Abstract
The ability of forests to provide ecosystem services and renewable goods faces several challenges related to insect defoliation. Spruce budworm outbreaks represent one of the major natural disturbances in the boreal forest of eastern North America. In this chapter, we will focus on the effects of defoliation by eastern spruce budworm in balsam fir and black spruce trees. We first describe tree water status depending on the duration of defoliation. We then present the response of springtime starch reserves and radial growth at different levels of defoliation. We summarize four mechanisms to explain mortality under defoliation and the consequences for forest management.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
1 Introduction
Insect outbreaks, fire, and drought are the major disturbances in forest ecosystems (Seidl et al., 2017). Worldwide, about 345 million ha of forests are affected annually by such disturbances (van Lierop et al., 2015). Because of their impact on growth and survival, insect outbreaks have serious economic and ecological implications for the boreal ecosystem (Sturtevant et al., 2015). Outbreaks of eastern spruce budworm (Choristoneura fumiferana (Clemens), hereafter SBW) occur periodically in eastern North America (Morin et al., 2010; Zhang et al., 2014). Cyclical SBW outbreaks during the twentieth century caused more than a 50% annual productivity loss and widespread mortality in spruce and fir species (Morin, 1994; Pureswaran et al., 2016). Since 2006, there has been a new and ongoing SBW outbreak in Québec, Canada, affecting more than 8 million ha of forest in 2018 (Bouchard et al., 2018; MFFP 2018).
Climate factors, such as temperature and precipitation, determine the distribution of SBW (Pureswaran et al., 2015). Ongoing climate change thus alters the interactions between plants and insects (Fleming & Volney, 1995; Haynes et al., 2014; Singer & Parmesan, 2010), directly influencing the frequency of outbreaks and their spatial distribution (Despland, 2018; Foster et al., 2013). Moreover, under global warming scenarios, future outbreaks will last six years longer than at present and produce 15% greater defoliation (Gray, 2008), thereby influencing the soil–plant-atmosphere continuum and the associated forest carbon, water, and energy dynamics (Balducci et al., 2020; Fierravanti et al., 2019; Liu et al., 2018).
Given the ongoing global change, a 20 to 40% reduction in soil water content is expected in eastern North America (Houle et al., 2015). During the twentieth century, SBW outbreaks increased in duration and severity in eastern Canada (Navarro et al., 2018). However, insect defoliation may offset the negative impact of water deficit on tree growth, thereby reducing mortality (Bouzidi et al., 2019; Itter et al., 2019). Nonetheless, the interactive effect of water availability and defoliation on tree water status remains puzzling (Quentin et al., 2012), especially when considering the short-term chewing effect of SBW (Bouzidi et al., 2019) and the long duration (ca. 10 years) of their outbreaks (Candau et al., 1998; Gray et al., 2000), which affect long-term water uptake and tree growth (Balducci et al., 2020).
Outbreaks result in a tree mortality of approximately 50% (Bergeron et al., 1995) and volume losses of 32–48% (Ostaff & MacLean, 1995) Thus, outbreaks play a significant role in the carbon (C) flux of forests, with losses estimated at 2.87 t C·ha−1 over the course of an outbreak. Insect outbreaks represent a major disturbance that affects the entire physiology of a tree. The changes in carbon allocation patterns under defoliation have important physiological consequences, including the modification of bud burst (Deslauriers et al., 2019), a drastic reduction in radial growth rate, i.e., wood formation (Bouzidi et al., 2019; Deslauriers et al., 2015), and increased tree mortality (Fierravanti et al., 2019). As the mortality of defoliated trees depends on many factors, including water status and carbon allocation, disentangling these effects can help predict tree mortality under defoliation.
This chapter aims to describe the effect of defoliation by SBW on the water status and carbon allocation of balsam fir (Abies balsamea L. Mill.) and black spruce (Picea mariana (Mill.) BSP). We first describe the short- and long-term effects of budworm defoliation on the tree and soil water status. We then describe how growth reduction following defoliation is associated with a reduction in carbon reserves. Last, we describe how changes in tree water status and carbon allocation affect tree mortality and discuss the related implications for forest management.
2 Change in the Water Status of Trees and Soils During Budworm Defoliation
Depending on the specific plant–insect interaction, the direct effects of defoliation on plant water status range from positive to negative (Aldea et al., 2005; Nardini et al., 2012; Pittermann et al., 2014). An improvement in tree water potential, i.e., an increase in leaf water potential, has been observed at the beginning of a defoliation event in partially defoliated eucalyptus trees (Eyles et al., 2013; Quentin et al., 2011, 2012). In contrast, insect attacks on broadleaf species reduce leaf size and, therefore, midday water potential, leading to morphological and physiological changes that are similar to drought responses (Nabity et al., 2009; Peschiutta et al., 2016). Similarly, lower water content has been observed on defoliated twigs in balsam fir (Deslauriers et al., 2015), black spruce (Bouzidi et al., 2019), and Scots pine (Pinus sylvestris L.) (Salmon et al., 2015), indicating a higher evaporative demand in defoliated trees and a consequent decrease in tree water status during defoliation. The responses of defoliated conifers to water must therefore consider the cumulative effect of defoliation over both shorter and longer periods to better understand the impact on tree mortality.
2.1 Short-Term Effects
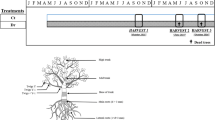
The plant water status of black spruce saplings defoliated by eastern SBW shows time-dependent effects (Bouzidi et al., 2019). During defoliation, i.e., when the budworm is feeding vigorously on the needles, a higher evaporative demand and lower midday leaf water potential (Ψmd) were observed, whereas there was an opposite pattern after the period of defoliation (Fig. 9.1) (Bouzidi et al., 2019). This was closely related to the timing of measurements linked with the period of larvae feeding. Although plant water status changes rapidly during and after defoliation, no effect of defoliation has been observed on soil moisture (Bouzidi et al., 2019).

Modified by permission of Springer Nature from Bouzidi et al. (2019)
Predawn (Ψpd, MPa) and midday (Ψmd, MPa) leaf water potential of black spruce saplings subjected to defoliation. The shaded gray areas indicate defoliation periods. Different letters indicate significant differences (P < 0.05) in the defoliated treatments per sampling period.
