Abstract
This medical device development tool (MDDT) is categorized as a non-clinical assessment model (NAM). This MDDT is a computational modeling and simulation tool. It can predict heating of metallic orthopedic implants with the radio frequency (RF) electromagnetic fields in the magnetic resonance imaging (MRI) coils while targeting a mid-aged and elderly female population primarily affected by osteoporosis and the associated bone fracture.
This MDDT uses a high resolution anatomical female CAD (computer aided design) model coupled with the proven multiphysics finite element method (FEM) software (Ansys Workbench) to simulate the complete MRI environment. The environment is consisting of a tuned MRI coil with the given output power, detailed heterogeneous human model within the coil at the given landmark and a properly embedded metallic implant within the anatomical model to compute the extent of heating generated around the implant.
Specifically, this MDDT is the in silico analog of an MRI scan for an elderly female subject with a metallic orthopedic implant at 1.5 T in a full-body birdcage RF coil.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- RF-induced heating
- MR safety assessment
- Passive implantable medical devices
- Medical device development tool
- Computational model validation
- Specific absorption rate
- Model mesh co-registration
- Birdcage MRI coil
1 Introduction
This tool partially described in Refs. [1,2,3,4] is a non-clinical assessment model used to predict the RF power deposition induced temperature rise near orthopedic implants as a function of implant geometry, location, material, and scan time. It is using the complete in silico multiphysics MRI environment and the anatomical human CAD model. The validated context of use is limited to a mid-aged or elderly female subject of 50–70 years old with a higher obesity (BMI or body mass index of 30–36) scanned in a 1.5 T full-body circularly polarized cylindrical MRI birdcage coil with two ports at 64 MHz. It is also limited to one yet critical implant geometry: a long femoral nail (a nearly straight long metal rod) which is subject to most excessive heating during long scan times at 1.5 T.
This tool can augment the widely used ASTM F2182 standard that measures RF implant heating in a homogeneous gel phantom by providing extra safety margins caused by the influence of the realistic heterogeneous human body. It can help to identify the appropriate worst-case implant size, configuration, and orientation as a function of the scan protocol and required scan time (the output RF power). The tool does provide the estimates of SAR values in the regions around the implant. The tool does not provide heating or SAR estimates for gradient-coil induced power deposition for orthopedic implants.
A number of modeling and physical processes were tested to validate this tool. They include: (i) topological validation of the entire female CAD model (required approximately 4000 man hours for its construction); (ii) anatomical validation of the constructed human model by anatomical experts; (iii) validation of the human model and FEM software compatibility; (iv) SAR deposition validation in the 1.5 T full-body MRI birdcage coil (accuracy of 10% or better); (v) temperature rise validation in a phantom with the actual long femoral nail implant (accuracy of 20% against the experiment) and; (v) temperature rise validation in the detailed human model with the same femoral nail implant located at approximately the same depth (accuracy of 25% or 2.4 °C on average with the standard deviation of or 0.2 °C against the experiments with the phantom).
In the last case, the tool testbed predicted the higher temperature rise (by approximately 25% or 2.4 °C higher on average) at the implant tips than the in vitro experiments with the simplified gel phantom. An additional validation of the MDDT was therefore made against in vivo measurements in living human arm which indicated the temperature deviation of the MDDT from the in vivo experiment of only 10%.
This tool addresses the growing number of patient MRI scans and expected prevalence of patients with implanted medical orthopedic devices specifically mid-aged and elderly women who are most affected by osteoporosis and the associated bone fracture. Newly developed implants, as well as legacy medical implants without MRI safety information, need to be evaluated for safety in the MRI environment.
The widely used ASTM F2182 standard allows measuring RF implant heating in a homogeneous gel phantom. However, the human body anatomy is different from this phantom. For safety and completeness, it may be therefore desired to additionally estimate the heating of the same implant in a realistic heterogeneous anatomical human model.
This MDDT tool may help to estimate the induced RF heating of a metallic orthopedic implant in the detailed model of an elderly woman aged ~60 at the frequency of 64 MHz (1.5 T MRI scan). This tool can also help to identify the appropriate worst-case implant size, configuration, orientation, and an allowable scan protocol (coil power) by performing multiple simulations to determine the RF-induced temperature rise as a function of the scan protocol and the required scan time.
This tool has demonstrated that it accurately predicts absolute temperature rise for RF-induced heating with acknowledgement of the following limitations:
-
Although all separate blocks of the modeling pipeline (human model, coil model, SAR values) were validated separately and in the general case, the end result – temperature distribution along an implant and as a function of time – is limited to one yet most critical implant geometry: a long femoral nail (a nearly straight metal rod) subject to the most excessive heating and possible resonant effects.
-
The focus of this tool is currently at 1.5 T/64 MHz cylindrical bore MRI systems using circularly polarized RF birdcage full body coils.
-
The tool currently does not include multi-parts orthopedic implants or implants with screws.
-
The tool currently does not provide heating estimates for gradient-coil induced power deposition for orthopedic implants.
-
The computational human model margins are limited to women aged 50–70 and with a high obesity (BMI of 30–36).
The main advantage of using this tool lies in the possibility to accurately estimate temperature rise near an orthopedic implant in the realistic high-resolution elderly female human model and thus additionally justify and assess measurements obtained with the ASTM F2182 standard and their safety margins.
While modeling SAR distributions near implants in realistic virtual human models is well understood, accurate modeling and prediction of temperature rise at and near implants in human models is much more difficult. The reason is a necessity to couple an electromagnetic software and a thermal software with a realistic heterogeneous human model including fine implant and tissue details which are best described with the finite element method that is developed for curved and fine geometries.
