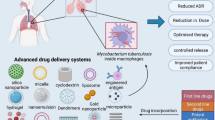
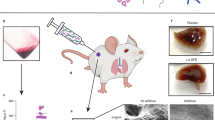
Abstract
Tuberculosis (TB) is a communicable disease caused by the bacterium Mycobacterium tuberculosis which is the second most lethal infectious disease after AIDS. The global problem of multidrug-resistant tuberculosis is nearing epidemic proportions, and it is a leading killer of young adults worldwide. It is endemic in the majority of developing countries and has resurfaced in both developed and emerging countries with high rates of HIV infection. This chapter reviews the clinical trials and drug delivery approaches for tuberculosis. A number of innovative implant, microparticulate, and other carrier-based drug delivery systems containing the main anti-tuberculosis medicines have been developed with the goal of improving patient outcomes by either targeting the location of tuberculosis infection or reducing dose frequency. To fully realize the promise of drug development, it will take creativity, perseverance, collaboration, and resources. A delicate balance must be struck between preserving medications from developing resistance and ensuring that regimens are low cost, readily available, and widely accepted by healthcare systems and clinicians.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. In: Lancet. Elsevier; 2003. p. 887–99.
Zumla A, Chakaya J, Centis R, D’Ambrosio L, Mwaba P, Bates M, et al. Tuberculosis treatment and management-an update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respiratory Med Elsevier. 2015;3:220–34.
Chaisson RE, Schecter GF, Theuer CP, Rutherford GW, Echenberg DF, Hopewell PC. Tuberculosis in patients with the acquired immunodeficiency syndrome. Clinical features, response to therapy, and survival. Am Rev Respir Dis. 1987;136(3):570–4.
Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10(6):605–12.
Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9(3):153–61.
Ehlers LP, Bianchi MV, Argenta FF, Lopes BC, Taunde PA, Wagner PGC, et al. Mycobacterium tuberculosis var. tuberculosis infection in two captive black capuchins (Sapajus nigritus) in Southern Brazil. Braz J Microbiol. 2020;51(4):2169–73.
Ruge CC, Kirch J, Lehr CM. Pulmonary drug delivery: from generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. Lancet Respiratory Med Elsevier. 2013;1:402–13.
Zeng XM, Martin GP, Marriott C. The controlled delivery of drugs to the lung. Int J Pharm. 1995;124:149–64.
Kellaway IW, Farr SJ. Liposomes as drug delivery systems to the lung. Adv Drug Deliv Rev. 1990;5:149–61.
Couvreur P, Fattal E, Andremont A. Liposomes and nanoparticles in the treatment of intracellular bacterial infections. Off J Am Assoc Pharm Sci Pharm Res. 1991;8:1079–86.
Heurtault B, Saulnier P, Pech B, Proust JE, Benoit JP. A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharm Res. 2002;19(6):875–80.
Byron PR. Prediction of drug residence times in regions of the human respiratory tract following aerosol inhalation. J Pharm Sci. 1986;75(5):433–8.
Hoet PHM, Brüske-Hohlfeld I, Salata OV. Nanoparticles – known and unknown health risks. J Nanobiotechnol BioMed Central. 2004;2:12.
Gref R, Domb A, Quellec P, Blunk T, Müller RH, Verbavatz JM, et al. The controlled intravenous delivery of drugs using PEG-coated sterically stabilized nanospheres. Adv Drug Deliv Rev Elsevier. 1995;16:215–33.
Lasic DD. On the thermodynamic stability of liposomes. J Colloid Interf Sci. 1990;140:302–4.
Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172(1–2):33–70.
Zeng XM, Martin GP, Marriott C. The controlled delivery of drugs to the lung. Int J Pharm. 1995;124(2):149–64.
Illum L, Davis SS, Müller RH, Mak E, West P. The organ distribution and circulation time of intravenously injected colloidal carriers sterically stabilized with a blockcopolymer – poloxamine 908. Life Sci. 1987;40(4):367–74.
Falzon D, Jaramillo E, Schünemann HJ, Arentz M, Bauer M, Bayona J, et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38(3):516–28.
Dorman SE, Johnson JL, Goldberg S, Muzanye G, Padayatchi N, Bozeman L, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009;180(3):273–80.