In the short term, Ψmd is often lower in defoliated plants than nondefoliated controls, indicating a higher evaporative demand during or immediately following defoliation (Salmon et al., 2015). Eyles et al. (2013) also report a decrease in Ψmd in saplings subjected to different levels of defoliation—50% or 100% apical bud damage. The decrease of Ψmd occurs over a short period (approximately two weeks) and is caused by the mechanical chewing action of larvae during active defoliation (Bouzidi et al., 2019). In contrast, predawn leaf water potential (Ψmd) is not affected during or after defoliation by SBW (Fig. 9.1). Budworm feeding habits damage many growing needles, resulting in a loss of turgor or even localized cavitation because of the entry of air into the water conduits of damaged needles; this likely decreases leaf water potential. However, an opposite pattern is observed afterward during the post-defoliation period when leaf Ψmd is higher in nondefoliated saplings (Fig. 9.1); this observation agrees with similar patterns for defoliated Larix decidua Mill., Pinus strobus L., and Quercus velutina Lam. (Vanderklein & Reich, 2000; Wiley et al., 2013). When direct defoliation ceases, the reduced leaf area leads to lower transpiring woody tissues (Schmid et al., 2017; Wiley et al., 2013), thereby decreasing water transport. In the short term, therefore, defoliation affects the plant–water relationship both negatively (during active defoliation by growing instars) and positively (after defoliation).
2.2 Long-Term Effects
An event of several years of needle loss reveals a different response than that observed for short-term defoliation, i.e., one year. We define long-term effects as mean various defoliation intensities lasting between 5 and 15 years. To study the long-term effects of recurrent defoliation on the water status of trees and soil, we measured soil volumetric water content (VWC), shoot relative water content (SWC), and midday water potential (Ψmd) along a defoliation gradient in black spruce and balsam fir during a two-year period (2014–2015) at several sites in Québec, Canada (Balducci et al., 2020). The decrease in SWC and Ψmd with increased total tree defoliation reveal that the plant water status reflects the quantity of foliage loss in mature trees (Fig. 9.2). In the long term, the water potential of both fir and spruce (Ψmd) decreases with greater defoliation; values range from −1.01 MPa at 0% defoliation to a maximum of –1.84 MPa in completely defoliated plants. Plant water status lowers as defoliation increases (Fig. 9.2). At low defoliation levels between 5 and 15%, Ψmd increases from −0.96 to −0.87 MPa but becomes more negative at higher defoliation levels (>20%), suggesting a threshold effect. Therefore, at the beginning of defoliation (<20%), the reduced leaf area shows a similar effect to the post-defoliation period (Fig. 9.1), also leading to lower leaf transpiration (Schmid et al., 2017; Wiley et al., 2013).

Modified with permission from Elsevier from Balducci et al. (2020)
Midday water potential (Ψmd) expressed as a function of vapor pressure deficit (VPD) and defoliation (%) in both balsam fir and black spruce. The solid lines represent the fitted linear regression, the dashed lines refer to the 95% confidence interval for the data points, and the vertical bars represent the standard deviation of the mean.
In defoliated trees, Ψmd is also more negative (reaching values of between −0.96 and −1.36 MPa) with an increasing vapor pressure deficit (VPD) (ranging from 0.1 to 0.8 kPa, Fig. 9.2). High VPD decreases the midday water potential, i.e., an increase in water tension in the xylem. Trees having a more negative water potential because of high VPD are also more likely to lose hydraulic conductivity, with a consequent increase in the risk of xylem embolism (Tyree & Sperry, 1989) and eventual death by hydraulic failure (Adams et al., 2017; Anderegg et al., 2013). In two consecutive years of defoliation, an increase in defoliation also resulted in a significant decrease in the SWC (Fig. 9.3). Similar results have been found in the twigs of mature balsam fir, where the SWC decreased by 8% in defoliated trees compared with nondefoliated trees (Deslauriers et al., 2015).
Soil volumetric water content (VWC, %) and shoot relative water content (SWC, %) expressed as a function of defoliation for sampled trees in 2014 and 2015. Data includes the fitted linear regression (solid black line), the 95% confidence intervals (dashed lines), and the standard error of the individual data points (vertical bars)
Although Ψmd dropped as defoliation increased (Fig. 9.2), soil VWC increased in both 2014 and 2015 (Fig. 9.3). In both years, VWC was close to 14% in lightly defoliated trees, whereas, in fully defoliated trees, VWC increased from 17% in 2014 to 25% in 2015. These results for the plant and soil water content of defoliated trees therefore suggest a reduced water transfer throughout the soil–plant–air continuum. As also observed by Dietze and Matthes (2014), a slight increase in soil water content occurs following defoliation. Such an increase can be explained by (i) a loss of foliage at a greater rate than fine root production and (ii) reduced transpiration. Defoliated trees markedly alter water storage in the soil and the water balance of a stand by changing the ratio between water input (precipitation) and output (evapotranspiration) (Dietze & Matthes, 2014; Hata et al., 2016).
When defoliation increases canopy transparency, such as via canopy openness (Hata et al., 2016), less precipitation is intercepted by the canopy, and, consequently, the soil receives more water (Sun et al., 2015). As vegetation cover reflects water movement in the soil–plant sphere, a higher percentage of cover (a larger leaf area index, LAI) increases evaporation from interception, thereby compensating for the reduced evaporation from the soil (Fatichi & Pappas, 2017). Defoliation thus changes the water storage, i.e., the difference between precipitation and evapotranspiration (Viglizzo et al., 2016) through a reduced interception and canopy evaporation. An ecophysiological framework applied to ponderosa pine (Pinus ponderosa Dougl. ex C. Lawson) in western North America showed that canopy transparency at higher defoliation levels increases soil moisture in response to reduced canopy transpiration and dryness of the soil surface because of increased evaporation (Dietze & Matthes, 2014). In the boreal forest of eastern Québec, however, temperatures are generally low and precipitation abundant, i.e., greater than soil evaporation (Gauthier et al., 2015); this is particularly true in boreal forest peatlands where the contribution of transpiration to evapotranspiration is limited to 1% (Warren et al., 2018).
3 Carbon Allocation in Conifers Defoliated by Budworm
The intra-annual carbon allocation is severely modified during defoliation (Figs. 9.4 and 9.5) because recurrent defoliation represents a special case of carbon source limitation (Körner, 2015). The changes will be reflected in carbon sink priority, which is ordered according to organ priorities with seed production having the highest priority and reserves the lowest (Minchin & Lacointe, 2005). In conifers, carbon reserves in the form of starch increase before the resumption of shoot and stem growth (Desalme et al., 2017; Hoch et al., 2003; Little, 1970; Martinez-Vilalta et al., 2016), and the highest amounts of starch are found in the needles (Deslauriers et al., 2015, 2019). Regardless of host species (balsam fir, white spruce, or black spruce), recurrent defoliation prevents the accumulation of starch during the spring (Deslauriers et al., 2015, 2019), thus reflecting sink priority. The reduction in the buildup of carbon reserves is followed by an earlier bud burst, promoting needle growth at the expense of cambium activity; thus, ring width decreases. Multiple studies on conifers have illustrated the changes occurring in the carbon reserves of nondefoliated and defoliated trees, the latter having a lower starch content (Webb, 1980, 1981; Webb & Karchesy, 1977).
Variations of starch (orange) and sugar (blue) concentrations over a year in the shoots of boreal conifers. The cases of no defoliation (top) and after several years of defoliation (bottom) are presented. Bud break generally occurs around the maximum of starch accumulation during spring. Under recurrent years of defoliation, needle loss impairs starch accumulation during the spring and can reduce soluble sugar concentrations in several tree organs (e.g., needles, twigs, stem, and roots).