The present tool addresses this knowledge gap by constructing and validating the state-of-the-art multiphysics FEM modeling pipeline. It couples the detailed accurate CAD human model, the world-best Ansys electromagnetic modeling software HFSS including the MRI coil modeling, and the Ansys thermal software, all within one single user-friendly shell – the Ansys Workbench.
The second advantage of using this tool is the type of the embedded anatomical female CAD model that is appropriate for elderly female subjects of 50–70 years old with a high obesity. There is no similar anatomical CAD model currently available.
The main disadvantage with the tool is that it currently requires long computation times necessary to obtain accurate and reliable temperature rise results. These times are on the order of 6–24 hours depending on the computer hardware used.
As stated previously, the tool’s context of use is currently restricted to the long femoral nails and does not include other types of the femoral and shoulder orthopedic implants. It is also is limited to the 1.5 T cylindrical bore MRI system only.
2 Tool Description and Validation
2.1 VHP Female v 5.0 Anatomical Human Model (DOI 10.20298/VHP-FEMALE-V.5.0)
The VHP Female v.3.0–5.0 computational human model (also known under the nickname ‘Nelly’ to users of Dassault Systèmes SIMULIA software CST), shown at left in Fig. 1, is an anatomically accurate heterogeneous female (~60 years old, ~88 kg, BMI of ~36, obese, with heart pathology) surface-based human body model. It was constructed from the photographic cryosection data of the Visible Human Project of the US National Library of Medicine with the world-best isotropic resolution of 0.33 mm. Its construction has been well documented in the literature [5,6,7,8,9,10], but several points are worth emphasizing here:
-
1.
There are 249 distinct components or triangular 2-manifold surface meshes (with an additional 40 characterizing embedded implants). No intersections or joint faces between discrete meshes are allowed, enabling unique assignment of electromagnetic, thermal, or other material properties.
-
2.
The source data for the model are freely and publicly available [11, 12]. The complete co-registration data for all model cross-sections are also made publicly available [13].
One advantage of the model is its topological and computational simplicity. The model size is purposely limited to approximately 0.4 M triangular facets in total with an attempt to keep the anatomical accuracy within the body within 1–7 mm. This makes it possible to apply virtually any commercial or custom-tailored finite-element or boundary element computational solver in a very reasonable amount of time. Nearly identical results are obtained when using different software packages and numerical methods [14]. Yet another advantage is the separation of the muscular system of the body into individual muscle objects (about 50 in total).
(a) Full-body Visible Human Project Female v5.0 CAD based computational phantom embedded into Ansys FEM software. The phantom is composed of 249 individual structures. Some individual muscles are removed for clarity. (b–e) Examples of co-registration maps for surface meshes in different transverse planes with tissue labeling. In case (b), non-anatomical separation between scalp and skull was corrected. In case (d), the tissue labeling list is not shown. The complete full-body co-registration maps with the vertical resolution of 1 mm and with tissue labeling in every cross-sectional plane are available online in *.mp4 format [13] for independent inspection and verification purposes
Some recent uses of the VHP Female v3.0–5.0 computational human model have been documented in the literature – cf. for example [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31] – and compared with experimental data on radio frequency propagation. A number of studies have focused on the characteristics and behaviors of antennas near the human body [15,16,17,18,19,20,21,22,23]. Others have examined through simulation various biomedical applications including transmission channel modeling [24], transcranial direct current stimulation [25], estimation of bone density [26], gastroenterology [27, 28], SAR simulations in MRI coils at 3 T [29], and safety of active implants under MRI procedures [30, 31].
The VHP-Female model has been licensed by several private entities engaged in medical device modeling including Ansys, Inc., Dassault Systèmes SIMULIA, Stryker Corp., Cambridge Consultants, Inc., and WIPL-D. A simplified version of the model, VHP-Female v. 2.2, has over 500 registered college users and is available for free download online.
2.2 Ansys FEM Computational Software Suite
2.2.1 Computation of SAR
The human model has been coupled with the proven Ansys Electronics Desktop (HFSS) finite-element electromagnetic software with adaptive mesh refinement. It has been employed to solve Maxwell’s equations in three-dimensional space. In this way, the model is considered as an arbitrary (inhomogeneous) isotropic medium with piecewise-constant electric permittivity ε having the units of F/m and with constant magnetic permeability μ having the units of H/m.
After Maxwell’s equations for an electric field (or the electric field intensity) E(r, t) [V/m] and for a magnetic field (or the magnetic field intensity) H(r, t) [A/m] are solved, the local SAR (W/kg) is defined through averaging the dissipated power per unit mass over a small (ideally infinitesimally small) volume V, that is
where σ is (generally piece-wise constant) medium conductivity with the units of S/m, ρ(r) is the local mass density, and |E(r)| is the electric field magnitude at the observation point. The body-averaged or the whole-body SARbody is given by averaging over the entire body volume, as
Similarly, SAR1g is given by averaging over a volume with the weight of 1 g
SAR10g(r) is found in a similar fashion.
2.2.2 Computation of Temperature Rise
The SAR is the density of volumetric heat sources, not the temperature rise itself. To compute the resulting local temperature rise, the solutions of electromagnetic simulations have been coupled to Ansys Mechanical/Thermal via Ansys Workbench. Pennes’ bioheat equation, based on the heat diffusion equation, is a standard approximation for heat transfer in biological tissues [39,40,41] implemented in the Ansys Mechanical software. It has the form
where T(r, t) is the local temperature, ρ(r) is density, C(r) is specific heat capacity (at constant pressure), Q is the metabolic heat generation rate, B is the perfusion rate [1/s]; index b is related to blood.
