Abstract
Erectile dysfunction (ED) is defined as the failure to achieve and/or maintain a penile erection that is satisfactory for sexual intercourse.
The implantation of a penile prosthesis may be considered in patients who are not suitable for different pharmacotherapies and do not respond to the first and the second line of medical treatment.
Intraoperative complications include hematoma formation, floppy glans, perforation of the corpora cavernosa, and urethral injury. Bladder, vascular, and bowel injury are the most dangerous events and should be prevented. Postoperative complications are represented by infections, erosions, and glandular ischemia.
There is an increasing interest in the hypothesis of “regenerative” cures for ED aimed at decreasing fibrosis of the corpora cavernosa and rebuilding their normal biological architecture. These new regenerative treatments include stem cell injections, platelet-rich plasma, and low-intensity shock wave therapy.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Erectile Dysfunction: Surgical Therapy
5.1.1 Introduction
Erectile dysfunction (ED) is defined as the failure to achieve and/or maintain a penile erection that is satisfactory for sexual intercourse [1]. It is postulated that more than 40% of men between the ages of 40 and 80 could suffer from different grades of ED [2]. The causes of ED are numerous but only in very few cases, ED is truly curable, such as the psychogenic one. In most cases, ED is only treatable [2].
Regardless of the cause, chronic ED is characterized by anatomical and functional alterations in the erectile cavernous tissue characterized by fibrosis [3]. Historically, the concept of penile fibrosis has been entirely linked to Peyronie’s disease and urethral stricture [4]. On the other hand, corpus cavernosum (CC) fibrosis was considered a rare disorder that was only seen after penile fracture or after prolonged erection [5].
However, recently, several studies have demonstrated that CC fibrosis is a common pathological sign underlying most cases of vasculogenic and/or neurogenic ED. The penile erection is regulated by a complex mechanism that involves the synergy of the nitrergic and adrenergic neuronal system, endothelium, and smooth muscle cells of the CC. Pathological disorders affecting one or more of these elements could cause CC fibrosis [6]. An impaired elasticity of the CC due to fibrosis leads to a diminished filling of the sinusoids and inadequate compression of the subtunical venules. This lack of compression will result in blood leaking out of the CC during an erection, which makes the penis incapable to become entirely erected.
The ED treatment has been standard for many years, and it was characterized by a limited range of therapeutic agents. First-level approach consists of lifestyle modification followed by medical therapy with phosphodiesterase-5 (PDE5i) inhibitors. For refractory patients, or those with intolerable side effects, European guidelines [7] suggest second- and third-level treatments such as vacuum devices, self-administered intracavernous injection of erectogenic substances, intraurethral creams, and placement of penile prostheses [8].
5.1.2 History of Penile Prosthesis
The world of penile prosthesis was born more than a hundred years ago, and the actual devices are the ultimate evolution of the earliest systems. The first mechanism similar to a penile prosthesis was created in 1930s by Bogoras, who implanted rib cartilage into an abdominal tube pedicle graft during war [9]. This technique evolved during years, but the material was not considered ideal, due to its firmness and its high risk of extrusion and reabsorption. In 1952, Goodwin implanted the first non-autologous device, made of an acrylic rob outside the corpora cavernosa, then replaced by the first silicone implants by Beheri in 1966, who used polyethylene rob into the corpora cavernosa [9, 10]. The modern era of penile prosthesis started in 1974 at the AUA Meeting, where Carrion proposed the silicone gel-filled penile implant, inspired by silicone gel-filled breast implants, with excellent outcomes: this was the ancestor of the actual semi-rigid prosthesis [9]. In 1970s, Scott, Timm, and Bradley laid the foundations of the first inflatable penile prosthesis, utilizing a fluid-based system to inflate an expandable cylinder in the corpora cavernosa [11]. Many adjustments have been made during the last 40 years, up until the current models, which remain recognizable from the original prototype.
5.1.3 Penile Prosthesis Implant
The implantation of a penile prosthesis may be considered in patients who are not suitable for different pharmacotherapies and do not respond to the first and the second line of treatment, that are pharmacological therapies [7]. An appropriate patient selection and preoperative counselling are required to achieve solid outcomes.
5.1.4 Types of Devices and Differences
Penile prosthesis can be divided in two main types: non-inflatable or malleable and inflatable (Table 5.1).
Non-inflatable or malleable penile prosthesis consists of a pair of rods made of spiral wire or silicone material, wrapped in fabric, like silicone (Fig. 5.1). There have been many features, that improved these kinds of devices: articulated segments, held by a central spring, providing a positional memory, and allowing the rods to remain hidden when not in use; a hydrophilic coating, which allows the choice of any antibiotic as a device preparation [12]. Malleable devices require less manual dexterity by the patient, given their malleable nature, they are less prone to malfunction; they are permanently firm and have lower overall satisfaction, also having a higher risk of erosion and chronic pain [13].