In conifers (except for larch), the older needles assimilate and store carbon, especially during the period preceding bud burst and growth. Under defoliation conditions, any change in carbon balance thus occurs initially in the starch reserves of older needles (Deslauriers et al., 2019; Vanderklein & Reich, 1999) because of reduced starch accumulation during the spring (Wiley & Helliker, 2012) before defoliation (Fig. 9.4). Therefore, the defoliation level closely corresponds to differences in springtime starch reserves (Fig. 9.5) and radial growth (Fig. 9.6); both variables decrease at higher defoliation levels. Within a wider annual context, the metabolism of starch and soluble sugars during the year (Figs. 9.4 and 9.5) becomes biologically meaningful compared with growth activity and bud dormancy. After bud break, starch levels slowly decrease during the summer to sustain primary and secondary growth (Deslauriers et al., 2019; Webb & Karchesy, 1977); however, when the starch reserves fail to increase under defoliation, radial growth decreases proportionally.

Modified from Deslauriers et al. (2015), CC BY 4.0 license
Variation in starch (mg·g−1dw) measured in leaf (right) and stem wood (left) of balsam fir during the growing season for the different defoliation classes (nondefoliated, moderately defoliated, and heavily defoliated). Vertical bars represent the standard deviation among six trees. Note the different scales of the vertical axes.

Reproduced from Fierravanti et al. (2019), CC BY 4.0 license
Tree-ring widths in trees belonging to the four defoliation classes in black spruce and balsam fir, from measurements obtained between 2006 and 2015. D0 represents nondefoliated trees, and D1, D2, and D3 represent 1%–33%, 33%–66%, and 66%–100% defoliation, respectively. Different letters indicate significant differences between defoliation classes. Rectangles indicate situations where data points sharing the same letter are overlapping on the plots.
The absence of starch buildup during the spring reveals the changes occurring in carbon allocation under defoliation. Rather than allocating carbon to starch reserves during the spring (Figs. 9.4 and 9.5), this carbon is allocated to shoot growth, which has a higher sink strength, at the expense of radial stem growth and reserve accumulation; this change represents a shift in the trees’ priorities for physiological resources to ensure survival (Deslauriers et al., 2019). In deciduous conifers such as larch (Larix decidua Mill.) affected by budmoth (Zeiraphera griseana Hübner), however, replenishment of carbon storage for refoliation is prioritized (Peters et al., 2020).
In conifers, newly assimilated carbon by older needles is mainly allocated to the canopy during primary growth (85% of the allocated 13C), with only a minor fraction (1.6%) translocated to the lower stem (Heinrich et al., 2015). During primary growth, the substantial loss of needles under defoliation produces a carbon limitation in other parts of the tree, mainly in the stem wood where starch starvation can occur (Fig. 9.5, right). Carbon allocation to the roots is also severely impaired after defoliation (Li et al., 2002; Reich et al., 1993). As bud growth competes with other sinks, such as wood formation (Antonucci et al., 2015; Deslauriers et al., 2016; Huang et al., 2014; Traversari et al., 2018) and root growth (Reich et al., 1993), prioritizing carbon allocation to the buds has a positive impact on bud opening and successive shoot growth but negatively affects secondary growth, i.e., tree-ring formation (Fig. 9.6; see also Peters et al. (2020) and Deslauriers et al. (2015) for impacts on wood formation and wood anatomy).
4 How Changes in Water Status and Carbon Allocation Affect Tree Mortality
Exploring the effect of SBW defoliation on tree water status and carbon allocation reveals that four mechanisms can trigger tree mortality: the difficulty in water reaching the remaining needles, hydraulic failure, the decrease in carbon storage during spring, and the decrease in radial growth.
4.1 Decreased Shoot Water Content in Defoliated Trees and the Related Mortality
Needle damage and death due to defoliation are visible effects of insect feeding, and these signs integrate a series of physiological changes within plants. First, they represent a direct constraint on transpiration (Pincebourde et al., 2006), linked to a decrease in water reaching the remaining needles as defoliation increases (Sack & Holbrook, 2006). Therefore, the relative water content of shoots gradually drops with increasing defoliation (Deslauriers et al., 2015). Consequently, water potential decreases and gradually impairs water relations in defoliated trees (Fig. 9.7; also see Sect. 9.1). In the twentieth century, cumulative climatic stresses—two cold springs that reduced photosynthetic activity followed by a warmer summer that induced higher evapotranspiration—preceded SBW outbreaks and induced tree mortality in black spruce and balsam fir (De Grandpré et al., 2019). A better understanding of water status imbalance by defoliation and its consequences on tree mortality must consider (i) tree ontogeny, as the physiology of young and mature trees can differ within the same species, and (ii) the interaction between defoliation (biotic stress) and environmental stress (reduced soil moisture and atmospheric drought, including a high VPD.

Modified with permission from Elsevier from Balducci et al. (2020)
Summary of the effect of environmental (VPD, vapor pressure deficit) and biotic stresses (spruce budworm) on the water status of trees (RWC, root water content; Ψleaf, leaf water potential) and soil in defoliated and nondefoliated trees. Symbols (−) and (+) represent the limiting and optimal soil water availability, respectively.
Under controlled conditions, four-year-old saplings of fir, white spruce, and black spruce affected by severe defoliation after a full year of SBW outbreak show mainly bud phenological shifts. This alteration modifies the carbon allocation within the trees to primary rather than secondary growth (Deslauriers et al., 2019) and thereby ensures tree survival. Similarly, in young black spruce, a mild defoliation intensity (~40%) observed in current-year defoliation does not compromise tree survival, although water status is negatively affected during active insect feeding (Bouzidi et al., 2019). Under concomitant defoliation and drought stresses, however, the effect of drought prevails over defoliation-related stress. Drought alone increases the sapling mortality of black spruce immediately after the stress period. Nondefoliated drought-related saplings show higher mortality rates during the first two weeks after resuming irrigation (0.3 dead saplings·day−1) compared with defoliated drought-related saplings (0 dead saplings·day−1) (Bouzidi et al., 2019). The detrimental effects observed in needles during active defoliation become positive for tree water balance in the first few weeks. After two weeks, the mortality rate in defoliated saplings remains similar (0.05–0.1 dead saplings·day−1) to defoliated drought-related spruce. This difference in mortality rates occurs mainly because of the water storage effect in defoliated plants, as the reduced foliar biomass temporarily decreases water loss through transpiration.
