Abstract
The main objective of this chapter is to provide a comprehensive and intuitive introduction to MRI gradient coil related PNS modeling with human body models. We will present the fundamental concepts and analytical processes behind gradient coil-induced peripheral nerve stimulation (PNS) modeling and also show some new results of our work. We first describe the process of performing electromagnetic simulation of a gradient coil, the neurodynamic simulation of nerves, and the gradient coil design. Then, we present improvements of two existing human body models by adding more nerve trajectories in the head and upper body to reduce the discrepancies between the simulated and measured results for PNS thresholds in head gradient coils. Further, we apply the modified human body models to analyze three folded and non-folded gradient coils and reveal the relationship between the eddy current flow in the human body and the gradient coil wire pattern and its impact on the PNS. We also show the connection between concomitant fields and PNS and assess the accuracy of PNS calculations in human body models with simplified tissue properties. Finally, we give our thoughts on the future direction of this work.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Peripheral nerve stimulation
- MRI gradient coil
- Human body modeling
- Eddy current
- Concomitant fields
- Nerve trajectories
- Neurodynamic simulation
1 Introduction
With the recent advancements in high-performance head gradient coil technology for magnetic resonance imaging (MRI), the assessment of peripheral nerve stimulation (PNS) has become increasingly important. In this chapter, we first briefly describe the basic concepts of PNS and gradient coils in an MRI system. We then describe and compare the significance of a head-only MRI system with respect to a whole-body system. We will also provide a general outline of the electromagnetic simulation, the neurodynamic simulation and the gradient coil design processes. In the Methods section, we deliberate the shortcomings of a widely used human body model in the head gradient coil PNS analysis and describe the modifications made to two human body models to improve the correlation of simulation and measurement results from two head gradient coils. After that, we apply these new human body models to analyze three folded and non-folded gradient coils and reveal the relationship between the eddy current flow in the human body and the gradient coil wire pattern and its impact on PNS. We also discuss the connection between PNS and concomitant fields, and the effects that a human body model with simplified tissue properties has on the PNS calculations. Finally, we will discuss the shortcomings of this study and future work and provide our conclusions.
1.1 MRI Gradient Coil and E-Field
An MRI scanner consists of several key hardware components, including the magnet, the gradient coils and the RF coils. In a typical MRI scanner, the magnet provides a homogenous magnetic field, usually 1.5 Tesla or 3 Tesla, in a field of view (FOV) that spans 45 ~ 50 cm in diameter [1]. The gradient coil system has 3 sub-coils: x, y and z. Each coil provides a linear Bz field along one axis, such that Bz, k = Gkk (k = x, y, z) [2], where Gk is the gradient strength of each sub coil. The RF transmit coil transmits RF waves at the Larmor frequency of the hydrogen nucleus to excite proton spins [2] while the RF receive coil measures the resultant MR signal from these spins.
The resonance frequency ω = γBz is proportional to the applied static magnetic field, where γ is the gyro-magnetic ratio. The locations of the spins in space can be encoded by the Bz field using a combination of the main static field (B0) and the gradient by \( {B}_z={B}_0+\sum \limits_3^{k=1}{G}_kk. \) In a modern MRI system, different gradient waveforms are applied to each gradient sub-coil to spatially encode spins such that they have spatially varying frequencies and phases. The image of the subject can then be reconstructed from the received RF signals by utilizing image reconstruction algorithms [3].