Inflatable devices consist of a pair of cylinders implanted in the corpora cavernosa and connected to a pump: when the pump is squeezed and released multiple times, the cylinders are filled with normal sterile saline, stimulating the corpora blood filing during erection. They can be distinguished into one-piece, two-piece, and three-piece implants, based on the dimension and the site of the reservoir. The single-piece penile prosthesis has a small reservoir in the end of each cylinder, that allows the transition of a small volume of fluid into a central core [12]. These prostheses have been shown inferior to two- and three-piece inflatable prostheses, due to their poor mechanical reliability and patients’ satisfaction rates [14].
The two-piece system consists of corporal cylinders and a pump-reservoir. The pump transfers fluid from the small reservoir in the proximal portion of the cylinders to the inflatable distal portion of them, causing an erection. With the lack of a distinct reservoir, the two-piece devices do not permit complete deflation of the penile cylinders. The main indications for the two-piece device is represented by patients with little dexterity, since its easier mechanism of inflation and deflation; prior abdominal or pelvic surgery, due to obliteration of the Retzius space; pelvic organ transplantation recipients, since the absence of the reservoir; female-to-male transgender patients, due to absence of the reservoir, although its more difficult pump mechanism [12, 15].
The three-piece system (Fig. 5.2), instead, consists of corporal cylinders, a scrotal pump, and a separate reservoir, placed in abdomen, which allows the patient to press the button once, then squeezing the cylinders in one single time, making the deflation easier. Many improvement have been made in many three-piece models: a three-layered fabric was introduced, to reduce the cylinder aneurism formation and mechanical failure; an additional coating to the surface of the silicone to increase the lubricity of silicone itself; a lock-out valve has been incorporated into the pump to prevent auto-inflation of the cylinders in case of sudden high pressure within the reservoir; permanent antibiotic elution device, that is an antibiotic formulation impregnated onto the external surface of prosthesis; hydrophilic coating, which reduces bacterial attachments and binds strongly the antibiotic with a low rate of infection; the momentary-squeeze pump, which allows the deflation only pressing once the button with a quick squeeze, avoiding the patient to hold continuingly the deflation button. The most common device implanted in penile surgery is the three-piece IPP, since its mechanical reliability and very high satisfaction rates. This represents the best option in case of Peyronie’s disease, since its greater rigidity; in patients with severe corporal fibrosis, due to the little elasticity of tissues; in case of long narrow penises, because of decreased axial support [12, 13, 16, 17].
5.1.5 Surgical Approach
There are many different techniques of penile prosthesis, but the most used approaches are mainly three as follows: the infrapubic approach, first applied by Scott in 1972, the penoscrotal approach, first described by Barry and Scott in 1979, and the subcoronal approach, popularized by Egidio in 2016 [18].
All the procedures start with accurate disinfection of the field and bladder drainage inserting a 16 Fr Foley urethral catheter (Video 5.1). The skin incision represents the next step: in the penoscrotal approach, after positioning a Scott ring retractor, a longitudinal skin incision (about 3–4 cm) at the penoscrotal junction is made (Video 5.2), exposing and then incising the dartos, placing six hooks for retraction, until the tunica albuginea appears and the dartos is incised; while in the infrapubic approach, an infrapubic 2 cm skin incision is made about 1 cm above the penopubic junction; in case of the subcoronal technique, a distal sub-coronal “circumcision” incision is made 2 cm proximal to the coronal sulcus of the glans, then degloving the penis to the level of the penoscrotal junction, placing silk sutures on the everted dartos (at 3, 6, 9, and 12 o’clock).
Corporotomy may now start: in the penoscrotal technique, four absorbable Vicryl 2.0 stay sutures are placed in both corpora cavernosa and a 2 cm longitudinal tunical incision is made in each corpus between the stay sutures (Video 5.3); during the infrapubic approach, the Scarpa fascia is opened with the guidance of the finger and, once isolated, the two corpora cavernosa, the bilateral corporotomies (1.5 cm each) are made, after positioning, two stay stitches on each corpus, that must be not too large preventing their bunching up during the tunica closing; in the subcoronal technique stay, sutures are placed proximal to the penoscrotal junction, taking care that the corporotomy will be proximal enough, then a corporotomy is made between the stay suture.
Thereafter, dilatators are then used to create the intracorporeal space and a Furlow insertion tool is used to measure the corpora length to choose the cylinder size (Video 5.4). At this point, irrigation of the site with rifampicin solution is demanded to prevent infections and to check for any urethral injury. Measurements of each corpus are performed both proximal and distal, and a rear-tip extender (RTE) is selected, according to the difference between the proximal and distal measurements.
