Abstract
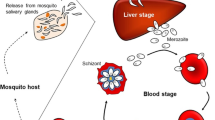
Malaria is a widespread well-known disease, causing many mortalities still today. Many drug therapies are available for the treatment of the disease, but still the scope for improvement is available. Nanocarriers indicate vast scope for research in antimalarial therapy. Dendrimers are one of the novel approaches in the everlasting research for antimalarial therapy. Dendrimers are largely branched, monodisperse macromolecules, which are having a structural perfection. Their surface and core are used to carry drug molecules along with them to the targeted delivery site. Many antimalarial drugs like chloroquine, primaquine, etc. are studied by conjugating with the dendrimers for their antimalarial properties along with the various formulation properties. They have been studied for the drug pharmacokinetic profiles, dose frequency and drug toxicity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
WHO, World malaria report 2020.
Malaria biology. Available at https://www.cdc.gov/malaria/about/biology/. Accessed on 20 Aug 2016.
Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228(4703):1049–55.
Meshnick SR. Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32(13):1655–60.
Posner GH, O’Neill PM. Knowledge of the proposed chemical mechanism of action and cytochrome P450 metabolism of antimalarial trioxanes like artemisinin allows rational design of new antimalarial peroxides. Acc Chem Res. 2004;37(6):397–404.
Wang J, Huang L, Li J, Fan Q, Long Y, Li Y, Zhou B. Artemisinin directly targets malarial mitochondria through its specific mitochondrial activation. PLoS One. 2010;5(3):e9582.
Vale N, Moreira R, Gomes P. Primaquine revisited six decades after its discovery. Eur J Med Chem. 2009;44(3):937–53.
Hiebsch RR, Raub TJ, Wattenberg BW. Primaquine blocks transport by inhibiting the formation of functional transport vesicles. Studies in a cell-free assay of protein transport through the Golgi apparatus. J Biol Chem. 1991;266(30):20323–8.
Fernando D, Rodrigo C, Rajapakse S. Primaquine in vivax malaria: an update and review on management issues. Malar J. 2011;10(1):1.
Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64(1):12–7.
Bray PG, Hawley SR, Mungthin M, Ward SA. Physicochemical properties correlated with drug resistance and the reversal of drug resistance in Plasmodium falciparum. Mol Pharmacol. 1996;50(6):1559–66.
Slater AF. Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol Ther. 1993;57(2–3):203–35.
Chou AC, Fitch CD. Control of heme polymerase by chloroquine and other quinolone derivatives. Biochem Biophys Res Commun. 1993;195(1):422–7.
Chai A, Chevli R, Fitch C. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980;19(8):1543–9.
Adaramoye OA, Osaimoje DO, Akinsanya AM, Nneji CM, Fafunso MA, Ademowo OG. Changes in antioxidant status and biochemical indices after acute administration of artemether, artemether- lumefantrine and halofantrine in rats. Basic Clin Pharmacol Toxicol. 2008;102(4):412–8.
Falade C, Makanga M, Premji Z, Ortmann CE, Stockmeyer M, de Palacios PI. Efficacy and safety of artemether–lumefantrine (Coartem®) tablets (six-dose regimen) in African infants and children with acute, uncomplicated falciparum malaria. Trans R Soc Trop Med Hyg. 2005;99(6):459–67.
Santos-Magalhaes NS, Mosqueira VC. Nanotechnology applied to the treatment of malaria. Adv Drug Deliv Rev. 2010;62(4):560–75.
Marques J, Valle-Delgado JJ, Urban P, Baro E, Prohens R, Mayor A, Cistero P, Delves M, Sinden RE, Grandfils C, de Paz JL. Adaptation of targeted nanocarriers to changing requirements in antimalarial drug delivery. Nanomedicine. 2017;13(2):515–25.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):1.
Moles E, Urbán P, Jimenez-Díaz MB, Viera-Morilla S, Angulo-Barturen I, Busquets MA, Fernàndez-Busquets X. Immunoliposome-mediated drug delivery to Plasmodium-infected and non-infected red blood cells as a dual therapeutic/prophylactic antimalarial strategy. J Control Release. 2015;210:217–29.
Moles E, Moll K, Ch’ng JH, Parini P, Wahlgren M, Fernandez-Busquets X. Development of drug-loaded immunoliposomes for the selective targeting and elimination of rosetting Plasmodium falciparum-infected red blood cells. J Control Release. 2016;241:57–67.
Pirson P, Steiger RF, Trouet A, Gillet J, Herman F. Liposomes in the chemotherapy of experimental murine malaria. Trans R Soc Trop Med Hyg. 1979;73(3):347.