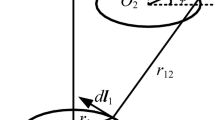
The alternating magnetic field \( \overset{\rightharpoonup }{B} \) generated by a gradient coil can induce an electric field \( \overset{\rightharpoonup }{E} \) in our body, which can be calculated from the vector potential \( \overset{\rightharpoonup }{A} \) and the scalar potential φ as \( \overset{\rightharpoonup }{E}=-\partial \overset{\rightharpoonup }{A}/\partial t-\nabla \varphi \), with \( \overset{\rightharpoonup }{A}\left(\overset{\rightharpoonup }{r}\right)=\frac{\mu_0I}{4\pi }{\int}_l1/\overset{\rightharpoonup }{r}-{\overset{\rightharpoonup }{r}}^{\prime }d\overset{\rightharpoonup }{l}\left({\overset{\rightharpoonup }{r}}^{\prime}\right), \)and t, I, \( d\overset{\rightharpoonup }{l}, \) \( {\overset{\rightharpoonup }{r}}^{\prime }, \) \( \overset{\rightharpoonup }{r} \) being time, current in the gradient coil, differential element in length direction, source vector and observation vector, respectively. The quantity φ in the body can be obtained from \( \nabla \cdotp \sigma \nabla \varphi +\nabla \cdotp \sigma \partial \overset{\rightharpoonup }{A}/\partial t=0 \) with \( \overset{\rightharpoonup }{j}=\sigma \overset{\rightharpoonup }{E} \) and the low-frequency approximation \( \nabla \cdotp \overset{\rightharpoonup }{j} \)=0, where σ and \( \overset{\rightharpoonup }{j} \) are electric conductivity and current density, respectively. Together with the boundary condition \( \frac{\partial \varphi }{\partial n}+\hat{n}\cdotp \frac{\partial \overset{\rightharpoonup }{A}}{\partial t}=0 \) from \( \hat{n}\cdotp \overset{\rightharpoonup }{J} \)=0,\( \hat{n} \) being the unit normal vector at the external surface, φ can be finally computed with numerical methods such as Finite Element Method (FEM) for complicated human body geometry with heterogenous electrical conductivity for different tissues [4]. One of the main safety-related bioeffects induced by this E-field is the stimulation of our peripheral nerves, which may cause tingling or even painful muscle contractions in severe cases. Hence, PNS is an important safety topic in gradient coil design. All gradient EM simulations in this study were performed using Sim4life Ver.6.0 (Zurich Med Tech, Zurich, Switzerland).
1.2 Head vs. Whole-Body Gradient
A very rough approximation of the E-field can give us some sense of the amplitude of the E-field. From \( \nabla \times \overset{\rightharpoonup }{E}=-\partial \overset{\rightharpoonup }{B}/\partial t \) we get \( {\oint}_l\overset{\rightharpoonup }{E}\cdotp d\overset{\rightharpoonup }{l}=\frac{\partial }{\partial t}\underset{A}{\int}\overset{\rightharpoonup }{B}\cdotp d\overset{\rightharpoonup }{s} \), where l is the integration path and the A is the area surrounded by the path, then [5]
It depends on the rate of change of the B-field and the volume (presented by r) to provide such a magnetic field.
A gradient coil is composed of many turns of wire as shown in Fig. 6 of reference [6]. Therefore, the gradient coil has a non-negligible inductance, which indicates that the current through it cannot change instantly. This means that ∂B/∂t and the gradient slew rate (SR), denoted by ∂G/∂t (subscript of G is removed later without loss of generality), cannot be infinite. A clinical whole-body gradient coil typically has a large FOV, resulting in a large-inductance coil that cannot support gradient waveforms with high slew rates. On the other hand, ultra-high performance head-only gradient coils designed for microstructure and functional imaging of the brain need a much smaller FOV that encompasses the head but require fast-changing gradient fields and high gradient waveform amplitudes. More importantly, head gradient coils with a smaller FOV will generate less E-field and thus have lower PNS risks.
We recently developed two high-performance head gradient systems HG2 [7] and MAGNUS [8] for research purposes. The former is designed for a compact 3 T head MRI scanner and the latter is a head gradient that can be inserted into a clinical 3 T whole-body scanner. Compared to typical clinical whole-body MRI scanners, whose gradient strength and slew rate are usually around 30 ~ 50 mT/m and 100 ~ 200 T/m/s, HG2 and MAGNUS achieve 85 mT/m, 700 T/m/s and 200mT/m, 500 T/m/s, respectively. In this study, these two coils are used in PNS model verification since we have measured data from volunteer scans.