After cycling and plumping the cylinders with saline solution to remove air bubbles and positioning the chosen RTE on both cylinders, one cylinder at time is placed in the corpora cavernosa with the guide of the insertion instrument laterally in the corpora to avoid urethral injury, placing it proximally and then distally. In case of subcoronal approach, the proximal end of the prosthesis is placed into the corpora, proximal to the penoscrotal junction, and then the distal tips of the prosthesis are pulled through the remaining corpora to the mid glans. The corporotomy closure begins with 2.0 Vycril horizontal sutures.
The next step consists of the pump placement (Video 5.5): in the penoscrotal approach, the pump space is created in the scrotum between both testes with the deflation button anteriorly; during the infrapubic approach, the pump is positioned in a dartos pouch in the scrotum, then brought down; in the subcoronal technique, a scrotal pouch is created posterior to the testes for placement of the pump, then placing the pump easily into the scrotum without a nasal speculum.
The reservoir placement is an essential step during the penile prosthesis implant (Video 5.6). During the penoscrotal approach, the reservoir usually is positioned into the Retzius space with the index finger as a guidance and, once pierced the transversalis fascia, a space near the bladder is made. The reservoir space during the subcoronal approach is created in the same way. In case of previous pelvic surgery, such as radical prostatectomy, the scarring of the prevesical space and effective “peritonealization” of the bladder, traditional three-piece IPP reservoir placement blindly into the space of Retzius via the external inguinal ring can take to severe complications given the close proximity of bowel, bladder, and major vascular structures. The reservoir can so be positioned ectopically, that is, above the transversalis fascia and below the transversus abdominis muscle. Another location of the reservoir is the submuscular or high submuscular (HSM) position, posterior to the rectus muscle but anterior to the transversalis fascia which can be achieved through a penoscrotal incision via the external inguinal ring, eliminating the risk of intraperitoneal placement and associated bowel, bladder, or vascular complications. The HSM technique is characterized by the so-called five steps: the first one consists of the access to the external inguinal ring with the index through the penoscrotal incision and the transversalis fascia is earned; then the 2/3 of the HSM tunnel is created by manual dissection, while in the next step, the remaining portion of the tunnel is obtained with a curved sponge stick, lifting the fibres of the rectus muscle from the transversalis fascia. At this point, the deflated reservoir is delivered into the newly created pocket and then filled with 120 cc, to flatten the reservoir and ensure the space; so the excess of saline is removed and then connected to the other components. In case of an infrapubic approach, the reservoir is positioned in a paravesical space, obtained with a nasal speculum, which passes through the external inguinal ring and then across the fascia transversalis, after blunting dissecting the fat off the pubic rami. In patients with compromised pelvis, the speculum is advanced less distance into the ring and thrust upward into the space anterior to transversalis fascia.
The cylinders, pump, and reservoir are all interconnected with kink-resistant tubes, and inflation and deflation of the device is done multiple times for testing the correct functioning and positioning (Video 5.7). A closed suction drain is placed in the scrotum, in case of the penoscrotal approach. The wounds are closed in two layers, that is, dartos fascia and the skin in the PS and IF approach; in the subcoronal technique, the dartos is reapproximated at the level of the glans and the sub-coronal skin incision is closed [13, 19,20,21].
5.1.6 Comparison Between the Three Techniques
The three main penile prosthesis implant techniques have all important advantages, but also considerable critical issues. The penoscrotal approach presents a minimal risk of dorsal nerve injury, compared to the infrapubic approach, even if this one allows a shorter operative time, which leads to a reduced risk of infections. This could be explained by the fact that, compared to the PS surgery, several steps are omitted in the IP approach, such as in the IP technique, the dilatation and measurement of the corpora is performed in a single step. The pump placement in the scrotum is quite easier in the penoscrotal technique, since in the infrapubic procedure, a pouch must be created ex novo and great attention is needed during the positioning and the orientation of the activator of the pump itself: the patient, in fact, can find more difficult to manage the pump compared to the penoscrotal system. In particular in obese patients, this last technique allows a better corporal exposure, in order to obtain a correct corporotomy and an appropriate measurement of the length of each corpus, allowing a more precise cylinder choice and positioning, while patients with previous abdominal surgical procedures, for whom reservoir placement can be difficult, may better benefit with the IP approach. As previously described, the reservoir placement both in the infrapubic and in the subcoronal approach is characterized by a direct visualization, avoiding pelvic organs and vessels injury, compared to the penoscrotal approach, where this procedure is completely blind and requires high precision and dexterity by the surgeon. The infrapubic technique includes a skin incision that inevitably creates a less acceptable cosmetic result, since the penoscrotal incision remains quite hidden on the scrotum. Urethral injury represents an issue, in particular during the penoscrotal technique, during the dissection of the corpora cavernosa at the level of the penoscrotal junction anteriorly. The penoscrotal approach seems to be more indicated in case of corporal fibrosis, mainly secondary to prior implant removal due to infection, as it allows more complete access to the corpora proximally and distally. About subcoronal approach, excellent visibility of corpora cavernosa and urethra are guaranteed and additional surgical reconstructive procedure, such as Peyronie’s disease, can be easily performed; however, it requires more operative time, compared to the two other techniques, and the degloving of the penis may cause sensorial alteration or skin loss; it is also limited by the number of studies about the surgical approach and the follow [13, 18].