As the hydraulic pathway from roots to canopy can be jeopardized by defoliation, trees maintain leaf water potential within functional limits to avoid critical levels of hydraulic failure and death (Benito Garzón et al., 2018). The hydraulic safety margin (HSM), which is the difference between the minimum leaf water potential measured in the plants (Ψmin) and that inducing 12 and 50% embolism, acts as an internal hydraulic buffer and provides information about the thresholds of leaf water potential inducing hydraulic failure. These physiological mechanisms involved in the resistance to cavitation are species-specific (Delzon et al., 2010); plants can differ in their safety margins. Atmospheric demand also negatively influences water potential (VPD). Trees experiencing a greater negative water potential because of an increase in VPD, i.e., increased summer temperatures and decreased air humidity, are also more likely to lose hydraulic conductivity following xylem embolism (Tyree & Sperry, 1989) and eventually die (Adams et al., 2017).
In young black spruce trees, the xylem tension induces a 50% loss of conductivity (P50) to −4.26 MPa, whereas the xylem air entry pressure (P12) is −2.9 MPa (Balducci et al., 2015). Therefore, the HSM of young defoliated trees is large (Bouzidi et al., 2019), explaining the low mortality rate. Juvenile wood is also more resistant to xylem failure by embolism than mature wood, decreasing the former’s vulnerability to cavitation relative to that of older trees (Domec et al., 2009). This greater resistance to xylem failure by embolism in younger trees is mainly due to their smaller xylem cell diameter. In mature stands during the period of active defoliation, shoot water potentials surpassed Ψmin thresholds, inducing 12% xylem embolism in mature black spruce but not in balsam fir (Balducci et al., 2020). The Ψmin reached −2.95 MPa, which corresponds to the onset of xylem embolism in balsam fir—average xylem air entry pressure, P12, being around −2.8 MPa in nondefoliated trees. Mature balsam fir might therefore have experienced greater xylem embolism during defoliation compared with defoliated black spruce and, thus, showing a narrower HSM under defoliation and a higher risk of hydraulic failure than the latter. Trees show a narrow HSM; therefore, they operate close to the hydraulic failure level (Choat et al., 2012). But populations that experience a narrower HSM are strongly associated with higher mortality (Benito Garzón et al., 2018). Conifers in North America, Europe, and Australia exhibit a large interspecific variability of cavitation resistance (Delzon et al., 2010). Although the phenotypic variability is considered negligible in the point of critical loss of xylem conductivity, intraspecific variability in P50 has recently been reported in some species (Anderegg, 2015; Benito Garzón et al., 2018). In this context, the HSM for young black spruce is greater than for mature black spruce, which is greater than that for mature balsam fir. Therefore, the mortality rate under defoliation follows an opposite pattern, suggesting that in future scenarios of more frequent and intense drought events and SBW attacks, balsam fir could be more vulnerable than black spruce.
The integration of knowledge regarding water status and the hydraulic conductivity of trees can help us understand mortality dynamics in natural conditions (Martinez-Vilalta et al., 2019). In mature trees, SBW outbreaks of longer duration and higher intensity limit tree survival (Chen et al., 2017; MacLean, 1980). Several studies agree that mature firs are more sensitive, i.e., higher tree mortality, after five years of severe defoliation by SBW than other host species, e.g., white and black spruce (MacLean, 1980, 2016; Virgin & MacLean, 2017). However, species susceptibility to SBW depends on the stand, with higher mortality rates in balsam fir–dominated stands than in mixed boreal stands, although mortality remains high for the host species (Bouchard et al., 2005). A greater abundance of hardwood in stands significantly reduces the dispersion of the second instars (L2) of SBW, thereby helping limit balsam fir defoliation (MacLean, 2016; Zhang et al., 2020). A similar consensus exists for the survival of balsam fir regeneration, where the type of forest overstory (as hardwood, softwood, and mixed) reduces or increases seedling mortality rate to 17% and 24% to 26%, respectively (Nie et al., 2018). Furthermore, seedling height is likely associated with a larval density increase, as taller seedlings of balsam fir and black spruce are more defoliated than shorter seedlings (10%) (Cotton-Gagnon et al., 2018; Lavoie et al., 2019; Nie et al., 2018). Balsam fir at 1.2 m height has a 50% probability of sustaining severe defoliation, whereas for black spruce the same probability of severe defoliation is attained only when seedlings are taller than 3.5 m. Thus, balsam fir is 15% more defoliated than black spruce (Cotton-Gagnon et al., 2018; Lavoie et al., 2021; Nie et al., 2018). Nonetheless, taller seedlings grown in open conditions should show greater survival than seedlings under shaded conditions, even if the defoliation level reaches 75% (Nie et al., 2018).
4.2 Decreased Carbon Storage and Growth in Defoliated Trees Leads to Greater Mortality
Boreal stands of spruce and fir can tolerate extended periods of spruce budworm defoliation because trees allocate most of their carbon resources to the production of new shoots and needles (Deslauriers et al., 2019; Piene, 1989a, b) rather than storage (Figs. 9.4 and 9.5) or stem radial growth (Fig. 9.6). This allocation strategy allows trees to endure several years of defoliation before eventually succumbing when very few needles remain. Compensatory mechanisms are also used to maximize carbon gain under defoliation, such as longer needle retention (Doran et al., 2017) and greater epicormic bud production (Piene, 1989a).
Under defoliation, drastic growth reductions in dying balsam fir trees occur simultaneously with declines in starch reserves, especially in May and June. A nominal logistic regression can calculate the probability of tree mortality on the basis of starch concentrations (Fig. 9.8). The starch concentration in needles in May explained the increase in tree mortality, with the minimum values indicating mortality during spring (Fig. 9.8). The starch concentration at which the probability of plant mortality exceeds 50% is significant in May, having a value of 28 mg·g−1dw of starch in needles (Fig. 9.8). This threshold is reached only in defoliation classes higher than 66%, although the probability of mortality begins to increase between 33 and 66% defoliation (Fierravanti et al., 2019).

Reproduced from Fierravanti et al. (2019), CC BY 4.0 license
Logistic function linking starch concentrations in May with the probability of balsam fir mortality. The box plot represents starch concentrations across the different defoliation classes. The gray rectangles represent the threshold of tree mortality at a probability of 50%. D0 represents nondefoliated trees, and D1, D2, and D3 represent 1%–33%, 33%–66%, and 66%–100% defoliation, respectively.
By using canonical correlation, Fierravanti et al. (2019) showed how defoliation intensity is inversely correlated to changes in both growth (Fig. 9.6) and carbon allocation (Fig. 9.8). In balsam fir, mortality is related to both reduced radial growth (i.e., as measured by tree-ring width) and starch reserves; however, a direct causal link requires further testing, including carbon allocation at the whole tree level in relation to the tree water status (see Sect. 9.1). The physiological mechanisms that lead to mortality and the associated change in growth rates remain poorly known, although mortality is preceded by growth reductions in 84% of cases (Cailleret et al., 2017), as observed in balsam fir (Fig. 9.6). Moreover, for shade-tolerant species such as balsam fir, the probability of tree mortality is a function of the growth rate with increasing size (Das et al., 2016; De Grandpré et al., 2019; Kneeshaw et al., 2006). In the absence of environmental changes, the common assumption is that the tree growth rate is inversely associated with the probability of mortality and the slowest-growing trees having a higher probability of dying (Das et al., 2016; De Grandpré et al., 2019; Kneeshaw et al., 2006). Co-occurring past disturbances—climatic and biotic stress events—result in a reduced tree growth (Das et al., 2016; De Grandpré et al., 2019; Kneeshaw et al., 2006); thus, a lower growth rate appears to be an early signal of tree vigor in boreal conifers.