Figure 1 shows the E-field intensity of MAGNUS and a typical whole-body gradient coil with a head scan position in the Yoon-sun human body model (v4.0b03, IT’IS Foundation, Zurich, Switzerland). It can be clearly seen that the high \( \mid \overset{\rightharpoonup }{E}\mid \)-field region of the whole-body gradient is much larger than that of the head gradient. The high field regions by MAGNUS X and Y are mainly in the head.
1.3 Peripheral Nerve Stimulation
Nerve stimulation means an action potential is initiated and propagates through the nerve tissue. The theory is that the opening of the ion channels within the membrane of a neuron requires work from a mechanical impulse, which is further produced by an electric field through time. The fundamental law for nerve stimulation by an external electric field can be expressed as [5].
where Er is the rheobase, the minimum E-field to activate the nerve while τc is the chronaxie, the duration with which the threshold is exactly twice of the rheobase. If the external field is less than the rheobase Er, no stimulation can happen even if the field is applied for an infinite time.
When the electric field is induced by the magnetic field, it is possible to use the magnetic field time derivative to describe the fundamental law. Since G and the SR ∂G/∂t, are two important performance parameters for every gradient coil, the equation can be further derived as a relationship between gradient strength, slew rate and the gradient pulse duration τ [9].
Because the E-field from the gradient coil for a certain scan position is diverse inside of the human body, the PNS response from each nerve is also different. The nerve that is easiest to be stimulated gives the threshold under this certain excitation condition.
1.4 Neurodynamic Simulation
To study the action potential initiation and propagation in detail, a closer look at the behavior of the neuron membrane is needed. The sodium and potassium gates, etc., on the neuron membrane, can be simplified as a set of voltage-controlled conductance, while other characteristics of the neuron can also be described as a set of distributed capacitance and conductance. A widely accepted computation model of myelinated mammalian nerve fibres is the MRG model [10], which utilizes double-cable RC-circuits to describe the Ranvier node, paranodal, internodal section of the axon and the myelin sheath of the axon. As a result, the action potential behavior can be simplified and described by the cable theory equation [11].
where Gm, Cm and Ga are membrane conductance, membrane capacitance and axial internodal conductance, respectively. Gm is further controlled by membrane potential reflecting the transient on-off status and the current-conducting ability of the sodium channel, potassium channel and other paths on the membrane. Vn(t) and Ve(t) are membrane potential response and applied potential by the external E-field, respectively (time dependency is omitted for simplicity in the equation). The excitation item (driving function) on the right side of the equation is about the second order difference of the electric potential, but actually relates the first order difference of the tangential E field along the nerve, where n is the index of the Ranvier node. With the E-field calculated from the electromagnetic simulation in the previous step, the voltage drop Ve along the nerve trajectory can be obtained by \( {V}_e={\int}_l\overset{\rightharpoonup }{E}\cdotp d\overset{\rightharpoonup }{l} \). After multiplying with the time domain modulation function, α(t) (E-field waveform through time, refer to Fig. 2), and an additional scaling coefficient T,
is finally fed into the Eq. (4) to calculate the membrane potential response for each nerve trajectory. The solver will iteratively change the scaling factor T to find the smallest field intensity to initiate an action potential. This is called the titration process. In this study, we use the NEURON module (Yale University, New Haven, CT) that is integrated in Sim4life for PNS calculations. Thus, ΔG can be calculated for every τ. Since the relationship between ΔG and τ is linear, only two values of τ are used in the simulation. Table 1 shows the parameters used for neuron simulation in Sim4Life.