5.1.7 Complications
Penile prosthesis implant complications can be distinguished into intraoperative and postoperative.
5.1.7.1 Intraoperative Complications
About intraoperative complications, we may include first a hematoma formation, typically in the scrotum, which can be prevented using a compressive dressing and placing a drainage; floppy glans represents an issue, due to inadequate prosthetic cylinder sizing or positioning, causing insufficient venous compression between Buck’s fascia and the corpora cavernosa; it is usually adjusted with the normal healing and so it is quite easily resolved. A relevant complication is corporal fibrosis, defined as the replacement of smooth muscle cells with fibrotic tissue within the corporal bodies: it is common in diabetic patients and in ones with a history of ischemic priapism and usually requires increased effort during corporal dilatation, increasing though the likelihood of perforation and so requiring high levels of accuracy. Corporal crossover may occur mostly during corporal dilatation or cylinder placement: the contralateral cylinder perforation may be caused by the needle used in the ipsilateral cylinder placing; to prevent this, both the needles should be placed correctly before placing the cylinders and it is safer to start the corpora dilatation laterally and gradually. Another issue is represented by perforation of the corpora cavernosa: proximal perforation of the corpora can be detected as a sudden loss of resistance during dilatation, and it can be treated by inserting corporotomy sutures, preventing the proximal migration of the prosthesis; distal perforation represents a more serious issue due to the risk of urethral injury and it is safe to interrupt the procedure. The rate of urethral injury is very low (0.1–0.4%) and it may be avoided staying as lateral as possible during the dilatation of the corpora; it should be repaired with a catheter and the procedure rescheduled after 6 weeks at least.
Bladder, vascular, and bowel injury are the most dangerous events: bladder injury, evident because of blood in the catheter, should be prevented by fully emptying the bladder itself before reservoir placing; while in case of both vascular and bowel damage, the procedure must be stopped to consult the specialists (general surgeons) [13, 22].
5.1.7.2 Postoperative Complications
Infection represents a serious issue: its rate reaches 4% and the most common organism involved is Staphylococcus epidermidis, due to the contamination of the skin flora during the procedure; risk factors are long operative time, immunosuppressed or transplanted patients and diabetic population. The main tips to prevent infections are intraoperatively the genital region bathing with an antiseptic soap and the use of a chlorhexidine-alcohol skin preparation, while perioperatively the administration of intravenous antibiotics (vancomycin) starting an hour prior the operation and continuing up to 24 h, constant irrigation of the field with antibiotic solution and the no-skin touch technique during surgery. In case of infection, resistant to antibiotic therapy, the removal of the prosthesis must be considered. Impending erosion, instead, can start from distal lateral corpora, urethra and glans and its rate increases in case of intraoperative urethral damage and in patients with spinal cord injuries, because of the absolute need of the catheter, which can easy the erosion mechanism. Then, glandular ischemia can be considered a very rare complication, more likely in patients with CVD, diabetes, and history of smoking; it can lead to penile gangrene and it is induced in case of an interruption of the blood supply to the glans through the dorsal penile arteries [13, 22].
5.2 Regenerative Therapies for Erectile Dysfunction
5.2.1 Introduction
Over the last decades, there has been an increasing interest in the hypothesis of “regenerative” cures for ED aimed at decreasing fibrosis of the CC and rebuilding their normal biological architecture. These new regenerative treatments include stem cell injections, platelet-rich plasma, and low-intensity shock wave therapy (Li-SWT). There are numerous data obtained on animal models of ED that indicate that these methods can result in angiogenesis and reducing fibrosis, thus “restoring” dysfunctional CC tissue [23].
To date, there are limited clinical data to support regenerative therapies as a first-line treatment for ED. However, evidence is growing every year, and these regenerative therapies are becoming a realty in the ED treatment clinical scenario.