5 Implications for Forest Management
Improved knowledge of the changes in water status and carbon allocation of fir and spruce in relation to water deficits, meteorological conditions, and defoliation will help refine current forest management practices to reduce the impacts of SBW outbreaks. A better understanding of the factors driving mortality is particularly critical in the case of SBW-caused mortality in eastern Canada, as trees that die following SBW defoliation generally decay too quickly to be salvaged and used in conventional transformation processes such as pulp and paper or sawmills.
We highlight that over several years of defoliation, a tree’s capacity to absorb and conduct water decreases as defoliation increases. Over the long term, i.e., 5 to 15 years (as described above), defoliated trees are therefore more susceptible to drought, potentially generating synchronous mortality in defoliated trees when severe drought conditions occur. Moreover, larger trees, which tend to have a higher portion of their energy budget devoted to supporting their existing biomass, are probably even more exposed to such events. This reinforces the idea that the most effective means of preventing defoliation-caused mortality, other than spraying insecticides, is probably to harvest susceptible trees relatively early during outbreak development, e.g., less than five years after defoliation is first observed at the stand level. The sudden bursts of mortality likely to occur during eventual droughts can therefore be anticipated and prevented, at least in part.
Although the main harvesting method in a SBW outbreak context is clear-cutting (followed or not by plantation), partial harvesting can be used under particular conditions. We suggest that when partial cuts are used, black spruce—less likely to be severely defoliated and more likely to maintain hydraulic conductivity—and smaller or younger trees should be left unfelled, as they are less likely to suffer mortality. To further refine guidelines for partial harvesting in a SBW outbreak context, future studies should look at differences in susceptibility to defoliation and water deficit in trees that belong to a range of size classes (from seedlings to large trees) and canopy positions, i.e., acclimated to different shade levels.
References
Adams, H. D., Barron-Gafford, G. A., Minor, R. L., et al. (2017). Temperature response surfaces for mortality risk of tree species with future drought. Environmental Research Letters, 12, 115014. https://doi.org/10.1088/1748-9326/aa93be.
Aldea, M., Hamilton, J. G., Resti, J. P., et al. (2005). Indirect effects of insect herbivory on leaf gas exchange in soybean. Plant, Cell and Environment, 28, 402–411. https://doi.org/10.1111/j.1365-3040.2005.01279.x.
Anderegg, W. R. L. (2015). Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytologist, 205, 1008–1014. https://doi.org/10.1111/nph.12907.
Anderegg, W. R. L., Plavcová, L., Anderegg, L. D. L., et al. (2013). Drought’s legacy: Multiyear hydraulic deterioration underlies widespread aspen forest die-off and portends increased future risk. Global Change Biology, 19(4), 1188–1196. https://doi.org/10.1111/gcb.12100.
Antonucci, S., Rossi, S., Deslauriers, A., et al. (2015). Synchronisms and correlations of spring phenology between apical and lateral meristems in two boreal conifers. Tree Physiology, 35, 1086–1094. https://doi.org/10.1093/treephys/tpv077.
Balducci, L., Deslauriers, A., Giovannelli, A., et al. (2015). How do drought and warming influence survival and wood traits of Picea mariana saplings? Journal of Experimental Botany, 66, 377–389. https://doi.org/10.1093/jxb/eru431.
Balducci, L., Fierravanti, A., Rossi, S., et al. (2020). The paradox of defoliation: Declining tree water status with increasing soil water content. Agricultural and Forest Meteorology, 290, 108025. https://doi.org/10.1016/j.agrformet.2020.108025.
Benito Garzón, M., González Muñoz, N., Wigneron, J.-P., et al. (2018). The legacy of water deficit on populations having experienced negative hydraulic safety margin. Global Ecology and Biogeography, 27(3), 346–356. https://doi.org/10.1111/geb.12701.
Bergeron, Y., Leduc, A., Joyal, C., et al. (1995). Balsam fir mortality following the last spruce budworm outbreak in northwestern Quebec. Canadian Journal of Forest Research, 25, 1375–1384. https://doi.org/10.1139/x95-150.
Bouchard, M., Kneeshaw, D., & Bergeron, Y. (2005). Mortality and stand renewal patterns following the last spruce budworm outbreak in mixed forests of western Quebec. Forest Ecology and Management, 204, 297–313. https://doi.org/10.1016/j.foreco.2004.09.017.
Bouchard, M., Martel, V., Régnière, J., et al. (2018). Do natural enemies explain fluctuations in low-density spruce budworm populations? Ecology, 99, 2047–2057. https://doi.org/10.1002/ecy.2417.
Bouzidi, H. A., Balducci, L., Mackay, J., et al. (2019). Interactive effects of defoliation and water deficit on growth, water status, and mortality of black spruce (Picea mariana (Mill.) B.S.P.). Annals of Forest Science, 76, 21. https://doi.org/10.1007/s13595-019-0809-z.
Cailleret, M., Jansen, S., Robert, E. M. R., et al. (2017). A synthesis of radial growth patterns preceding tree mortality. Global Change Biology, 23(4), 1675–1690. https://doi.org/10.1111/gcb.13535.
Candau, J., Fleming, R. A., & Hopkin, A. (1998). Spatiotemporal patterns of large-scale defoliation caused by the spruce budworm in Ontario since 1941. Canadian Journal of Forest Research, 28, 1733–1741. https://doi.org/10.1139/x98-164.
Chen, C., Weiskittel, A., Bataineh, M., et al. (2017). Evaluating the influence of varying levels of spruce budworm defoliation on annualized individual tree growth and mortality in Maine, USA and New Brunswick, Canada. Forest Ecology and Management, 396, 184–194. https://doi.org/10.1016/j.foreco.2017.03.026.
Choat, B., Jansen, S., Brodribb, T. J., et al. (2012). Global convergence in the vulnerability of forests to drought. Nature, 491, 752–755. https://doi.org/10.1038/nature11688.
Cotton-Gagnon, A., Simard, M., De Grandpre, L., et al. (2018). Salvage logging during spruce budworm outbreaks increases defoliation of black spruce regeneration. Forest Ecology and Management, 430, 421–430. https://doi.org/10.1016/j.foreco.2018.08.011.
Das, A. J., Stephenson, N. L., & Davis, K. P. (2016). Why do trees die? Characterizing the drivers of background tree mortality. Ecology, 97, 2616–2627. https://doi.org/10.1002/ecy.1497.