All electromagnetic, mechanical, and thermal tissue properties used in the present study are catalogued in the reputable IT’IS database [42]. The electromagnetic material properties are given as a function of frequency, which is ideal for the present analysis.
2.3 Validation of Overall Segmentation and Anatomical Correctness of the Human Model
2.3.1 Topological Validation
All 249 distinct components of the human model have been proven to be strictly 2-manifold (watertight) surface meshes or a reasonable mesh quality. All intersection or joint faces between discrete meshes have been eliminated enabling unique assignment of electromagnetic, thermal, or other material properties.
2.3.2 FEM Software Compatibility Validation
The entire model (including the implant parts) was so constructed that is passes the optional built-in Ansys mesh intersection and mesh quality checker with the highest option “Strict”. Such a check can be independently performed and confirmed by any MDDT user.
2.3.3 Anatomical Validation
To validate the model anatomically, the co-registration method has been used, which implies direct superposition of transverse cross-sections of all surface tissue meshes onto the original cryosection images. An in-house MATLAB module (Fig. 1b–e) was written that performs such superposition with the resolution of 1 mm and simultaneously labels all tissue meshes which are present for a given cross-section. Its output is a scanning sequence in *.mp4 format [13].
After resolving multiple mesh intersections, the validation of the model segmentation shown in Fig. 1a was performed by
-
(i)
co-registration of surface meshes superimposed onto the original cryo-section images (Fig. 1b–e) and;
-
(ii)
correcting all intersection flaws.
Further visual anatomical validation was performed by a number of anatomical experts in their respective areas from Beth Israel Deaconess Med. Ctr. and Massachusetts General Hospital, Boston MA including Profs A. Nazarian (orthopaedics), A. R. Opotowsky, (cardiovascular systems), V. Poylin, (gastroenterology), E. K. Rodriguez (nusculoskeletal tissue components), A. Pascual-Leone (cranial and intracranial anatomy) and Prof. G. Haleblian (urology). The questionable surface meshes (mostly bones but also soft tissues including scalp, bladder, uterus, etc.) were corrected. An example of a non-anatomical flaw is shown in Fig. 1b where the scalp was non-anatomically separated from the skull during the cryogenic process, which required proper mesh adjustment.
The surface deviation for all meshes was found not to exceed 1–8 mm. The highest accuracy was achieved for
-
(i)
extracerebral and intracranial volume;
-
(ii)
vertebral column/spinal cord.
The complete full-body co-registration maps (movies) with the vertical resolution of 1 mm and with tissue labeling in every cross-sectional plane are available online to the MDDT users in *.mp4 format [13] for independent inspection, verification and design/development purposes.
2.4 Validation of Overall SAR Prediction by the MDDT During a 1.5 T MRI Scan
The first step of the MDDT workflow – the computation of local SAR – has been validated by comparison of normalized SAR predicted by two different modeling techniques in a 1.5 T birdcage whole body MRI coil.
A generic, whole-body high-pass birdcage coil with 16 rungs and 32 matching capacitors, loaded with the VHP Female model, has been considered. The coil has a diameter of 64 cm and length of 69 cm consistent with [46]. The simulation geometry is shown in Fig. 2a. Simulations have been conducted at shoulder/heart and abdominal landmarks. For the former, the coil center is oriented to coincide with the top of the T7 vertebra; the latter has the coil center located at the top of the L1 vertebra. The coil was tuned to the desired frequency of 64 MHz (B0 = 1.5 T) when loaded with the subject at each landmark (cf. [53]). Similar to [53], an ideal excitation was applied with 32 sources placed in the two end-rings to perform the function of the capacitors. This excitation provides results which are very similar to the conventional two-port or four-port excitations [53].
Local SAR distribution in the coronal plane for a high pass full-body RF coil operating at 64 MHz loaded with the VHP-Female v5.0 computational phantom given \( {B}_1^{+} \) amplitude of 1 μT at the coil center. (a) Positioning of model within the birdcage at shoulder/heart landmark. (b) Ansys HFSS (Electronics Desktop) solution with one adaptive pass. (c) Ansys HFSS (Electronics Desktop) solution with eight adaptive passes
A comparison was further made with the results of Ref. [53] which was using a nearly identical high-pass birdcage coil (diameter of 63 cm and length of 70 cm), the nearly identical heart landmark, and the identical coil excitation type. However, an in-house voxel model for the same VHP-Female dataset was employed in [53] followed by the FDTD simulation method with the resolution of 5 mm.
To compare the solution variation as a function of FEM mesh density and the solution convergence trends, solutions were generated first with 1 and then with 8 adaptive mesh refinement passes, created approximately 0.5 M and 2.0 M tetrahedra, respectively. As an example, Fig. 2 shows the corresponding local SAR distributions at two different FEM resolutions in the coronal plane for the coil loaded with the VHP-Female v3.0 computational human model given \( {B}_1^{+} \) amplitude of 1 μT at the coil center. Fields solutions from Ref. [53] were also normalized given the desired magnitude of \( {B}_1^{+} \) at the coil center of 1 μT. The normalization is done in the form
The local SAR was computed in Ansys HFSS and then exported to MATLAB over a uniform 3D grid of 2 mm in size. Whole-body SARbody was computed from this data directly in MATLAB. SAR1g was also calculated by finding a volume surrounding the observation point having the mass of exactly 1 g, and then performing averaging according to Eq. (3c). This averaging volume contains approximately 5 × 5 × 5 individual voxels (2 × 2 × 2 mm each) closest to the observation point. The observation points form a 3D sub-grid spaced of 20 mm and 10 mm, respectively. The tissue density was set at 1 g/cm3 uniformly in space. SAR10g was computed in the same way. In this case, the averaging volume contained approximately 1250 individual voxels (2 × 2 × 2 mm each) closest to the observation point.