Qiu L, Jing N, Jin Y. Preparation and in vitro evaluation of liposomal chloroquine diphosphate loaded by a transmembrane pH-gradient method. Int J Pharm. 2008;361(1):56–63.
Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2):165–96.
Utreja S, Jain NK. Solid lipid nanoparticles. Adv Control Novel Drug Deliv. 2001:408–24.
Mueller RH, Maeder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–77.
Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1):121–8.
Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–55.
Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123–7.
Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft Matter. 2016;12(11):2826–41.
Tang Z, He C, Tian H, Ding J, Hsiao BS, Chu B, Chen X. Polymeric nanostructured materials for biomedical applications. Prog Polym Sci. 2016;60:86.
Barratt G. Colloidal drug carriers: achievements and perspectives. Cell Mol Life Sci CMLS. 2003;60(1):21–37.
Couvreur P, Barratt G, Fattal E, Vauthier C. Nanocapsule technology: a review. Crit Rev Ther Drug Carrier Syst. 2002;19(2):99–134.
Dos Santos PP, Flôres SH, de Oliveira RA, Chisté RC. Biodegradable polymers as wall materials to the synthesis of bioactive compound nanocapsules. Trends Food Sci Technol. 2016;53:23–33.
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, et al. Dendritic macromolecules— synthesis of starburst dendrimers. Macromolecules. 1986;19:2466–8.
Newkome GR, Yao ZQ, Baker GR, Gupta VK, Russo PS, Saunders MJ. Cascade molecules: 2. Synthesis and characterization of a benzene [9]3-arborol. J Am Chem Soc. 1986;108:849–50.
Sayed S, Hedstrand DM, Spinder R, Tomalia DA. Hydrophobically modified poly (amidoamine) (PAMAM) dendrimers: their properties at the air-water interface and use as nanoscopic container molecules. J Mater Chem. 1997;7:1199–205.
Svenson S, Tomalia DA. Dendrimers in biomedical applications—reflections on the field. Adv Drug Deliv Rev. 2012;64:102–15.
Tomalia DA. Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog Polym Sci. 2005;30:294–324.
Noriega-Luna B, Godínez LA, Rodríguez FJ, Rodríguez A, Zaldívar-Lelo de Larrea G, Sosa-Ferreyra CF, Mercado-Curiel RF, Manríquez J, Bustos E. Applications of dendrimers in drug delivery agents, diagnosis, therapy, and detection. J Nanomater. 2014;2014:5072–3.
Gómez R, de la Mata FJ, Jiménez-Fuentes JL, Ortega P, Klajnert B, Pedziwiatr-Werbicka E, Shcharbin D, Bryszewska M, Maly M, Maly J. Cationic carbosilane dendrimers as non-viral vectors of nucleic acids (oligonucleotide or siRNA) for gene therapy purposes. In: Dendrimers in biomedical applications, vol. 6. Cambridge: RSC Publishing; 2013. p. 40–55.
Yang Y, Sunoqrot S, Stowell C, Ji J, Lee CW, Kim JW, Khan SA, Hong S. Effect of size, surface charge, and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules. 2012;13:2154–62.
Yang Y, Pearson RM, Lee O, Lee CW, Chatterton RT Jr, Khan SA, Hong S. Dendron-based micelles for topical delivery of endoxifen: a potential chemo-preventive medicine for breast cancer. Adv Funct Mater. 2014;24:2442–9.
Sherje AP, Jadhav M, Dravyakar BR, Kadam D. Dendrimers: a versatile nanocarrier for drug delivery and targeting. Int J Pharm. 2018;548:707–20.
Tomalia DA, Naylor AM, Goddard Iii WA. Starburst dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. ACIEAY. 1990;29:138–75.
De Brabander-van den Berg EMM, Meijer EW. Poly(propylene imine) dendrimers: large-scale synthesis by hetereogeneously catalyzed hydrogenations. ACIEAY. 1993;32:1308–11.
Sadler K, Tam JP. Peptide dendrimers: applications and synthesis. J Biotechnol. 2002;90:195–229.
Hawker CJ, Frechet JMJ. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J Am Chem Soc. 1990;112:7638–47.
Grinstaff MW. Biodendrimers: new polymeric biomaterials for tissue engineering. Chemistry. 2002;8:2839–46.
Ihre H, Hult A, Söderlind E. Synthesis, characterization, and 1H NMR self-diffusion studies of dendritic aliphatic polyesters based on 2,2-bis(hydroxymethyl)propionic acid and 1,1,1-tris(hydroxyphenyl)ethane. J Am Chem Soc. 1996;118:6388–95.