1.5 Gradient Coil Design
Modern gradient coil design can be regarded as an optimization problem that seeks the optimal current distribution on a pre-defined design surface that produces a target field distribution while subjected to a set of optimization constraints [12, 13]. The current density can be decomposed into basis functions with unknown coefficients.
and the interested physical quantities such as magnetic energy and the Bz field in FOV can be expressed as quadratic form or linear form of the unknown coefficients, respectively
where \( \mathcal{L}=\left[{\mathcal{L}}_{ij}\right] \), \( {\mathcal{B}}_z=\left[{\mathcal{B}}_{zj}\right] \) and
for i, j = 1, …, n. Finally, the problem usually could be solved by a quadratic programming algorithm:
To find: c = [c1, c2, …, cn]T
Minimizing: \( \frac{1}{2}{c}^T\mathcal{L}\kern0.28em c \) + other quadratic forms of the unknown coefficients…
Gradient constraint: \( {G}_xx-\varepsilon \le {\mathcal{B}}_zc\le {G}_xx+\varepsilon \) for each point in X coil FOV, ε is the tolerance.
Other constraints such as force, torque, eddy current and fringe field having linear form of the unknown coefficients can also be included.
2 Methods
2.1 Augmentation of Nerves in Yoon-Sun and Duke Models
We used Yoon-sun female model v4.0b03 and Duke male model v3.1b01 from IT’IS foundation in this study. The Yoon-sun model is reconstructed from high-resolution cryosection images and it has a relatively complete nerve trajectory atlas for further neurodynamic simulation while the Duke model is based on MRI data of a volunteer without any nerve trajectories. In our previous study of PNS response for MAGNUS [14], we found that the calculated PNS thresholds for the X and Y coils are higher than those in the measured data. We hypothesized that this was because the existing nerve trajectories in the head of the Yoon-sun model are located mainly in the deeper recesses of the skull, which are in the low gradient field regions. The Yoon-sun model lacks extracranial nerves that are located in the high gradient field region.
To address this, we added extracranial nerves and superficial nerves in the neck and shoulder region to both Yoon-sun and Duke based on our knowledge of the high field area of the head gradient coils and the anatomy of the body (Fig. 3). The reason we only added a portion of the nerves is that the human body and the X-coil are mainly left-right symmetric, and it is time-consuming to add anatomically realistic nerve trajectories into the existing human body model. Table 2 summarizes the nerve branches that have been added to the human body model. The nerve branches selected in Duke differ slightly from Yoon-sun based on our early calculations on the latter by omitting nerves that have a low likelihood of being stimulated.
2.2 Non-folded and Folded Gradient Coil Design
We used the gradient specifications (Table 3) from Tang’s paper [12] to design three different gradient coils for this study (Fig. 4). Coil A is a non-folded design, in which the primary layer is separated from the shield layer. The second design is folded at the patient end, allowing more turns close to FOV which makes it more efficient. However, the wire turns that are close to the FOV are also close to the human body, which increases the E-field exposure. The third coil is also folded, but the coil turn number in the primary/shield connecting region is reduced to about half of the second coil. The idea is to seek a balance between the first and second design, and to examine how the wire pattern strategy affects the PNS in the human body.
It needs to be noted that PNS constraints were not applied in the gradient coil optimization process in this study. Relatively mature algorithms have been developed during recent years and an interested audience could read Davids’ work [15] for further information. In this work, however, we mainly focus on the mechanism of how the wire pattern changes can affect the E-field, and thus, PNS.
3 Results and Discussions
3.1 Model Verification on MAGNUS and HG2 X Coils
The simulated PNS threshold by MAGNUS and HG2 X coils are within the observed range and have shown much better agreement with the measurement than before (Fig. 5a) [14]. Figure 6 shows the most sensitive places for PNS in Yoon-sun and Duke. Overall, MAGNUS and HG2 X coils have very similar PNS performances. PNS was observed to be strongest at the nasion/glabella region and also occurs with high probabilities in regions close to the eye, forehead and cheeks [16, 17]. These places are obviously different from those in [14] since there were no such nerve trajectories in the original Yoon-sun models. PNS threshold is lower in Duke than in Yoon-sun, mainly because the size of Duke is larger than Yoon-sun, and as such, the nerves in Duke can extend to higher E-field regions than in Yoon-sun. All these results are consistent with observations from volunteer scans that were reported in our previous work [16].