De Grandpré, L., Kneeshaw, D. D., Perigon, S., et al. (2019). Adverse climatic periods precede and amplify defoliator-induced tree mortality in eastern boreal North America. Journal of Ecology, 107, 452–467. https://doi.org/10.1111/1365-2745.13012.
Delzon, S., Douthe, C., Sala, A., et al. (2010). Mechanism of water-stress induced cavitation in conifers: Bordered pit structure and function support the hypothesis of seal capillary-seeding. Plant, Cell and Environment, 33, 2101–2111. https://doi.org/10.1111/j.1365-3040.2010.02208.x.
Desalme, D., Priault, P., Gerant, D., et al. (2017). Seasonal variations drive short-term dynamics and partitioning of recently assimilated carbon in the foliage of adult beech and pine. New Phytologist, 213, 140–153. https://doi.org/10.1111/nph.14124.
Deslauriers, A., Caron, L., & Rossi, S. (2015). Carbon allocation during defoliation: Testing a defense-growth trade-off in balsam fir. Frontiers in Plant Science, 6, 338. https://doi.org/10.3389/fpls.2015.00338.
Deslauriers, A., Huang, J. G., Balducci, L., et al. (2016). The contribution of carbon and water in modulating wood formation in black spruce saplings. Plant Physiology, 170, 2072–2084. https://doi.org/10.1104/pp.15.01525.
Deslauriers, A., Fournier, M. P., Carteni, F., et al. (2019). Phenological shifts in conifer species stressed by spruce budworm defoliation. Tree Physiology, 39, 590–605. https://doi.org/10.1093/treephys/tpy135.
Despland, E. (2018). Effects of phenological synchronization on caterpillar early-instar survival under a changing climate. Canadian Journal of Forest Research, 48, 247–254. https://doi.org/10.1139/cjfr-2016-0537.
Dietze, M. C., & Matthes, J. H. (2014). A general ecophysiological framework for modelling the impact of pests and pathogens on forest ecosystems. Ecology Letters, 17, 1418–1426. https://doi.org/10.1111/ele.12345.
Domec, J. C., Warren, J. M., Meinzer, F. C., et al. (2009). Safety factors for xylem failure by implosion and air-seeding within roots, trunks and branches of young and old conifer trees. IAWA Journal, 30, 101–120. https://doi.org/10.1163/22941932-90000207.
Doran, O., MacLean, D. A., & Kershaw, J. A. (2017). Needle longevity of balsam fir is increased by defoliation by spruce budworm. Trees, 31, 1933–1944. https://doi.org/10.1007/s00468-017-1597-4.
Eyles, A., Pinkard, E. A., Davies, N. W., et al. (2013). Whole-plant versus leaf-level regulation of photosynthetic responses after partial defoliation in Eucalyptus globulus saplings. Journal of Experimental Botany, 64, 1625–1636. https://doi.org/10.1093/jxb/ert017.
Fatichi, S., & Pappas, C. (2017). Constrained variability of modeled T:ET ratio across biomes. Geophysical Research Letters, 44, 6795–6803. https://doi.org/10.1002/2017GL074041.
Fierravanti, A., Rossi, S., Kneeshaw, D., et al. (2019). Low non-structural carbon accumulation in spring reduces growth and increases mortality in conifers defoliated by spruce budworm. Frontiers for Global Change, 2, 15. https://doi.org/10.3389/ffgc.2019.00015.
Fleming, R. A., & Volney, W. J. A. (1995). Effects of climate change on insect defoliator population processes in Canada’s boreal forest: Some plausible scenarios Water. Water, Air, and Soil Pollution, 82, 445–454. https://doi.org/10.1007/BF01182854.
Foster, J. R., Townsend, P. A., & Mladenoff, D. J. (2013). Mapping asynchrony between gypsy moth egg-hatch and forest leaf-out: Putting the phenological window hypothesis in a spatial context. Forest Ecology and Management, 287, 67–76. https://doi.org/10.1016/j.foreco.2012.09.006.
Gauthier, S., Bernier, P., Kuuluvainen, T., et al. (2015). Boreal forest health and global change. Science, 349(6250), 819–822. https://doi.org/10.1126/science.aaa9092.
Gray, D. R. (2008). The relationship between climate and outbreak characteristics of the spruce budworm in eastern Canada. Climatic Change, 87, 361–383. https://doi.org/10.1007/s10584-007-9317-5.
Gray, D. R., Régnière, J., & Boulet, B. (2000). Analysis and use of historical patterns of spruce budworm defoliation to forecast outbreak patterns in Quebec. Forest Ecology and Management, 127(1), 217–231. https://doi.org/10.1016/S0378-1127(99)00134-6.
Hata, K., Kawakami, K., & Kachi, N. (2016). Increases in soil water content after the mortality of non-native trees in oceanic island forest ecosystems are due to reduced water loss during dry periods. Science of the Total Environment, 545–546, 372–380. https://doi.org/10.1016/j.scitotenv.2015.12.007.
Haynes, K. J., Allstadt, A. J., & Klimetzek, D. (2014). Forest defoliator outbreaks under climate change: Effects on the frequency and severity of outbreaks of five pine insect pests. Global Change Biology, 20, 2004–2018. https://doi.org/10.1111/gcb.12506.
Heinrich, S., Dippold, M. A., Werner, C., et al. (2015). Allocation of freshly assimilated carbon into primary and secondary metabolites after in situ 13C pulse labelling of Norway spruce (Picea abies). Tree Physiology, 35, 1176–1191. https://doi.org/10.1093/treephys/tpv083.
Hoch, G., Richter, A., & Körner, C. (2003). Non-structural carbon compounds in temperate forest trees. Plant, Cell and Environment, 26, 1067–1081. https://doi.org/10.1046/j.0016-8025.2003.01032.x.
Houle, D., Paquette, A., Côté, B., et al. (2015). Impacts of climate change on the timing of the production season of maple syrup in eastern Canada. PLoS ONE, 10, e0144844. https://doi.org/10.1371/journal.pone.0144844.
Huang, J. G., Deslauriers, A., & Rossi, S. (2014). Xylem formation can be modeled statistically as a function of primary growth and cambium activity. New Phytologist, 203, 831–841. https://doi.org/10.1111/nph.12859.
Itter, M. S., D’Orangeville, L., Dawson, A., et al. (2019). Boreal tree growth exhibits decadal-scale ecological memory to drought and insect defoliation, but no negative response to their interaction. Journal of Ecology, 107(3), 1288–1301. https://doi.org/10.1111/1365-2745.13087.
Kneeshaw, D. D., Kobe, R. K., Coates, K. D., et al. (2006). Sapling size influences shade tolerance ranking among southern boreal tree species. Journal of Ecology, 94, 471–480. https://doi.org/10.1111/j.1365-2745.2005.01070.x.
Körner, C. (2015). Paradigm shift in plant growth control. Current Opinion in Plant Biology, 25, 107–114. https://doi.org/10.1016/j.pbi.2015.05.003.