Figures 2b, c show the local SAR within the various tissues of the VHP-Female v3.0 model after 1 pass and 8 passes, respectively, for the shoulder/heart landmark. All results are normalized to the \( {B}_1^{+} \) amplitude of 1 μT at the coil center. By analyzing Fig. 2 we can see that
-
1.
The accurate solution with eight adaptive mesh refinement passes generates a more realistic SAR distribution, especially with regard to the local SAR – see Table 1. In particular, two non-physical maxima of the SAR observed at the top of the head are no longer present.
-
2.
The accurate solution with eight adaptive mesh refinement passes and the coarse solution with one adaptive pass generate approximately the same whole-body SAR and SAR distribution maps, but considerably different peak local SAR values – see Table 1. Note that the coil has been retuned separately in both cases.
-
3.
The maximum SAR for the present landmark is observed in the upper shoulder/neck area and in the arms area.
-
4.
A gap between the arms and the body may generate large SAR values.
Table 1 compares the computed SAR values with the values obtained in Ref. [53] under nearly identical conditions. For both models and both methods, the whole body SAR and the maximum SAR1g(r) differ by 8% and 10%, respectively.
A comparison with a variety of other modeling reference sources [35,36,37, 46,47,48,49,50,51,52,53,54,55,56] has also been made both at 1.5 T and 3 T. It was found that the SAR results calculated when using the VHP Female v3.0 model are strictly within the bounds of all other reported values.
2.5 Validation of Temperature Rise Prediction by the MDDT Near a Long Femoral Nail Implant Against Measurements with an ASTM-Like Phantom
ASTM and ASTM-like phantoms have been used as the standard method for testing compliance of implants within MRI environment [54]. These phantoms are not an equivalent of intricate human body structures. Here, the comparison is made with the original experiments performed in a series of papers [43,44,45].
2.5.1 Modeling Testbed
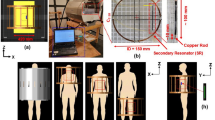
Prior to conducting simulations using the VHP Female v5.0 model, a necessary workflow and RF power calibration have been established. Figure 3 depicts to scale simulation of the original experiment performed in a series of papers [32,33,34]. It was also used for initial calibration purposes. At left in Fig. 3, a homogenous experimental AGAR gel phantom with electromagnetic properties matching that of the human body is positioned within a 1.5 T birdcage MRI coil operating at 64 MHz.
Simulations to establish calibration for experiments conducted in [32,33,34]. At left, the metallic nail implant placed within a homogeneous loop-like Agar phantom [32,33,34] at a depth of 2 cm. At top right, the current density produced with a whole-phantom SAR of 4.0 W/kg. At bottom right, the simulated temperature given a total volumetric power loss of 120 W exactly corresponding to experiment [32,33,34]. Simulation results produced a temperature rise of 10.02 °C, slightly less than the 12.6 °C experimentally observed in [32,33,34]
This phantom (40 cm long by 20 cm wide) in Fig. 3 is not the exactly the ASTM F2182 phantom. However, it does support and even enhances the loop-like distribution of the electric field in the birdcage coil and thus describes a realistic heating scenario, perhaps even the worst-case scenario with respect to implant heating.
All examinations [32,33,34] were performed with a 1.5 T MR scanner (MAGNETOM Symphony, SIEMENS) with the phantom at the center of the coil. A birdcage shaped transmit/receive body coil was used there with the inner diameter of 60 cm and the length of 70 cm, which is close to the present modeling setup (64 cm and 69 cm, respectively). Coil tuning and excitation was performed as described in the previous example. We again use ideal excitation which is very similar [53] to the two-port excitation used in [32,33,34].
A 24 cm long metallic orthopaedic nail implant of Zimmer, Inc. made of nonmagnetic stainless still (originally very slightly bent but modeled as a straight rod of the same diameter) has been used. Such implants are normally used to treat fractures in clinical practice.
The implant was embedded in this phantom such that it is 2 cm away from the top and side edges of the phantom. Both the phantom and metallic rod have been assigned dimensions and material properties consistent with experiment [32,33,34].
2.5.2 Replication of Original Experiments
The computed volumetric current density within the phantom is shown at top right in Fig. 3. This density matches well with the published result; it was produced by adjusting coil power to exactly replicate the RF exposure given in [32,33,34] – a whole-phantom SAR of 4.0 W/kg and a volumetric power loss density of 120 W in the phantom. The temperature simulation is shown at bottom right of Fig. 3. Given the above power loss density as an internal heat generation source, the total temperature rise within the human model was 10.02 °C. This value is slightly less than the 12.6 °C observed in [32,33,34]. However, it is close enough (a 20% temperature difference) to give confidence in the adequate simulation setup, enabling the extension of this methodology to the case involving the VHP female human model.