We also observed that simulated thresholds are lower than the measurements. It is probably because, in simulations, the action potentials are initiated mostly at the ends of the nerve trajectories, which seem to be induced by the rather abrupt termination of the nerve trajectories that is unreal. In reality, these nerves may extend longer and become narrower in diameter, having telodendria or dendrites at the ends. We have reported this effect in our previous study [14], but for comparison purposes, the simulation result is still close to measurement.
3.2 Bz and ∣E∣ Field in Free Space and Human Body for Folded/Non-folded X Coils
The Bz and E-field distributions in free space in the three gradient coil designs are plotted in Fig. 7. While the B-field will be largely unperturbed by the presence of a human body model, the E-field distribution could be very different when a body model is introduced into the simulations. It could be seen that for the three designs, although the Bz fields are similar in FOV, the E-field in free space and the human body could be different. Coil A generates a relatively large E-field in the head region, while coil B results in a larger E-field in the neck and shoulders. Coil C lowers the E-field in the body from coil B by limiting the coil turns in the fold region. We should also note here that due to interference of the shoulder of Yoon-sun and the gradient coil, Yoon-sun has to be shifted 4 cm inferior to Duke in these three coils. This is another reason why augmentation of nerves was introduced into both Yoon-sun and Duke models.
3.3 PNS Simulation Results for Folded/Non-folded X Coils
While Figure 8 provides the E-field intensity, Figure 9 shows the most PNS-sensitive locations in Yoon-sun and Duke in the three newly designed gradient coils. For Coil A, the action potentials are initiated mostly in the forehead region in Yoon-sun. For the same coil, the action potentials are formed in both the forehead and glabellar regions in Duke. For coil B, the strongest PNS is observed in the neck or shoulder region. For Coil C, the most PNS-sensitive regions have a situation between coil A and coil B. Coil C yields the highest threshold so the lowest risk in the Yoon-sun model but has similar performance to coil B for Duke. For coil C, although the E-field in the body is higher than the E-field in the head (Fig. 8), the PNS threshold in the body is not necessarily lower. This is because, from the nerve cable equation, it is the first order difference of E-field intensity along the nerve that determines PNS, not the E-field intensity itself. But overall, PNS-sensitive locations are correlated to the high field region of the gradient coil. The difference in PNS results between the models can be explained by the difference in the size of the subjects and their relative positions in the coil. Figure 5b summarizes the PNS thresholds of the three coils.
3.4 E-Field Streamlines
We recommend using E-field streamline plot to further reveal the relationship between the gradient coil wire pattern and PNS as what is shown in Fig. 10. E-field follows the eddy current \( \overset{\rightharpoonup }{J} \) in the human body since \( \overset{\rightharpoonup }{J}=\sigma \overset{\rightharpoonup }{E} \), so that using E-field streamline is a better way to represent how the eddy current flows in the human body than E-field intensity map. It can be seen that eddy current should follow the wire pattern of the gradient coil and is also affected by the geometry and tissue properties of the human body.
For Coil A, since most of the conductor turns are at the upper head region, higher intensity E-fields and thus eddy currents, will flow through this region. On the other hand, since Coil B has many wire turns in the lower part of the coil, the eddy current loops concentrate on the neck and shoulders. This also leads to reduced eddy currents in the forehead region. Coil C yields a situation in between Coils A and B. It is clear that the E-fields that form the eddy current determine the PNS. Thus, to design the gradient coil with PNS constraints, one would seek to shape the eddy current paths in the human body to avoid producing high E-fields (driving function) in regions where peripheral nerves are located. While previous studies propose algorithms to constrain PNS in the gradient coil design process, here, we provide a more intuitive explanation of how they work.