5.2.2 Li-SWT for Erectile Dysfunction
In the last 10 years, the use of LI-SWT has been increasingly offered as an alternative treatment for vasculogenic ED, being the only currently approved therapy that might provide a “cure,” which is the most wanted result for men affected by ED. LI-SWT has gotten recognition in the treatment of ED, based on the assumption that LI-SWT application may result in neoangiogenesis and thus increased blood flow to the corpora cavernosa. The usage of LI-SWT was for long time against the EAU guidelines; however, the last 2020 EAU guideline on sexual health [7] promoted LI-SWT as treatment for ED. EAU Guideline suggested to use LI-SWT in patients with mild vasculogenic ED or as an alternative first-line therapy in well-informed patients who do not wish or are not suitable for oral vasoactive therapy or desire a curable option. More importantly, a recent study has shown that an increased proportion of urologist had suggested a wider use of LISWT, and some even encouraged its application in neovasculogenic ED [7].
5.2.2.1 Mechanism of Action
A shockwave is defined as a high-pressure acoustic wave with the capacity to conduct energy and spread through a medium [24]. The waveform itself is characterized by a high peak pressure inducing a focal tissue compression followed by extension. This causes tissue injury which is postulated to induce a wound healing process activation characterized by neovascularization and activation of local stem cells. Another theory is the shockwaves can activate the neovascularization using a process called “mechanotransduction” [25], which is defined as a biochemical response to mechanical stimuli [26].
Several basic science reports showed LI-SWT improved levels of VEGF and endothelial nitric oxide synthase (eNOS), and that caveolin-1 and ß1-integrin, constitutive proteins of caveolae, which are invaginated organelles found in the plasma membrane and accountable for cell homing, are integral for LI-SWT-induced angiogenesis [25].
LI-SWT can promote neurogenesis through local mechanisms [27]. In a rat model of pelvic neurovascular injuries, a recent report proved that LI-SWT amended erectile function by penile nerve regeneration [28].
In conclusion, the mechanism of action at the base of LI-SWT regenerative effects on CC tissue is not totally comprehended but likely include angiogenesis and neurogenesis. Local activation and recruitment of stem cells may also play a role. Thus, from a theoretical point of view, LI-SWT has the potential to cure ED conversely to the other standard treatment.
5.2.2.2 Type of Li-SWT Machine
These waves are generated by machines called lithotripters. There are mainly three types of lithotripters in common use: electrohydraulic, electromagnetic, and piezoelectric (Table 5.2).
Contemporary lithotripter machines differ from each other regarding specific settings, namely energy flux density (EFD), penetration depth, and frequency (Table 5.3).
Also, each company has its own suggested protocol, including number and frequency of sessions and number of shocks per each session. Disparities amongst machine protocols or type of lithotripters and the lack of head-to-head reports make it puzzling to define the advantage of one machine and/or protocol over another [29].
5.2.2.3 Efficacy
Numerous single-arm studies have reported encouraging effects of LI-SWT on ED patients. However, results from randomised prospective are contradictory, and many issues wait to be solved specifically because of the several types of lithotripters used; type of energy or frequency parameters and treatment protocols [30]. The large part of the studies has reported that LI-SWT can significantly increase the IIEF in patients with vasculogenic ED [7]. More importantly, few studies have demonstrated an enhancement in penile haemodynamic at penile doppler after LI-SWT. Likewise, several reports suggest that LI-SWT could improve erectile function even in severe ED men who are PDE5Is non-responders, thus dropping the urgent need for second-line treatments like injection or penile implant insertion [31].
On the other hand, high-quality prospective randomize trials with long follow-up are needed to provide urologists and sexual medicine clinicians with more assurance concerning the efficacy of LI-SWT. Further clarity is also needed in defining treatment protocols that can result in greater clinical benefits [31].
5.3 Platelet-Rich Plasma for Erectile Dysfunction
Platelet-rich plasma (PRP) is defined as autologous blood plasma with supraphysiologic concentrations of activated platelets. Its regenerative capacities were first reported in the 1987 within the field of reconstructive surgery. In the last four decades, PRP has been utilized in a myriad of fields such as plastic surgery, cardio surgery, dermatology, and more recently in andrology [32].
5.3.1 Mechanism of Action
Notwithstanding PRP’s extensive usage, its biological characteristics and outcomes continue to be inadequately comprehended and debateable. The preparation of PRP is very simple, and it can be done in outpatient setting. Autologous blood is drawn and centrifuged to obtain a platelet-rich plasma fluid with a concentration reaching up to seven times physiological levels (Fig. 5.3) [33]. Preclinical results show that PRP can release in the system a wide range of growth factors (Fig. 5.4) and activated platelets which act synergistically to assist mitogenesis and neoangiogenesis, thus reconstructing injured tissues. Other constituents inside PRP have also been reported to work as a scaffold for healing process [34].