2.5.3 Heating of the Same Implant in the MDDT Human Female Phantom
Figure 4 at left shows the VHP Female computational human model in the 1.5 T birdcage MRI coil. The human model has been oriented so that the center of the femur bone is aligned with the center of the coil. The left quadriceps muscle within the VHP model is not shown in Fig. 4 so that the position of the femur can clearly be seen. Within the left femur, the same 24 cm long cylindrical metallic implant has been inserted as required in the surgical practice.
The VHP-Female computational phantom positioned with the 1.5 T MRI birdcage coil. Some body parts are removed for clarity. At left, the femur position is illustrated to show its orientation within the model – the metallic nail implant is aligned to reside within the trabecular bone structure. At top right, each individual object within Ansys Mechanical model is assigned specific thermal properties. At bottom right, the temperature rise is shown after 900 seconds of continuous coil operation. These values correspond well with published experimental data [32,33,34]
This rod is assigned material properties consistent with [32,33,34]. The coil is driven at 64 MHz and at a power such that the volumetric power loss within the human model is again 120 W, also consistent with the published experimental results.
A length-based mesh constraint of no edge larger than 2.5 mm was enforced for the metallic rod mesh and a total of about 636,000 tetrahedral elements were used in the Ansys HFSS simulation.
Once the electromagnetic simulation was complete, the results were passed to Ansys Mechanical software by linking the two simulations in Ansys Workbench. A mesh refinement was again employed on the faces of the rod to ensure that a dense enough mesh was created to capture the local temperature changes. Approximately 629,000 tetrahedral elements were used in the transient thermal simulation. Volumetric losses (SAR) produced by the Ansys HFSS simulation were imported into Ansys Mechanical and used as the internal heat generation source density. The RF coil and the heat sources were active for 900 s and the model was allowed to cool for another 600 s. The values for the implant temperature captured from 0 to 900 s are shown in Fig. 5.
Comparison of the simulated numerical values (red stars) obtained using the VHP Female computational phantom with published experimental data (two black curves) [32,33,34] for the maximum temperature rise near the implant. The depth of the implant within the VHP model is approximately 4 cm and the maximum temperature value after 900 seconds of coil operation time is represented by the top red star. The VHP model predicts a slightly higher (by 2.75°) maximum temperature rise than what was measured in [32,33,34]. This is likely due to the different (non-homogeneous) material properties employed in the present study and the slightly angled orientation of the nail
Figure 5 shows temperature dynamics: it compares simulated numerical values (red stars) obtained using the VHP Female computational phantom with published experimental data (two black curves) [32,33,34] for the maximum temperature rise near the implant. The depth of the implant within the VHP model is approximately 3–4 cm and the maximum temperature value after 900 seconds of coil operation time is represented by the top red star.
Peak simulated temperature values for the implant within the human model are shown in the bottom right of Fig. 4. A maximum temperature rise of approximately 11 °C is computed at the very ends of the metallic implant against 8.25 °C observed in experiment at 4 cm depth [32,33,34]. Thus, the MDDT testbed predicts a higher (by 2.75 °C) maximum temperature rise of 8.25 °C than what was measured in [32,33,34] which constitutes the difference of 33% or 2.75 °C.
This is likely due to the different (non-homogeneous) material properties employed in the present study, the realistic angled orientation of the nail, and a possible resonant behavior of the long implant. In the VHP Female model, the rod is aligned with the femur and represents a more realistic position that would be encountered in a clinical setting.
2.6 Variation and Uncertainty of Temperature Rise Measurements Near the Implant
Table 2 gives the least mean square difference in the temperature rise (maximum implant temperature at the tip is recorded) over the entire interval from 0 to 15 min between the modeled data and the in-vitro data at 2 and 4 cm implant depth, respectively, in degrees C. Statistical significance of the deviation is quantified via the p-value for the paired-sample t-test with p ≤ 0.05 considered statistically significant.
2.7 Validation of Temperature Rise Prediction by the MDDT Against In Vivo Measurements
In the previous case validation example, the MDDT testbed predicted the higher maximum temperature rise (up to 33% higher) at the implant tips than the experiment in vitro with the simplified gel phantom. An additional validation of temperature rise was therefore made against in vivo measurements in living human.
Since in-vivo measurements in the MRI coil at 1.5 T have not been reported, a comparison was made with tissue heating due to a small single-loop coil close to skin surface at 165 MHz [38]. The replication of the experimental setup [38] is shown in Fig. 6. The forearm of the VHP Female computational human model has been isolated to speed up the simulation. Internal structures, including the humerus, ulna and radius bones, extensor, flexor, triceps and biceps muscles, radial nerve, and various arteries and veins, are encapsulated within concentric layers of muscle, fat and skin tissue. The coil [38] is modeled as a 80 mm diameter copper torus with a minor diameter of 2 mm driven at 165 MHz by a 50 Ohm lumped antenna port and a thin sheet of Teflon separates the antenna from the forearm. These settings exactly correspond to experiment. All thermal and electromagnetic material properties associated with the internal body structures correspond to those supplied in [38].
According to [38], the coil antenna is provided sufficient power to dissipate approximately 31 W within the forearm and produce peak local SAR values in the lower corners of the forearm of approximately 450 W/kg. These power conditions have been replicated in simulations with the forearm of the VHP Female model. Adaptive mesh refinement produced a volumetric mesh consisting of approximately 0.1 M tetrahedra.
Following simulation in Ansys HFSS, the peak SAR value experienced in the outermost model layer is computed as approximately 450 W/kg, consistent with the value reported in [38] to within 5%.