Lavoie, J., Montoro Girona, M., & Morin, H. (2019). Vulnerability of conifer regeneration to spruce budworm outbreaks in the eastern Canadian boreal forest. Forests, 10(10), 850. https://doi.org/10.3390/f10100850.
Lavoie, J., Montoro Girona, M., Grosbois, G., et al. (2021). Does the type of silvicultural practice influence spruce budworm defoliation of seedlings? Ecosphere, 12(4), 17. https://doi.org/10.1002/ecs2.3506.
Li, M., Hoch, G., & Korner, C. (2002). Source/sink removal affects mobile carbohydrates in Pinus cembra at the Swiss treeline. Trees, 16, 331–337. https://doi.org/10.1007/s00468-002-0172-8.
Little, C. H. A. (1970). Derivation of the springtime starch increase in balsam fir (Abies balsamea). Canadian Journal of Botany, 48, 1995–1999. https://doi.org/10.1139/b70-291.
Liu, Z., Peng, C., De Grandpré, L., et al. (2018). Development of a new TRIPLEX-insect model for simulating the effect of spruce budworm on forest carbon dynamics. Forests, 9, 513. https://doi.org/10.3390/f9090513.
MacLean, D. A. (2016). Impacts of insect outbreaks on tree mortality, productivity, and stand development. The Canadian Entomologist, 148, S138–S159. https://doi.org/10.4039/tce.2015.24.
MacLean, D. A. (1980). Vulnerability of fir-spruce stands during uncontrolled spruce budworm outbreaks: A review and discussion. The Forestry Chronicle, 56(5), 13–221. 5558/tfc56213-5.
Martinez-Vilalta, J., Sala, A., Asensio, D., et al. (2016). Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecological Monographs, 86, 495–516. https://doi.org/10.1002/ecm.1231.
Martinez-Vilalta, J., Anderegg, W. R. L., Sapes, G., et al. (2019). Greater focus on water pools may improve our ability to understand and anticipate drought-induced mortality in plants. New Phytologist, 223, 22–32. https://doi.org/10.1111/nph.15644.
Minchin, P. E., & Lacointe, A. (2005). New understanding on phloem physiology and possible consequences for modelling long-distance carbon transport. New Phytologist, 166, 771–779. https://doi.org/10.1111/j.1469-8137.2005.01323.x.
Ministère des Forêts de la Faune et des Parcs (MFFP). (2018). Aires infestées par la tordeuse des bourgeons de l’épinette au Québec en 2018-Version 1.0. Québec: Direction de la protection des forêts, Gouvernement du Québec.
Morin, H., Jardon, Y., & Simard, S. (2010). Détection et reconstitution des épidemies de la tordeuse des bourgeons de l'épinette (Choristoneura fumiferana Clem.) a l'aide de la dendrochronologie. In S. Payette, L. Filion (Eds.), La dendroécologie: Principes, methodes et applications (pp. 415–436). Québec: Presses de l’Universite Laval.
Morin, H. (1994). Dynamics of balsam fir forests in relation to spruce budworm outbreaks in the Boreal Zone of Quebec. Canadian Journal of Forest Research, 24(4), 730–741. 1139/x94-097.
Nabity, P. D., Zavala, J. A., & DeLucia, E. H. (2009). Indirect suppression of photosynthesis on individual leaves by arthropod herbivory. Annals of Botany, 103, 655–663. https://doi.org/10.1093/aob/mcn127.
Nardini, A., Pedà, G., & Rocca, N. (2012). Trade-offs between leaf hydraulic capacity and drought vulnerability: Morpho-anatomical bases, carbon costs and ecological consequences. New Phytologist, 196, 788–798. https://doi.org/10.1111/j.1469-8137.2012.04294.x.
Navarro, L., Morin, H., Bergeron, Y., et al. (2018). Changes in spatiotemporal patterns of 20th century spruce budworm outbreaks in eastern Canadian boreal forests. Frontiers in Plant Science, 9, 1905. https://doi.org/10.3389/fpls.2018.01905.
Nie, Z., MacLean, D. A., & Taylor, A. R. (2018). Forest overstory composition and seedling height influence defoliation of understory regeneration by spruce budworm. Forest Ecology and Management, 409, 353–360. https://doi.org/10.1016/j.foreco.2017.11.033.
Ostaff, D. P., & MacLean, D. A. (1995). Patterns of balsam fir foliar production and growth in relation to defoliation by spruce budworm. Canadian Journal of Forest Research, 25, 1128–1136. https://doi.org/10.1139/x95-125.
Peng, C., Ma, Z., Lei, X., et al. (2011). A drought-induced pervasive increase in tree mortality across Canada’s boreal forests Nat. Nature Climate Change, 1, 467–471. https://doi.org/10.1038/nclimate1293.
Peschiutta, M. L., Bucci, S. J., Scholz, F. G., et al. (2016). Compensatory responses in plant-herbivore interactions: Impacts of insects on leaf water relations. Acta Oecologica, 73, 71–79. https://doi.org/10.1016/j.actao.2016.03.005.
Peters, R. L., Miranda, J. C., Schonbeck, L., et al. (2020). Tree physiological monitoring of the 2018 larch budmoth outbreak: Preference for leaf recovery and carbon storage over stem wood formation in Larix decidua. Tree Physiology, 40, 1697–1711. https://doi.org/10.1093/treephys/tpaa087.
Piene, H. (1989a). Spruce budworm defoliation and growth loss in young balsam fir: Defoliation in spaced and unspaced stands and individual tree survival. Canadian Journal of Forest Research, 19, 1211–1217. https://doi.org/10.1139/x89-185.
Piene, H. (1989b). Spruce budworm defoliation and growth loss in young balsam fir: Recovery of growth in spaced stands. Canadian Journal of Forest Research, 19, 1616–1624. https://doi.org/10.1139/x89-244.
Pincebourde, S., Frak, E., Sinoquet, H., et al. (2006). Herbivory mitigation through increased water-use efficiency in a leaf-mining moth-apple tree relationship. Plant, Cell and Environment, 29, 2238–2247. https://doi.org/10.1111/j.1365-3040.2006.01598.x.
Pittermann, J., Lance, J., Poster, L., et al. (2014). Heavy browsing affects the hydraulic capacity of Ceanothus rigidus (Rhamnaceae). Oecologia, 175, 801–810. https://doi.org/10.1007/s00442-014-2947-1.
Pureswaran, D. S., De Grandpré, L., Paré, D., et al. (2015). Climate-induced changes in host tree–insect phenology may drive ecological state-shift in boreal forests. Ecology, 96, 1480–1491. https://doi.org/10.1890/13-2366.1.
Pureswaran, D. S., Johns, R., Heard, S. B., et al. (2016). Paradigms in eastern spruce budworm (Lepidoptera: Tortricidae) population ecology: A century of debate. Environmental Entomology, 45, 1333–1342. https://doi.org/10.1093/ee/nvw103.