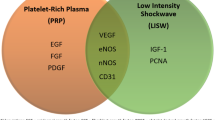
Overview of PRP content. PRP platelet-rich plasma, PDGF platelet-derived growth factor, TGF-b transforming growth factor beta, VEGF vascular endothelial growth factor, EGF epidermal growth factor, IGF insulin-like growth factor, FGF fibroblast growth factor, PF-4 platelet-factor 4, VSM vascular smooth muscle
5.3.2 Effectiveness
Up to the present time, few basic science studies have assessed PRP efficacy in ED animal model. Based on their results, PRP was able to (1) increase erectile function, (2) boost neural regeneration, and (3) decrease expression of pro-fibrotic molecules within the corporal cavernosa tissue. Nevertheless, these reports are characterized by several limitations that can jeopardize the value of the results like (1) reduced sample sizes, (2) dissimilar methods of PRP extraction, and (3) poor regulation of PRP concentrations [33,34,35,36,37,38,39]. In humans, conversely, PRP proved promising results on ED. In fact, few small reports and phase 1–2 trials showed that PRP injections significantly improved EF based on intracavernosal peak systolic velocity (PSV), IIEF-5, and sexual encounter profile scores independently of whether PRP was activated by calcium or used in conjunction to another treatment. It appears that the concurrent use of PRP could prolong Li-ESWT notable improvements for up to 6 months [32].
Notwithstanding early excitement for PRP as a regenerative cure for ED, the existing data to support its value in the ED therapy is deficient. Solid records on its safety and efficacy are still missing with only two clinical trials completed until now. The largest clinical trial assessing the efficacy of PRP involved in only 75 men with heterogeneous of ED severities [40].
Presently, no randomized controlled blinded clinical trials have delivered enough proof to sustain the extensive use of PRP for ED therapy. More importantly, approximately half of the clinical reports are abstracts and needed important details. Also, protocols required homogeneity; it is crucial to offer longer follow-up to allow beneficial outcomes to establish. Furthermore, PRP extraction varied noticeably. While, reports described a supplement of hyaluronic acids to the PRP, one reported on the efficacy of Li-ESWT combined with CaCl2-activated PRP [32].
5.4 Stem Cells for Erectile Dysfunction
5.4.1 Introduction
In the context of ED, the effects of stem cells from a broad range of sources have been reported, including adipose-derived stem cells (ADSCs), bone marrow stem cells (BMSCs), embryonic stem cells (ESCs), endothelial progenitor cells (EPCEPCs), urine-derived stem cells (USCs), skeletal muscle-derived stem cells (SkMSCs), and stromal vascular fraction (SVF) (Table 5.4). In the past decade, several studies have evaluated the effect of stem cell therapy on the recovery of erectile function in several animal models of ageing, diabetes mellitus, and cavernous nerve injury [41]. More important several phase 1 and phase 2 studies have evaluated the safety and the efficacy of stem cell-based therapy in man suffering ED [42,43,44,45,46,47].
5.4.2 Mechanism of Action
The mechanism of action of stem cells in the treatment of ED has generated, in the last 15 years, considerable attention in molecular biology, genetics, and bioengineer. Stem cells are well known for capacity of self-renewal and their potential for differentiation into mature cell types or tissue. Depending on their potential for differentiation, stem cells are classified as totipotent stem cells, pluripotent stem cells, or multipotent stem cells. ESCs are an example of pluripotent whereas Mesenchymal stem cells are multipotent stem cells. MSC can be isolated from organs and can differentiate into any cell type within their germ tissue. ESCs have two main advantages over MSCs and SVF, the first of which is their ability to proliferate for longer periods of time, and the second is their capacity to differentiate into a broader range of cell types [3]. However, owing to the ethical conflict that surrounds ESCs, their use in research has been limited and, as such, MSCs and SVF are a more feasible option for research and therapeutic applications.
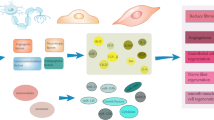
Although the mechanism of action of stem cells on ED is not yet very well understood, it is one of the most popular targets of both preclinical studies and clinical trials in the current decade. Despite their potential for differentiation into mature cell types or tissue, there is another capacity of the stem cells that makes them appealing for therapeutic purpose. The latest theory identified stem cells as a sort of drug store able to secrete several different molecules acting via paracrine way di complex mechanisms including, modulation of the innate and adaptive immune system, stimulation of neo-angiogenesis and neurogenesis, reducing apoptosis, fibrosis, and myofibroblast activation [3, 48] (Fig. 5.5).
5.4.3 Efficacy
While basic science results for stem cells and SVF are encouraging and have generated significant findings about the mechanisms of penile tissue regeneration, clinical results are limited and not robust. There are quite a few of small trial sharing similar protocols and involving few patients. Two studies looked at ESC. One involving seven diabetic patients who had regained morning erections and two who achieved erections hard enough for sexual penetrative intercourse [47]. The other recruited eight patients with organic ED for at least 6 months and those with baseline IIEF scores of 21 or higher were treated with placental matrix derived stem cells injected into the corpora cavernosal [49]. This trial showed a significantly improved systolic velocity (PSV) at penile Doppler ultrasonography [49].