Then, the geometry and resulting fields were passed via Ansys Workbench to Ansys Mechanical. Here, the human model was again remeshed, producing just over 0.06 M elements. The volumetric power losses determined in Ansys HFSS were imported and evaluated as the heat generation source density. Heat generation sources were active for 120 s and the model then cooled via convection for 19.2 s; temperatures were recorded throughout the duration of the simulation. The corresponding temperature rise after 139 seconds in total was computed and compared with experiment.
The simulated and measured [38] thermal maps within the forearm in a plane directly above the coil center have been examined. The peak simulated temperature change is about 6.6 °C, which is slightly higher that the measured 6 °C change reported in [38]. The deviation is within 10%.
The spatial temperature map are also consistent with the measured map despite the different wrist geometry and tissue composition. There is some difference in the observed depth of temperature change. This is likely mostly due to the fat layer of the VHP Female model which is thicker than the subject reported in [38].
3 Conclusions
Based on the evidence provided, this non-clinical assessment tool entitled “Computational Tool Comprising Visible Human Project® Based Anatomical Female CAD Model and Ansys HFSS/Mechanical® FEM Software for Temperature Rise Prediction near an Orthopedic Femoral Nail Implant during a 1.5 T MRI Scan” was found to reliably predict both temperature distribution and its evolution in time along the long femoral nail metal implant caused by RF power deposition from the 1.5 T birdcage MRI full body coil. The tool can also help to identify the appropriate worst-case device and coil size, configuration, and orientation by performing multiple simulations to determine the RF-induced temperature rise as a function of a scan protocol and required scan time.
All separate blocks of the modeling pipeline – the human model topology and anatomy including co-registration, surgically correct implant embedding, the RF coil model, and the resulting SAR and temperature behavior – have been validated independently and all the validation results have been made available to the user.
References
G.M. Noetscher, P. Serano, W.A. Wartman, K. Fujimoto, S.N. Makarov, Visible Human Project® female surface based computational phantom (Nelly) for radio-frequency safety evaluation in MRI coils. PLoS One 16(12), e0260922 (2021). https://doi.org/10.1371/journal.pone.0260922. PMID: 34890429; PMCID: PMC8664205
S.N. Makarov, G.M. Noetscher, J. Yanamadala, M.W. Piazza, S. Louie, A. Prokop, A. Nazarian, A. Nummenmaa, Virtual human models for electromagnetic studies and their applications. IEEE Rev. Biomed. Eng. 10, 95–121 (2017). https://doi.org/10.1109/RBME.2017.2722420. Epub 2017 Jun 30
H. Tankaria, X.J. Jackson, R. Borwankar, G.N. Srichandhru, A. Le Tran, J. Yanamadala, G.M. Noetscher, A. Nazarian, S. Louie, S.N. Makarov, VHP-female full-body human CAD model for cross-platform FEM simulations: recent development and validations. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2016, 2232–2235 (2016). https://doi.org/10.1109/EMBC.2016.7591173
M. Kozlov, G.M. Noetscher, A. Nazarian, S.N. Makarov, Comparative analysis of different hip implants within a realistic human model located inside a 1.5T MRI whole body RF coil. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 7913–7916 (2015). https://doi.org/10.1109/EMBC.2015.7320227
G.M. Noetscher et al., VHP-Female v3.0 FEM/BEM Computational Human Phantom, 24th Int. Meshing Roundtable (IMR24), Austin, TX, Oct. 12–14, 2015
J. Yanamadala et al., Multi-purpose VHP-female version 3.0 cross-platform computational human model, 10th European Conf. on Antennas and Propagation (EuCAP16), Davos, Switzerland, April 2016, pp. 1–5
H. Tankaria et al., VHP-female full-body human CAD model for cross-platform FEM simulations – recent development and validations, 38th Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society (EMBC 2016), Orlando, FL, Aug. 16–20, 2016
G. Noetscher et al., Creating a computational human model, IEEE Pulse, April 27th 2016. Available: http://pulse.embs.org/march-2016/creating-a-computational-human-model/
G. Noetscher et al., Computational human model VHP-FEMALE derived from datasets of the national library of medicine, 38th Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society (EMBC 2016), Orlando, FL, Aug. 16–20 2016
G.M. Noetscher et al., VHP-female CAD human model family for antenna modeling, 2016 IEEE Int. Sym. on Antennas and Propagation/USNC-URSI National Radio Science Meeting, Puerto Rico, June 2016
The Visible Human Project, US National Library of medicine. Online: https://www.nlm.nih.gov/research/visible/visible_human.html
M.J. Ackerman, The visible human project: from body to bits. IEEE Pulse 8(4), 39–41 (2017). https://doi.org/10.1109/MPUL.2017.2701221