Quentin, A. G., O’Grady, A. P., Beadle, C. L., et al. (2011). Responses of transpiration and canopy conductance to partial defoliation of Eucalyptus globulus trees. Agricultural and Forest Meteorology, 151, 356–364. https://doi.org/10.1016/j.agrformet.2010.11.008.
Quentin, A. G., O’Grady, A. P., Beadle, C. L., et al. (2012). Interactive effects of water supply and defoliation on photosynthesis, plant water status and growth of Eucalyptus globulus Labill. Tree Physiology, 32, 958–967. https://doi.org/10.1093/treephys/tps066.
Reich, P. B., Walters, M. B., Krause, S. C., et al. (1993). Growth, nutrition and gas exchange of Pinus resinosa following artificial defoliation. Trees, 7, 67–77. https://doi.org/10.1007/BF00225472.
Sack, L., & Holbrook, N. M. (2006). Leaf hydraulics. Annual Review of Plant Biology, 57, 361–381. https://doi.org/10.1146/annurev.arplant.56.032604.144141.
Salmon, Y., Torres-Ruiz, J. M., Poyatos, R., et al. (2015). Balancing the risks of hydraulic failure and carbon starvation: A twig scale analysis in declining Scots pine. Plant, Cell and Environment, 38, 2575–2588. https://doi.org/10.1111/pce.12572.
Schmid, S., Palacio, S., & Hoch, G. (2017). Growth reduction after defoliation is independent of CO2 supply in deciduous and evergreen young oaks. New Phytologist, 214, 1479–1490. https://doi.org/10.1111/nph.14484.
Seidl, R., Thom, D., Kautz, M., et al. (2017). Forest disturbances under climate change. Nature Climate Change, 7(6), 395–402. https://doi.org/10.1038/nclimate3303.
Singer, M. C., & Parmesan, C. (2010). Phenological asynchrony between herbivorous insects and their hosts: Signal of climate change or pre-existing adaptive strategy? Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 365, 3161–3176. https://doi.org/10.1098/rstb.2010.0144.
Sturtevant, B. R., Cooke, B. J., Kneeshaw, D. D., et al. (2015). Modeling insect disturbance across forested landscapes: Insights from the spruce budworm. In A. H. Perera, B. R. Sturtevant, & L. J. Buse (Eds.), Simulation modeling of forest landscape disturbances (pp. 93–134). Springer International Publishing.
Sun, G., Caldwell, P. V., & McNulty, S. G. (2015). Modelling the potential role of forest thinning in maintaining water supplies under a changing climate across the conterminous United States. Hydrological Processes, 29, 5016–5030. https://doi.org/10.1002/hyp.10469.
Traversari, S., Emiliani, G., Traversi, M. L., et al. (2018). Pattern of carbohydrate changes in maturing xylem and phloem during growth to dormancy transition phase in Picea abies (L.) Karst. Dendrobiology, 80, 12–23. https://doi.org/10.12657/denbio.080.002.
Tyree, M. T., & Sperry, J. S. (1989). Vulnerability of xylem to cavitation and embolism. Annual Review of Plant Physiology and Plant Molecular Biology, 40, 19–36. https://doi.org/10.1146/annurev.pp.40.060189.000315.
van Lierop, P., Lindquist, E., Sathyapala, S., et al. (2015). Global forest area disturbance from fire, insect pests, diseases and severe weather events. Forest Ecology and Management, 352, 78–88. https://doi.org/10.1016/j.foreco.2015.06.010.
Vanderklein, D. W., & Reich, P. B. (1999). The effect of defoliation intensity and history on photosynthesis, growth and carbon reserves of two conifers with contrasting leaf lifespans and growth habits. New Phytologist, 144, 121–132. https://doi.org/10.1046/j.1469-8137.1999.00496.x.
Vanderklein, D. W., & Reich, P. B. (2000). European larch and eastern white pine respond similarly during three years of partial defoliation. Tree Physiology, 20, 283–287. https://doi.org/10.1093/treephys/20.4.283.
Viglizzo, E. F., Jobbágy, E. G., Ricard, M. F., et al. (2016). Partition of some key regulating services in terrestrial ecosystems: Meta-analysis and review. Science of the Total Environment, 562, 47–60. https://doi.org/10.1016/j.scitotenv.2016.03.201.
Virgin, G. V. J., & MacLean, D. A. (2017). Five decades of balsam fir stand development after spruce budworm-related mortality. Forest Ecology and Management, 400, 129–138. https://doi.org/10.1016/j.foreco.2017.05.057.
Warren, R. K., Pappas, C., Helbig, M., et al. (2018). Minor contribution of overstorey transpiration to landscape evapotranspiration in boreal permafrost peatlands. Ecohydrology, 11, e1975. https://doi.org/10.1002/eco.1975.
Webb, W. L. (1980). Starch content of conifers defoliated by the Douglas-fir tussock moth. Canadian Journal of Forest Research, 10, 535–540. https://doi.org/10.1139/x80-087.
Webb, W. L. (1981). Relation of starch content to conifer mortality and growth loss after defoliation by the Douglas-fir tussock moth. Forest Sciences, 27, 224–232.
Webb, W. L., & Karchesy, J. J. (1977). Starch content of Douglas-fir defoliated by the tussock moth. Canadian Journal of Forest Research, 7, 186–188. https://doi.org/10.1139/x77-026.
Wiley, E., & Helliker, B. (2012). A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. New Phytologist, 195, 285–289. https://doi.org/10.1111/j.1469-8137.2012.04180.x.
Wiley, E., Huepenbecker, S., Casper, B. B., et al. (2013). The effects of defoliation on carbon allocation: Can carbon limitation reduce growth in favour of storage? Tree Physiology, 33, 1216–1228. https://doi.org/10.1093/treephys/tpt093.
Zhang, X., Lei, Y., Ma, Z., et al. (2014). Insect-induced tree mortality of boreal forests in under a changing climate. Ecology and Evolution, 4, 2384–2394. https://doi.org/10.1002/ece3.988.
Zhang, B., MacLean, D. A., Johns, R. C., et al. (2020). Hardwood-softwood composition influences early-instar larval dispersal mortality during a spruce budworm outbreak. Forest Ecology and Management, 463, 118035. https://doi.org/10.1016/j.foreco.2020.118035.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Deslauriers, A., Balducci, L., Fierravanti, A., Bouchard, M. (2023). Changes in Water Status and Carbon Allocation in Conifers Subjected to Spruce Budworm Defoliation and Consequences for Tree Mortality and Forest Management. In: Girona, M.M., Morin, H., Gauthier, S., Bergeron, Y. (eds) Boreal Forests in the Face of Climate Change. Advances in Global Change Research, vol 74. Springer, Cham. https://doi.org/10.1007/978-3-031-15988-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-15988-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15987-9
Online ISBN: 978-3-031-15988-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)