INtra-cavernous STem-cell INjection (INSTIN) clinical trial [45] focused on men suffering from post-radical prostatectomy (RP) iatrogenic ED. This was a phase 1 trial that used bone marrow-derived stem cells. The authors reported no adverse events. At 180 days, they reported significant progresses in the sexual satisfaction and erectile function domains of the IIEF-15 and EHS. It should be noted that these trials were phase 1, powered only for safety, and adverse events were no reported. Notably, the group reported a decline in the improved erectile function over time, advocating a role for multiple SC treatments. Similarly, at 2 years post RP, no PSA recurrence was reported. Consequently, this trial indicates a relative safety of stem cell treatment in prostate cancer patients.
Two clinical studies published looking at SVF [43, 44]. One study investigated SVF treatment in 21 men suffering from ED post RP, and another trial in 30 patients [43]. No serious adverse events were observed. The most common being bruising or pain at the site of SVF injection or liposuction within the first 48 h. In both studies, IIEF-5 scores demonstrated an improvement.
Notwithstanding the encouraging outcomes of these phase 1–2 trials, it is imperative to acknowledge that these studies include small number of patients, are open label, and asses the safety rather than the efficacy of stem cells. All together, these trials involve around 70 ED patients treated with different protocols and type of stem cells. Most studies evaluated the safety as the primary end point and no trial reported any significant adverse event. Consequently, to overcome the substantial bias that characterized this research, the next studies need to be larger, placebo-controlled, double-blinded, and randomized trials.
References
Castiglione F, Montorsi F, Briganti A. Tadalafil for erectile dysfunction prevention after radiotherapy for prostate cancer. JAMA. 2014;312:748. https://doi.org/10.1001/jama.2014.7920.
Goldstein I, Goren A, Li VW, Tang WY, Hassan TA. Epidemiology update of erectile dysfunction in eight countries with high burden. Sex Med Rev. 2020;8:48–58. https://doi.org/10.1016/J.SXMR.2019.06.008.
Milenkovic U, Albersen M, Castiglione F. The mechanisms and potential of stem cell therapy for penile fibrosis. Nat Rev Urol. 2019;16:79–97. https://doi.org/10.1038/s41585-018-0109-7.
Pozzi E, Muneer A, Sangster P, Alnajjar HM, Salonia A, Bettocchi C, et al. Stem-cell regenerative medicine as applied to the penis. Curr Opin Urol. 2019;29:443–9. https://doi.org/10.1097/MOU.0000000000000636.
Capece M, la Rocca R, Mirone V, Bivalacqua TJ, Castiglione F, Albersen M, et al. A systematic review on ischemic priapism and immediate implantation: do we need more data? Sex Med Rev. 2019;7:530–4. https://doi.org/10.1016/j.sxmr.2018.10.007.
Milenkovic U, Albersen M, Castiglione F. The mechanisms and potential of stem cell therapy for penile fibrosis. Nat Rev Urol. 2018;16:79–97. https://doi.org/10.1038/s41585-018-0109-7.
Compilations of all guidelines | Uroweb n.d.. https://uroweb.org/guidelines/compilations-of-all-guidelines/. Accessed 4 Nov 2021.
Hazir B, Haberal HB, Asci A, Muneer A, Gudeloglu A. Erectile dysfunction management: a critical appraisal of clinical practice guidelines with the AGREE II instrument. Int J Impot Res. 2021;34(5):471–9. https://doi.org/10.1038/S41443-021-00442-7.
Carrion H, Martinez D, Parker J, Hakky T, Bickell M, Boyle A, et al. A history of the penile implant to 1974. Sex Med Rev. 2016;4:285–93. https://doi.org/10.1016/J.SXMR.2016.05.003.
Beheri GE. Surgical treatment of impotence. Plast Reconstr Surg. 1966;38:92–7. https://doi.org/10.1097/00006534-196608000-00002.
Brantley Scott F, Bradley WE, Timm GW. Management of erectile impotence. Use of implantable inflatable prosthesis. Urology. 1973;2:80–2. https://doi.org/10.1016/0090-4295(73)90224-0.
Chung E. Penile prosthesis implant: scientific advances and technological innovations over the last four decades. Transl Androl Urol. 2017;6:37–45. https://doi.org/10.21037/TAU.2016.12.06.
Ignacio MI, Pramod K. Textbook of urogenital prosthetic surgery: erectile restoration & urinary incontinence 2021.
Wilson SK, Cleves M, Delk JR II. Long-term results with Hydroflex and Dynaflex penile prostheses: device survival comparison to multicomponent inflatables. J Urol. 1996;155(5):1621–3. https://pubmed.ncbi.nlm.nih.gov/8627837/. Accessed 19 Dec 2021.