VHP Female v.3.0–5.0 Co-Registration Standard. 08/23/2017. Online: https://www.dropbox.com/sh/f1unwyan9e0sh2v/AACXzcQ7EpR8wYXowbnhC-eAa?dl=0
J. Yanamadala, G. Noetscher, S. Louie, A. Prokop, M. Kozlov, A. Nazarian, S.N. Makarov, Multi-purpose VHP-female version 3.0 cross-platform computational human model, 2016 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 2016, pp. 1–5, https://doi.org/10.1109/EuCAP.2016.7481298
R. Lemdiasov, A. Venkatasubramanian, Transmit coil design for Wireless Power Transfer for medical implants, 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, 2017, pp. 2158–2161. https://doi.org/10.1109/EMBC.2017.8037282
A. Venkatasubramanian, B. Gifford, Modeling and design of antennas for implantable telemetry applications, 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, 2016, pp. 6469–6472. https://doi.org/10.1109/EMBC.2016.7592210
D. Nikolayev, M. Zhadobov, P. Karban, R. Sauleau, Conformal antennas for miniature in-body devices: the quest to improve radiation performance. URSI Radio Sci. Bull. 2017(363), 52–64 (2017). https://doi.org/10.23919/URSIRSB.2017.8409427
D. Nikolayev, Modeling and characterization of in-body antennas, 2018 IEEE 17th International Conference on Mathematical Methods in Electromagnetic Theory (MMET), Kiev, 2018, pp. 42–46. https://doi.org/10.1109/MMET.2018.8460279
M. Barbi, C. Garcia-Pardo, N. Cardona, A. Nevarez, V. Pons, M. Frasson, Impact of receivers location on the accuracy of capsule endoscope localization, 2018 IEEE 29th annual international symposium on personal, indoor and Mobile radio communications (PIMRC), Bologna, 2018, pp. 340–344. https://doi.org/10.1109/PIMRC.2018.8580862
C. Garcia-Pardo et al., Ultrawideband technology for medical in-body sensor networks: an overview of the human body as a propagation medium, phantoms, and approaches for propagation analysis. IEEE Antennas Propagat. Magaz. 60(3), 19–33 (2018). https://doi.org/10.1109/MAP.2018.2818458
D. Nikolayev, M. Zhadobov, L. Le Coq, P. Karban, R. Sauleau, Robust Ultraminiature capsule antenna for ingestible and implantable applications. IEEE Trans. Antennas Propag. 65(11), 6107–6119 (2017). https://doi.org/10.1109/TAP.2017.2755764
S. Perez-Simbor, C. Andreu, C. Garcia-Pardo, M. Frasson, N. Cardona, UWB path loss models for ingestible devices. IEEE Trans. Antennas Propagat.. https://doi.org/10.1109/TAP.2019.2891717
L. Chen et al., Radiofrequency propagation close to the human ear and accurate ear canal models, 40th Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society (EMBC 2018), Honolulu, HI, Jul. 17–21 2018
J.M. Elloian, G.M. Noetscher, S.N. Makarov, A. Pascual-Leone, Continuous wave simulations on the propagation of electromagnetic fields through the human head. IEEE Trans. Biomed. Eng. 61, 1676–1683 (2014)
G.M. Noetscher et al., Comparison of cephalic and extracephalic montages for transcranial direct current stimulation – a numerical study. I.E.E.E. Trans. Biomed. Eng. 61(9), 2488–2498 (2014)
S.N. Makarov, G.M. Noetscher, S. Arum, et al., Concept of a radiofrequency device for osteopenia/osteoporosis screening. Sci. Rep. 10, 3540 (2020). https://doi.org/10.1038/s41598-020-60173-5
S. Perez-Simbor, C. Andreu, M. Frasson, N. Cardona, UWB path loss models for ingestible devices. IEEE Trans. Antennas Propagat. 67(8), 5025–5034 (2019)
S.P. Simbor, In-Body to on-Body Experimental UWB Channel Characterization for the Human Gastrointestinal Area. PhD Thesis. Universitat Politecnica de Valencia. Spain. Oct. 2019
A. Prokop, T. Wittig, A. Morey, Using anatomical human body model for FEM SAR simulation of a 3T MRI system. Ch. 16. pp. 273–281, in Brain and Human Body Modeling 2020, vol. 2, (Springer Nature, New York, 2020) Open access. ISBN 978-3-030-45622
J.E. Brown, R. Qiang, P.J. Stadnik, L.J. Stotts, J.A. Von Arx, Calculation of MRI RF-induced voltages for implanted medical devices using computational human models. Ch. 16. pp. 283–294, in Brain and Human Body Modeling 2019, vol. 1, (Springer Nature, New York, 2019) Open access. ISBN 978-3-030-21293-3. Online: https://link.springer.com/chapter/10.1007/978-3-030-21293-3_14
J.E. Brown, R. Qiang, P.J. Stadnik, L.J. Stotts, J.A. Von Arx, RF-induced unintended stimulation for implantable medical devices in MRI. Ch. 17. pp. 283–292, in Brain and Human Body Modeling 2020, vol. 2, (Springer Nature, New York) Open access. ISBN 978-3-030-45622
H. Muranaka, T. Horiguchi, S. Usui, Y. Ueda, O. Nakamura, F. Ikeda, K. Iwakura, G. Nakaya, Evaluation of RF heating on humerus implant in phantoms during 1.5 T MRI imaging and comparisons with electromagnetic simulation. Magn. Reson. Med. Sci. 5(2), 79–88 (2006)
H. Muranaka, T. Horiguchi, S. Usui, Y. Ueda, O. Nakamura, F. Ikeda, Dependence of RF heating on SAR and implant position in a 1.5T MR system. Magn. Reson. Med. Sci. 6(4), 199–209 (2007)
H. Muranaka, T. Horiguchi, Y. Ueda, N. Tanki, Evaluation of RF heating due to various implants during MR procedures. Magn. Reson. Med. Sci. 10(1), 11–19 (2011)