Turnbull WB, Stoddart JF. Design and synthesis of glycodendrimers. J Biotechnol. 2002;90:231–55.
Nilsen TW, Grayzel J, Prensky W. Dendritic nucleic acid structures. J Theor Biol. 1997;187:273–84.
Li Y, Tseng YD, Kwon SY, D’Espaux L, Bunch JS, McEuen PL, Luo D. Controlled assembly of dendrimer-like DNA. Nat Mater. 2004;3:38–42.
Sandoval-Yanez C, Castro RC. Dendrimers: amazing platforms for bioactive molecule delivery systems. Materials. 2020;13:570.
Falanga A, Tarallo R, Carberry T, Galdiero M, Weck M, Galdiero S. Elucidation of the interaction mechanism with liposomes of gH625-peptide functionalized dendrimers. PLoS One. 2014;9:e112128.
SPL7013 COVID-19 Nasal Spray Virucidal against SARS-CoV-2. Available online: https://starpharma.com/news/story/spl7013-covid-19-nasal-spray-virucidal-against-sars-cov-2. Accessed on 14 Sept 2020.
Santos A, Veiga F, Figueiras A. Dendrimers as pharmaceutical excipients: synthesis, properties. Toxic Biomed Appl Mater. 2019;13:65.
Javanbakht S, Hemmati A, Namazi H, Heydari A. Carboxymethylcellulose-coated 5-fluorouracil@MOF-5 nano-hybrid as a bio-nanocomposite carrier for the anticancer oral delivery. Int J Biol Macromol. 2020;155:876–82.
ZadehMehrizi T, Khamesipour A, ShafieeArdestani M, EbrahimiShahmabadi H, Haji MollaHoseini M, Mosaffa N, Ramezani A. Comparative analysis between four model nanoformulations of amphotericin B-chitosan, amphotericin Bdendrimer, betulinic acid-chitosan and betulinic acid-dendrimer for treatment of Leishmania major: real-time PCR assay plus. Int J Nanomedicine. 2019;14:7593–607.
Li Z, Yang H, Hu M, Zhang L, Ge S, Cui K, Yu J. Cathode photoelectrochemical paper device for microRNA detection based on cascaded photoactive structures and hemin/Pt nanoparticle-decorated DNA dendrimers. ACS Appl Mater Interfaces. 2020;12:17177–84.
Vidal L, Ben Aissa A, Salabert J, Jara JJ, Vallribera A, Pividori MI, Sebastian RM. Biotinylated phosphorus dendrimers as control line in nucleic acid lateral flow tests. Biomacromolecules. 2020;21:1315–23.
Ray S, Li Z, Hsu CH, Hwang LP, Lin YC, Chou PT, Lin YY. Dendrimer- and copolymer-based nanoparticles for magnetic resonance cancer theranostics. Theranostics. 2018;8:6322–49.
Nagpal K, Mohan A, Thakur S, Kumar P. Dendritic platforms for biomimicry and biotechnological applications. Artif Cells Nanomed Biotechnol. 2018;46:861–75.
Ye M, Qian Y, Tang J, Hu H, Sui M, Shen Y. Targeted biodegradable dendritic MRI contrast agent for enhanced tumor imaging. J Control Release. 2013;169(3):239–45.
Svenningsen SW, Frederiksen RF, Counil C, Ficker M, Leisner JJ, Christense JB. Synthesis and antimicrobial properties of a ciprofloxacin and PAMAM-dendrimer conjugate. Molecules. 2020;25:1389.
Ben Jeddou F, Falconnet L, Luscher A, Siriwardena T, Reymond JL, van Delden C, Kohler T. Adaptive and mutational responses to peptide dendrimer antimicrobials in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2020;64(4):e02040-19.
Bosch P, Staneva D, Vasileva-Tonkova E, Grozdanov P, Nikolova I, Kukeva R, Stoyanova R, Grabchev I. New poly (propylene imine) dendrimer modified with Acridine and its Cu(II) complex: synthesis, characterization and antimicrobial activity. Materials. 2019;12:3020.
Kamaruzzaman NF, Tan LP, Hamdan RH, Choong SS, Wong WK, Gibson AJ, Chivu A, Pina MF. Antimicrobial polymers: the potential replacement of existing antibiotics? Int J Mol Sci. 2019;20:2747.
