:1024
Ruthven DM (1984) Principles of adsorption and adsorption process. John Wiley & Sons
Sarubbo LA, Rocha RB Jr, Luna JM, Rufino RD, Santos VA, Banat IM (2015) Some aspects of heavy metals contamination remediation and role of biosurfactants. Chem Ecol 31:707–723
Sarubbo LA, Silva MGC, Durval IJB, Bezerra KGO, Ribeiro BG, Silva IA, Twigg MS, Banat IM (2022) Biosurfactants: production, properties, applications, trends and general perspectives. Biochem Eng J 181:108377
Schmidt VKO, Carvalho JS, Oliveira D, Andrade CJ (2021) Biosurfactant inducers for enhanced production of surfactin and rhamnolipids: an overview. World J Microbiol Biotechnol 37:1–15
Sharma RK, Wang S-C, Maity JP, Banerjee P, Dey G, Huang Y-H, Bundschuh J, Hsiao P-G, Chen T-H, Chen C-Y (2021) A novel BMSN (biologically synthesized mesoporous silica nanoparticles) material: synthesis using a bacteria-mediated biosurfactant and characterization. RSC Adv 11:32906–32916
She A-Q, Gang H-Z, Mu B-Z (2012) Temperature influence on the structure and interfacial properties of Surfactin micelle: a molecular dynamics simulation study. J Phys Chem B 116:12735–12743
Shin K-H, Kim K-W, Kim J-Y, Lee K-E, Han S-S (2008) Rhamnolipid morphology and phenanthrene solubility at different pH values. J Environ Qual 37:509–514
Silva VN, Dilarri G, Lovaglio RB, Gonçalves RB, Montagnolli RN, Contierro J (2021) Rhamnolipid from Pseudomonas aeruginosa can improve the removal of direct Orange 2GL in textile dye industry effluents. J Mol Liq 321:114753
Sips R (1948) On the structure of a catalyst surface. J Chem Phys 16:490–495
Sonowal S, Joshi SJ, Borah SN, Islam NF, Pandit S, Prasad R, Sarma H (2022) Biosurfactant-assisted phytoremediation of potentially toxic elements in soil: green technology for meeting the United Nations sustainable development goals. Pedosphere 32:0198–0210
Sophia CA, Lima EC (2018) Removal of emerging contaminants from the environment by adsorption. Ecotoxicol Environ Saf 150:1–17
Srivastava S, Mondal MK, Agrawal SB (2021) Biosurfactants for heavy metal remediation and bioeconomics. In: Biosurfactants for a Sustainable Future, pp 79–98
Temkin MI (1941) Adsorption equilibrium and the kinetics of processes on nonhomogeneous surfaces and in the interaction between adsorbed molecules. Zhurnal Fiziche- skoi Khimii 15:296–332
Usman MM, Dadrasnia A, Lim KT, Mahmud AF, Ismail S (2016) Application of biosurfactants in environmental biotechnology; remediation of oil and heavy metal. Bioengineering 3:289–304
Van Hamme JD, Singh A, Ward OP (2006) Physiological aspects: part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol Adv 24:604–620
Vance SJ, Mcdonald RE, Cooper A, Smith BO, Kennedy MW (2013) The structure of latherin, a surfactant allergen protein from horse sweat and saliva. J R Soc Interface 10:20130453
Varjani SJ, Upasani VN (2017) Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour Technol 232:389–397
Vauter-Giongo C, Bales BL (2003) Estimate of the ionization degree of ionic micelles based on Krafft temperature measurements. J Phys Chem B 107:5398–5403
Vinson PK, Talmon Y, Walter A (1989) Vesicle-micelle transition of phosphatidylcholine and octyl glucoside elucidated by cryo-transmission electron microscopy. The Biophysical Journal 56:669–681
Wang S, Liu Y, Lü Q (2020) Facile preparation of biosurfactant-functionalized Ti2CTX MXene nanosheets with an enhanced adsorption performance for Pb(II) ions. J Mol Liq 297:111810
Wu W, Hu Y, Guo Q, Yan J, Chen Y, Cheng J (2015) Sorption/desorption behavior of triclosan in sediment–water–rhamnolipid systems: effects of pH, ionic strength, and DOM. J Hazard Mater 297:59–65
Yagub MT, Sem TK, Afroze S, Ang HM (2014) Dye and it as removal from aqueous solution by adsorption: a review. Adv Colloid Interf Sci 209:172–184
Zhu Z, Gao C, Wu Y, Sun L, Huang X, Ran W, Shen Q (2013) Removal of heavy metals from aqueous solution by lipopeptides and lipopeptides modified Na-montmorillonite. Bioresour Technol 147:378–386
Zhu Z, Zhang B, Cai Q, Cao Y, Ling J, Lee K, Chen B (2021) A critical review on the environmental application of lipopeptide micelles. Bioresour Technol 339:125602
Zuim DR (2010) Study of the adsorption of coffee aroma components (benzaldehyde and acetic acid) lost during the soluble coffee production process. In: Dissertation (postgraduate program in food technology). Federal University of Paraná
Acknowledgments
The authors thank the Coordination of Superior Level Staff Improvement (CAPES—Financing Code 001), National Council for Scientific and Technological Development (CNPQ - Project Code 140541/2021-7), Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS) and the University of Passo Fundo (UPF).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Machado, T.S. et al. (2023). Biosurfactants and Their Perspectives for Application in Drug Adsorption. In: Aslam, R., Mobin, M., Aslam, J., Zehra, S. (eds) Advancements in Biosurfactants Research. Springer, Cham. https://doi.org/10.1007/978-3-031-21682-4_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-21682-4_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-21681-7
Online ISBN: 978-3-031-21682-4
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)