H. Homann, P. Börnert, H. Eggers, K. Nehrke, O. Dössel, I. Graesslin, Towards individualized SAR models and in vivo validation. Magn. Reson. Med. 66(6), 1767–1776 (2011) PMID 21630346
H. Homann, SAR prediction and SAR management for parallel transmit MRI. Karlsruhe Translat. Biomed. Eng. 16, 1–124 (2012)
M. Murbach, E. Neufeld, M. Capstick, W. Kainz, D.O. Brunner, T. Samaras, K.P. Pruessmann, N. Kuster, Thermal tissue damage model analyzed for different whole-body SAR and scan durations for standard MR body coils. Magn. Reson. Med. 71(1), 421–431 (2014)
S. Oh, Y.-C. Ryu, G. Carluccio, C.T. Sica, C.M. Collins, Measurement of SAR-induced temperature increase in a phantom and in vivo with comparison to numerical simulation. Magn. Reson. Med. 71(5), 1923–1931 (2014)
A. Lienhard, Heat Transfer Textbook (Phlogiston, Lexington, MA, 2005)
E.H. Wissler, Pennes’ 1948 paper revisited. J. Appl. Phys. 85, 35–41 (1998)
H. Arkin, Recent developments in modeling heat transfer in blood perfused tissues. I.E.E.E. Trans. Biomed. Eng. 41(2), 97–107 (1994)
P.A. Hasgall, E. Neufeld, M.C. Gosselin, A. Klingenböck, N. Kuster, A. Klingenbock, P. Hasgall, M. Gosselin, IT’IS Database for thermal and electromagnetic parameters of biological tissues, Version 3.0, Sept. 1st, 2015. Available: www.itis.ethz.ch/database
Z. Xiaotong, L. Jiaen, B. He, Magnetic-resonance-based electrical properties tomography: a review. IEEE Rev. Biomed. Eng. 7, 87–95 (2014)
E.K. Insko, L. Bolinger, Mapping of the radiofrequency field. J. Magn. Reson. A 103(1), 82–85 (1993)
J. Wang, M. Qiu, Q.X. Yang, M.B. Smith, R.T. Constable, Measurement and correction of transmitter and receiver induced nonuniformities in vivo. Magn. Reson. Med. 53(2), 408–417 (2005)
E. Cabot, A. Christ, N. Kuster, Whole body and local SAR in anatomical phantoms exposed to RF fields from birdcage coils. Proceedings of the 29th General Assembly of the International Union of Radio Science, 2008 August 7–16. Chicago, USA
S. Oh, A.G. Webb, T. Neuberger, B. Park, C.M. Collins, Experimental and numerical assessment of MRI-induced temperature change and SAR distributions in phantoms and in vivo. Magn Reson Med. 63(1), 218–223 (2010) PMCID: PMC2836721
G. Bonmassar, P. Serano, L.M. Angelone, Specific absorption rate in a standard phantom containing a deep brain stimulation lead at 3 Tesla MRI, 2013 6th International IEEE/EMBS Conference on Neural Engineering (NER), San Diego, CA, 2013, pp. 747–750. https://doi.org/10.1109/NER.2013.6696042
C.M. Collins, S. Li, M.B. Smith, SAR and B1 field distribution in a heterogeneous human head model with a birdcage coil. Magn. Reson. Med. 40(6), 847–856 (2005)
A. Rennings, L. Chen, S. Otto, D. Erni. B1-field inhomogeneity problem of MRI: basic investigation on a head- tissue-simulating cylinder phantom excited by a birdcage-mode. 42nd European Microwave Conference, The Netherlands, Amsterdam, 2012 Nov 1; pp. 542–545
B.P. Tomas, H. Li, M.R. Anjum, Design and simulation of a birdcage coil using CST studio suite for application at 7T. IOP Conf. Ser. Mater. Sci. Eng. 51(1), 1–6 (2013)
D.T.B. Yeo, Z. Wang, W. Loew, M.W. Volgel, I. Hancu, Local SAR in high pass birdcage and TEM body coils for multiple human body models in clinical landmark positions at 3T. J. Magn. Reson. Imaging 33(5), 1209–1217 (2011) PMCID: PMC3081105
W. Liu, C.M. Collins, M.B. Smith, Calculation of B1 distribution, SAR, and SNR for a body-size birdcage coil loaded with different human subjects at 64 and 128 MHz. Appl. Magn. Reson. 29(1), 5–18 (2005) PMID:23565039. PMCID: PMC3615460
L. Winter, F. Seifert, L. Zilberti, M. Murbach, B. Ittermann, MRI-related heating of implants and devices: a review. J. Magn. Reson. Imaging (2020). https://doi.org/10.1002/jmri.27194
M. Kozlov, R. Turner. RF transmit performance comparison for several MRI head arrays at 300 MHz. Progress in Electromagnetic Research Symposium Proceedings. 2013 March 28 Taipei, pp. 1052–1056
M. Kozlov, P.L. Bazin, H.E. Möller, N. Weiskopf. Influence of cerebrospinal fluid on specific absorption rate generated by 300 MHz MRI transmit array. 10th European Conference on Antennas and Propagation (EuCAP). 2016 April, Davos, Switzerland, pp. 1–5
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Noetscher, G., Serano, P., Nazarian, A., Makarov, S. (2023). Computational Tool Comprising Visible Human Project® Based Anatomical Female CAD Model and Ansys HFSS/Mechanical® FEM Software for Temperature Rise Prediction Near an Orthopedic Femoral Nail Implant During a 1.5 T MRI Scan. In: Makarov, S., Noetscher, G., Nummenmaa, A. (eds) Brain and Human Body Modelling 2021. Springer, Cham. https://doi.org/10.1007/978-3-031-15451-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-15451-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15450-8
Online ISBN: 978-3-031-15451-5
eBook Packages: EngineeringEngineering (R0)