3.5 Concomitant Field
Conventionally, only Bz is controlled in gradient coil design since only Bz is involved in image encoding. Previous studies [18, 19] showed it is possible to reduce the PNS risk by manipulating the concomitant field of the gradient coil. The concomitant field exists because the magnetic field is harmonic at places of no source, and it is impossible to obtain a linearly changing Bz without involving changing Bx and/or By [20]. Figure 11 shows the concomitant Bx fields together with the Bz fields for the three X coils. We can see that while Bz fields of these coils are identical in FOV, the Bx fields are different. The correlation between \( \mid \overset{\rightharpoonup }{E}\mid \) and \( \mid \overset{\rightharpoonup }{B}\mid \) can again be roughly explained by Eq. (1) and be verified with Figs. 11 and 12. \( \mid \overset{\rightharpoonup }{E}\mid \) field can be further manipulated by involving an additional uniform field coil [19] since essentially G is used in MR imaging process. Basically, the concomitant field can be changed by the changing of the wire pattern, as the same as that the eddy current loop in the human body can be changed by the wire pattern. But it should be noted that directly controlling the concomitant field does not involve the information of the human body, which includes the geometry and the tissue properties.
Free space \( \mid \overset{\rightharpoonup }{B}\mid \) field of three folded and non-folded coils in XZ plane (SR scaled to 10 mT/m). Coil A provides relatively higher \( \mid \overset{\rightharpoonup }{B}\mid \) values in the upper FOV region and Coil B and Coil C provide relatively higher \( \mid \overset{\rightharpoonup }{B}\mid \) values in the lower part (neck and shoulder region)
3.6 Effects of Homogeneous/Simplified Tissue Properties
The homogeneous human body model is attractive because it may simplify numerical computations and enable the utilization of the boundary element method (BEM), which might be faster than the FEM. But BEM only has a computational advantage when the boundaries or numbers of tissue types are relatively small. In the IEC 60601-2-33 standard, it has been recommended to use a homogeneous cylinder for a rough estimation of the \( \mid \overset{\rightharpoonup }{E}\mid \) field. Although 0.2 S/m is mentioned in this standard, which is roughly the average electrical conductivity in the human body, the E-field will not be affected by any electric conductivity value for the homogeneous model. Another situation is to use the human body model with as few tissue properties as possible. This is interested because segmentations for different tissues are very time expensive. The creation and use of subject-specific human body models for accurate PNS estimation in high-performance gradient systems are gaining more attention, which parallels the need for similar models for local specific absorption rate (SAR) estimation in ultra-high field MRI. The original Yoon-sun and Duke model are composed of over 70 tissues of different electrical conductivities. We conducted several simulation cases to study the impact on PNS results by simplifying the tissues, including (a) a homogenous human body model; (b) a model with only 3 tissue properties (fat, muscle and bone, all the other tissues are treated as muscle); (c) a model with 6 tissue properties (lung, liver and skin are further distinguished according to case (b)) and (d) a model with 8 tissue properties (averaged electric conductivities of tissues inside of the skull and tissues inside of the belly are further added in simulation to represent tissue inside of skull and tissue inside of belly, respectively).