Garcia-Gallego S, Franci G, Falanga A, Gomez R, Folliero V, Galdiero S, de la Mata FJ, Galdiero M. Function oriented molecular design: dendrimers as novel antimicrobials. Molecules. 2017;22:1581.
Garcia-Gallego S, Diaz L, Jimenez JL, Gomez R, de la Mata FJ, Munoz-Fernandez MA. HIV-1 antiviral behavior of anionic PPI metallo-dendrimers with EDA core. Eur J Med Chem. 2015;98:139–48.
Tarallo R, Carberry TP, Falanga A, Vitiello M, Galdiero S, Galdiero M, Weck M. Dendrimers functionalized with membrane-interacting peptides for viral inhibition. Int J Nanomedicine. 2013;8:521–34.
Carberry TP, Tarallo R, Falanga A, Finamore E, Galdiero M, Weck M, Galdiero S. Dendrimer functionalization with a membrane-interacting domain of herpes simplex virus type 1: towards intracellular delivery. Chemistry. 2012;18:13678–85.
Bosman AW, Janssen HM, Meijer EW. About dendrimers: structure, physical properties, and applications. Chem Rev. 1999;99(7):1665–88.
Hecht S, Vladimirov N, Frechet JM. Encapsulation of functional moieties within branched star polymers: effect of chain length and solvent on site isolation. J Am Chem Soc. 2001;123(1):18–25.
Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D’Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252:263–6.
Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of I-125-labelled poly(amidoamine) dendrimers in vivo. J Control Release. 2000;65:133–48.
El-Sayed M, Ginski M, Rhodes C, Ghandehari H. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J Control Release. 2002;81:355–65.
Yoo H, Juliano RL. Enhanced delivery of antisense oligonucleotides with fluorophore-conjugated PAMAM dendrimers. Nucleic Acids Res. 2000;28:4225–31.
D’Emanuele A, Attwood D. Dendrimer-drug interactions. Adv Drug Deliv Rev. 2005;57:2147–62.
D’Emanuele A, Attwood D, Abu Rmaileh R. In: Swarbrick J, editor. Dendrimers. Encyclopedia of pharmaceutical technology. New York: Marcel Dekker; 2003. p. 1–21.
Ringsdorf H. Structure and properties of pharmacologically active polymers. J Polym Sci Polym Symp. 1975;51:135–53.
Bielinska AU, Kukowska-Latallo JF, Johnson J, Tomalia DA, Baker JR. Regulation of in vitro gene expression using antisense oligonucleotides or antisense expression plasmids transfected using starburst PAMAM dendrimers. Nucleic Acids Res. 1996;24:2176–82.
Kukowska-Latallo JF, Raczka E, Quintana A, Chen CL, Rymaszewski M, Baker JR. Intravascular and endobronchial DNA delivery to murine lung tissue using a novel, nonviral vector. Hum Gene Ther. 2000;11:1385–95.
Gajbhiye V, Kumar PV, Tekade RK, Jain NK. Pharmaceutical and biomedical potential of PEGylated dendrimers. Curr Pharm Des. 2007;13(3):415–29.
Sakthivel T, Toth I, Florence AT. Distribution of a lipidic 2.5 nm diameter dendrimer carrier after oral administration. Int J Pharm. 1999;183:51–5.
D’Emanuele A, Jevprasesphant R, Penny J, Attwood D. The use of dendrimer-propanolol prodrug to bypass efflux transporters and enhance oral bioavailability. J Control Release. 2004;95:447–53.
Yiyun C, Na M, Tongwen X, Rongqiang F, Xueyuan W, Xiaomin W, et al. Transdermal delivery of nonsteroidal anti-inflammatory drugs mediated by polyamidoamine (PAMAM) dendrimers. J Pharm Sci. 2007;96(3):595–602.
Roberts J, Adams YE, Tomalia D, Mercer-Smith JA, Lavallee DK. Using starburst dendrimers as linker molecules to radiolabel antibodies. Bioconjug Chem. 1990;1:305–8.
Wiener EC, Auteri FP, Chen JW, Brechbiel MW, Gansow OA, Schneider DS. Molecular dynamics of ion-chelate complexes attached to dendrimers. J Am Chem Soc. 1996;118:7774–82.
Bryant LH, Brechbiel MW, Wu C, Bulte JWM, Herynek V, Frank JA. Synthesis and relaxometry of high-generation (G=5, 7, 9, and 10) PAMAM dendrimer-DOTA-gadolinium chelates. J Magn Reson Imaging. 1999;9:348–52.