Ahmad Z, Tyagi S, Minkowski A, Peloquin CA, Grosset JH, Nuermberger EL. Contribution of moxifloxacin or levofloxacin in second-line regimens with or without continuation of pyrazinamide in murine tuberculosis. Am J Respir Crit Care Med. 2013;188(1):97–102.
Migliori GB, Langendam MW, D’Ambrosio L, Centis R, Blasi F, Huitric E, et al. Protecting the tuberculosis drug pipeline: stating the case for the rational use of fluoroquinolones. Eur Respiratory J Eur Respiratory Soc. 2012;40:814–22.
Benator D, Bhattacharya M, Bozeman L, Burman W, Catanzaro A, Chaisson R, et al. Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet. 2002;360(9332):528–34.
Dorman SE, Goldberg S, Stout JE, Muzanyi G, Johnson JL, Weiner M, et al. Substitution of Rifapentine for Rifampin during intensive phase treatment of pulmonary tuberculosis: study 29 of the tuberculosis trials consortium. J Infect Dis. 2012;206(7):1030–40.
Mitchison DA. Pharmacokinetic/pharmacodynamic parameters and the choice of high-dosage rifamycins. Int J Tuberc Lung Dis. 2012;16(9):1186–9.
Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. Three months of Rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365(23):2155–66.
Wallis RS, Jakubiec W, Kumar V, Bedarida G, Silvia A, Paige D, et al. Biomarker-assisted dose selection for safety and efficacy in early development of PNU-100480 for tuberculosis. Antimicrob Agents Chemother. 2011;55(2):567–74.
Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, et al. The Diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360(23):2397–405.
Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3(11):2131–44.
Dawson R, Diacon A. PA-824, moxifloxacin and pyrazinamide combination therapy for tuberculosis. Expert Opin Investig Drugs. 2013;22(7):927–32.
Dey T, Brigden G, Cox H, Shubber Z, Cooke G, Ford N. Outcomes of clofazimine for the treatment of drug-resistant tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2013;68(2):284–93.
Van Deun A, Maug AKJ, Salim MAH, Das PK, Sarker MR, Daru P, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182(5):684–92.
Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE, Blanchard JS. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science (80-). 2009;323(5918):1215–8.
Sacksteder KA, Protopopova M, Barry CE, Andries K, Nacy CA. Discovery and development of SQ109: a new antitubercular drug with a novel mechanism of action. Future Microbiol NIH Public Access. 2012;7:823–37.
Pasca MR, Degiacomi G, Lopes Ribeiro ALDJ, Zara F, De Mori P, Heym B, et al. Clinical isolates of Mycobacterium tuberculosis in four European hospitals are uniformly susceptible to benzothiazinones. Antimicrob Agents Chemother. 2010;54(4):1616–8.
Lechartier B, Hartkoorn RC, Cole ST. In vitro combination studies of benzothiazinone lead compound BTZ043 against mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56(11):5790–3.
Johnson JL, Hadad DJ, Dietze R, Maciel ELN, Sewali B, Gitta P, et al. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. Am J Respir Crit Care Med. 2009;180(6):558–63.
Merle CSC, Sismanidis C, Sow OB, Gninafon M, Horton J, Lapujade O, et al. A pivotal registration phase III, multicenter, randomized tuberculosis controlled trial: design issues and lessons learnt from the Gatifloxacin for TB (OFLOTUB) project. Trials. 2012;61:13.
Blomberg B, Spinaci S, Fourie B, Laing R. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis. Bull World Health Organ. 2001;79:61–8.
Albanna AS, Smith BM, Cowan D, Menzies D. Fixed-dose combination antituberculosis therapy: a systematic review and meta-analysis. Eur Respir J. 2013;42(3):721–32.
Nagelkerke NJD, De Vlas SJ, Mahendradhata Y, Ottenhoff THM, Borgdorff M. The search for a tuberculosis vaccine: an elusive quest? Tuberculosis. 2006;86(1):41–6.
Conflicts of interest
Authors declares that there is no any competing interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alom, S., Ali, F., Choudhury, D., Gohain, A., Ahmed, A.B., Zaman, M.K. (2023). Tuberculosis and Drug Delivery System: Clinical Trials in TB. In: Shegokar, R., Pathak, Y. (eds) Tubercular Drug Delivery Systems. Springer, Cham. https://doi.org/10.1007/978-3-031-14100-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-14100-3_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-14099-0
Online ISBN: 978-3-031-14100-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)