The PNS responses of simplified tissue models are compared with the original heterogeneous Yoon-sun model on MAGNUS X and Z coils. It is noted that it is beneficial to retain accurate tissue model properties for tissues in closest proximity to nerve tissue. Tissues that are far away from the nerves can be assigned tissue properties that are averaged across the local tissue types. This is a compromise that reduces computation time while increasing the likelihood that the \( \overset{\rightharpoonup }{E} \) field distribution in closest proximity to nerve tissues conform closely to that in a fully segmented human body model. Table 4 lists the conductivities used in the simplified models. To compare the PNS response from different cases, we first convert the PNS response lines of different nerve trajectories in τ − ∆G plane to be points in ΔGmin − SR plane. Then we examine how the responses of the nerves shifted in the ΔGmin − SR plane due to the property simplification. We can calculate the pseudo-distance between the corresponding points to quantify the difference but only the most sensitive nerves (for example 20) matter. We can also count how many trajectories remain in the list of the original 20 most sensitive nerve trajectories. As an example (Fig. 13 and Table 5), for the 8-tissue model, stimulated by MAGNUS X coil, the averaged distance is 13.61 for the 20 most sensitive nerves and 20 of them are remained as the most sensitive nerves in Yoon-sun, while these numbers for the homogenous model become 48.62 and 16, respectively. Table 5 summarizes all the cases performed for the Yoon-sun model. It shows that compared to the homogenous model, the 3-tissue model provides a closer result to the original heterogeneous model and that the 6-tissue model is even closer to the latter. The 8-tissue model is a little better than the 6-tissue one because the averaged electric conductivity for tissues in the skull and in the belly is close to that of the muscle. Another observation is that fewer tissue properties are needed for the MAGNUS X coil than for the Z coil. This is because there are inherently fewer types of tissue outside of the skull than in the body. Overall, the result shows that the homogenous model could be used to give a rough estimation on the E-field but might be not accurate enough to estimate the PNS. Six tissue properties or more might provide a better estimation.
4 Shortcomings and Future Works
There are two shortcomings in this study. The first is that only a portion of the nerves has been reconstructed, limiting PNS analysis for X Coils. More nerves are needed to enable full X/Y/Z coil design and analysis. Moreover, the nerve trajectories’ geometry might not be perfectly accurate anatomically since a detailed image-derived nerve model was not available. However, compared to the lack of nerve trajectories in the interested places in the original body models, these added trajectories provide richer information to capture PNS responses for gradient coil analysis and design. A similar situation has been reported in [17], where virtual human bodies models are used. The second shortcoming is that the PNS constraint is not yet integrated into the gradient coil design in this study. It is preferred to include this constraint in the future.
5 Conclusions
In this work, extracranial nerves and upper body superficial nerves have been added into both Yoon-sun and Duke models. PNS analysis for gradient coil in the human body models can give results of reasonable accuracy with a relative complete nerve trajectory atlas in the places of interest. Three folded and non-folded head gradient coils of different wire patterns have been designed to reveal the relationship between the E-field/eddy current flow in human body and PNS. E-field streamline plots inside of the human body can provide more information than intensity projection plots. To manipulate PNS through concomitant field generally is to tune the E-field distribution through changing the B-field distribution but does not include the information of the human body. A homogenous model is not accurate enough for PNS threshold estimation, instead, six or more tissues are needed for simplified tissue models.
References
T.C. Cosmus, M. Parizh, Advances in whole-body MRI magnets. IEEE Trans. Appl. Supercond. 21(3), 2104–2109 (2011). https://doi.org/10.1109/TASC.2010.2084981