Bourne MW, Margerun L, Hylton N, Campion B, Lai JJ, Derugin N, Higgins CB. Evaluation of the effects of intravascular MR contrast media (gadolinium dendrimer) on 3D time of flight magnetic resonance angiography of the body. J Magn Reson Imaging. 1996;6:305–10.
Stevelmans S, Hest J, Jansen J, Boxtel D, Berg E, Meijer EW. Synthesis, characterization, and guest-host properties of inverted unimolecular dendritic micelles. J Am Chem Soc. 1996;118:7398–9.
Twyman LJ, Beezer AE, Esfand R, Hardy MJ, Mitchell JC. The synthesis of water soluble dendrimers, and their application as possible drug delivery systems. Tetrahedron Lett. 1999;40:1743–6.
Liu M, Kono K, Fréchet JMJ. Water- soluble dendritic unimolecular micelles: their potential as drug delivery agents. J Control Release. 2000;65:121–31.
Sigal GB, Mammen M, Dahmann G, Whitesides GM. Polyacrylamides bearing pendant-sialoside groups strongly inhibit agglutination of erythrocytes by influenza virus: the strong inhibition reflects enhanced binding through cooperative polyvalent interactions. J Am Chem Soc. 1996;118:3789–800.
Roy R, Zanini D, Meunier SJ, Romanowska A. Solid-phase synthesis of dendritic sialoside inhibitors of influenza A virus haemagglutinin. J Chem Soc Chem Commun. 1993:1869–72.
Zanini D, Roy R. Practical synthesis of starburst PAMAM thiosialodendrimers for probing multivalent carbohydrate-lectin binding properties. J Organomet Chem. 1998;63:3486–91.
Chen CZ, Cooper SL. Interactions between dendrimer biocides and bacterial membranes. Biomaterials. 2002;23(16):3359–68.
Hawthorne MF. The role of chemistry in the development of boron neutron capture therapy of cancer. Angew Chem. 1993;32:950–84.
Barth RF, Adams DM, Soloway AH, Alam F, Darby MV. Boronated starburst dendrimer-monoclonal antibody immunoconjugates: evaluation as a potential delivery system for neutron captures therapy. Bioconjug Chem. 1994;5:58–66.
Liu L, Barth RF, Adams DM, Soloway AH, Reisefeld RA. Bispecific antibodies as targeting agents for boron neutron capture therapy of brain tumors. J Hematother. 1995;4:477–83.
Capala J, Barth RF, Bendayam M, Lauzon M, Adams DM, Soloway AH, et al. Boronated epidermal growth factor as a potential targeting agent for boron neutron capture therapy of brain tumors. Bioconjug Chem. 1996;7:7–15.
Bhadra D, Bhadra S, Jain NK. PEGylated peptide dendrimeric carriers for the delivery of antimalarial drug chloroquine phosphate. Pharm Res. 2006;23:623–33.
Bhadra D, Bhadra S, Jain NK. PEGylated peptide-based dendritic nanoparticulate systems for delivery of artemether. J Drug Deliv Sci Technol. 2005;15:65–73.
Movellan J, Urban P, Moles E, de la Fuente JM, Sierra T, Serrano JL, Fernandez-Busquets X. Amphiphilic dendritic derivatives as nanocarriers for the targeted delivery of antimalarial drugs. Biomaterials. 2014;35:7940–50.
Janaszewska A, Lazniewska J, Trzepinski P, Marcinkowska M, Klajnert-Maculewicz B. Cytotoxicity of dendrimers. Biomol Ther. 2019;9:330.
Elmi T, Shafiee Ardestani M, Hajialiani F, Motevalian M, Mohamadi M, Sadeghi S, Zamani Z, Tabatabaie F. Novel chloroquine loaded curcumin based anionic linear globular dendrimer G2: a metabolomics study on Plasmodium falciparum in vitro using (1) H NMR spectroscopy. Parasitology. 2020;147:747–59.
Fröhlich T, Hahn F, Belmudes L, Leidenberger M, Friedrich O, Kappes B, Couté Y, Marschall M, Tsogoeva SB. Synthesis of artemisinin-derived dimers, trimers and dendrimers: investigation of their antimalarial and antiviral activities including putative mechanisms of action. Chemistry. 2018;24:8103–13.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sunita, C., Akruti, K., Ankit, C., Patel, J.K. (2023). Dendrimers in Malaria. In: Shegokar, R., Pathak, Y. (eds) Malarial Drug Delivery Systems. Springer, Cham. https://doi.org/10.1007/978-3-031-15848-3_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-15848-3_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15847-6
Online ISBN: 978-3-031-15848-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)