J. Jin, Electromagnetic Analysis and Design in Magnetic Resonance Imaging, 1st edn. (CRC Press, Boca Raton, 1998)
R.W. Brown, Y.-C.N. Cheng, E.M. Haacke, M.R. Thompson, R. Venkatesan, Magnetic Resonance Imaging: Physical Principles and Sequence Design, 2nd edn. (Wiley-Blackwell, Hoboken, 2014)
F. Liu, H. Zhao, S. Crozier, On the induced electric field gradients in the human body for magnetic stimulation by gradient coils in MRI. IEEE Trans. Biomed. Eng. 50(7), 804–815 (2003). https://doi.org/10.1109/TBME.2003.813538
W. Irnich, F. Schmitt, Magnetostimulation in MRI. Magn. Reson. Med. 33(5), 619–623 (1995). https://doi.org/10.1002/mrm.1910330506
S.S. Hidalgo-Tobon, Theory of gradient coil design methods for magnetic resonance imaging. Concepts Magn. Reson. 36A(4), 223–242 (2010). https://doi.org/10.1002/cmr.a.20163
T.K.F. Foo et al., Lightweight, compact, and high-performance 3 T MR system for imaging the brain and extremities. Magn. Reson. Med. 80(5), 2232–2245 (2018). https://doi.org/10.1002/mrm.27175
T.K.F. Foo et al., Highly efficient head-only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magn. Reson. Med. 83(6), 2356–2369 (2020). https://doi.org/10.1002/mrm.28087
B.A. Chronik, B.K. Rutt, Simple linear formulation for magnetostimulation specific to MRI gradient coils. Magn. Reson. Med. 45(5), 916–919 (2001). https://doi.org/10.1002/mrm.1121
C.C. McIntyre, A.G. Richardson, W.M. Grill, Modeling the excitability of mammalian nerve fibers: influence of Afterpotentials on the recovery cycle. J. Neurophysiol. 87(2), 995–1006 (2002). https://doi.org/10.1152/jn.00353.2001
D.R. McNeal, Analysis of a model for excitation of myelinated nerve. IEEE Trans. Biomed. Eng. BME-23(4), 329–337 (1976). https://doi.org/10.1109/TBME.1976.324593
F. Tang et al., An improved asymmetric gradient coil design for high-resolution MRI head imaging. Phys. Med. Biol. 61(24), 8875–8889 (2016). https://doi.org/10.1088/1361-6560/61/24/8875
H.S. Lopez, L. Feng, M. Poole, S. Crozier, Equivalent magnetization current method applied to the design of gradient coils for magnetic resonance imaging. IEEE Trans. Magn. 45(2), 767–775 (2009). https://doi.org/10.1109/TMAG.2008.2010053
Y. Hua, D.T.B. Yeo, T.K. Foo, PNS Estimation of a High Performance Head Gradient Coil by a Coupled Electromagnetic Neurodynamic Simulation Method, in 2020 50th European Microwave Conference (EuMC), Jan. 2021, pp. 1071–1074. https://doi.org/10.23919/EuMC48046.2021.9338172
M. Davids, B. Guérin, V. Klein, L.L. Wald, Optimization of MRI gradient coils with explicit peripheral nerve stimulation constraints. IEEE Trans. Med. Imaging 40(1), 129–142 (2021). https://doi.org/10.1109/TMI.2020.3023329
E.T. Tan et al., Peripheral nerve stimulation limits of a high amplitude and slew rate magnetic field gradient coil for neuroimaging. Magn. Reson. Med. 83(1), 352–366 (2020). https://doi.org/10.1002/mrm.27909
M. Davids, B. Guérin, A. vom Endt, L.R. Schad, L.L. Wald, Prediction of peripheral nerve stimulation thresholds of MRI gradient coils using coupled electromagnetic and neurodynamic simulations. Magn. Reson. Med. 81(1), 686–701 (2019). https://doi.org/10.1002/mrm.27382
P.B. Roemer, B.K. Rutt, Minimum electric-field gradient coil design: theoretical limits and practical guidelines. Magn. Reson. Med. 86(1), 569–580 (2021). https://doi.org/10.1002/mrm.28681
S.S. Hidalgo-Tobon, M. Bencsik, R. Bowtell, Reducing peripheral nerve stimulation due to gradient switching using an additional uniform field coil. Magn. Reson. Med. 66(5), 1498–1509 (2011). https://doi.org/10.1002/mrm.22926
M.A. Bernstein, K.F. King, Z.J. Zhou, Handbook of MRI Pulse Sequences (Academic, Amsterdam/Boston, 2004)
Acknowledgements
This work was funded in part by CDMRP W81XWH-16-2-0054.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Hua, Y., Yeo, D.T.B., Foo, T.K.F. (2023). Peripheral Nerve Stimulation (PNS) Analysis of MRI Head Gradient Coils with Human Body Models. In: Makarov, S., Noetscher, G., Nummenmaa, A. (eds) Brain and Human Body Modelling 2021. Springer, Cham. https://doi.org/10.1007/978-3-031-15451-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-15451-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15450-8
Online ISBN: 978-3-031-15451-5
eBook Packages: EngineeringEngineering (R0)