Kocjancic E, Jaunarena JH, Schechter L, Acar Ö. Inflatable penile prosthesis implantation after gender affirming phalloplasty with radial forearm free flap. Int J Impot Res. 2020;32:99–106. https://doi.org/10.1038/S41443-019-0153-8.
Abdelsayed GA, Levine LA. Ambicor 2-piece inflatable penile prosthesis: who and how? J Sex Med. 2018;15:410–5. https://doi.org/10.1016/J.JSXM.2017.12.010.
Levine LA, Becher EF, Bella AJ, Brant WO, Kohler TS, Martinez-Salamanca JI, et al. Penile prosthesis surgery: current recommendations from the international consultation on sexual medicine. J Sex Med. 2016;13:489–518. https://doi.org/10.1016/j.jsxm.2016.01.017.
Otero JR, Manfredi C, Wilson SK. The good, the bad, and the ugly about surgical approaches for inflatable penile prosthesis implantation. Int J Impot Res. 2020;34:128–37. https://doi.org/10.1038/S41443-020-0319-4.
Weinberg AC, Pagano MJ, Deibert CM, Valenzuela RJ. Sub-coronal inflatable penile prosthesis placement with modified no-touch technique: a step-by-step approach with outcomes. J Sex Med. 2016;13:270–6. https://doi.org/10.1016/J.JSXM.2015.12.016.
Baumgarten AS, Kavoussi M, VanDyke ME, Ortiz NM, Khouri RK, Ward EE, et al. Avoiding deep pelvic complications using a “Five-Step” technique for high submuscular placement of inflatable penile prosthesis reservoirs. BJU Int. 2020;126:457–63. https://doi.org/10.1111/BJU.15106.
Vollstedt A, Gross MS, Antonini G, Perito PE. The infrapubic surgical approach for inflatable penile prosthesis placement. Transl Androl Urol. 2017;6:620–7. https://doi.org/10.21037/TAU.2017.07.14.
Scherzer ND, Dick B, Gabrielson AT, Alzweri LM, Hellstrom WJG. Penile prosthesis complications: planning, prevention, and decision making. Sex Med Rev. 2019;7:349–59. https://doi.org/10.1016/J.SXMR.2018.04.002.
Towe M, Peta A, Saltzman RG, Balaji N, Chu K, Ramasamy R. The use of combination regenerative therapies for erectile dysfunction: rationale and current status. Int J Impot Res. 2021; https://doi.org/10.1038/S41443-021-00456-1.
Katz JE, Clavijo RI, Rizk P, Ramasamy R. The basic physics of waves, soundwaves, and shockwaves for erectile dysfunction. Sex Med Rev. 2020;8:100–5. https://doi.org/10.1016/J.SXMR.2019.09.004.
Hatanaka K, Ito K, Shindo T, Kagaya Y, Ogata T, Eguchi K, Kurosawa R, Shimokawa H. Molecular mechanisms of the angiogenic effects of low-energy shock wave therapy: roles of mechanotransduction. Am J Physiol Cell Physiol. 2016;311:C378–85. https://doi.org/10.1152/AJPCELL.00152.2016.
Young Academic Urologists Men’s Health Group, Fode M, Hatzichristodoulou G, Serefoglu EC, Verze P, Albersen M. Low-intensity shockwave therapy for erectile dysfunction: is the evidence strong enough? Nat Rev Urol. 2017;14:593–606. https://doi.org/10.1038/NRUROL.2017.119.
Murata R, Ohtori S, Ochiai N, Takahashi N, Saisu T, Moriya H, Takahashi K, Wada Y. Extracorporeal shockwaves induce the expression of ATF3 and GAP-43 in rat dorsal root ganglion neurons. Auton Neurosci. 2006;128:96–100. https://doi.org/10.1016/J.AUTNEU.2006.04.003.
Li H, Matheu MP, Sun F, Wang L, Sanford MT, Ning H, Banie L, Lee Y-C, Xin Z, Guo Y, Lin G, Lue TF. Low-energy shock wave therapy ameliorates erectile dysfunction in a pelvic neurovascular injuries rat model. J Sex Med. 2016;13:22–32. https://doi.org/10.1016/J.JSXM.2015.11.008.
Porst H. Review of the current status of low intensity extracorporeal shockwave therapy (Li-ESWT) in erectile dysfunction (ED), Peyronie’s disease (PD), and sexual rehabilitation after radical prostatectomy with special focus on technical aspects of the different marketed ESWT devices including personal experiences in 350 patients. Sex Med Rev. 2021;9:93–122. https://doi.org/10.1016/J.SXMR.2020.01.006.
Capogrosso P, Frey A, Jensen CFS, Rastrelli G, Russo GI, Torremade J, Albersen M, Gruenwald I, Reisman Y, Corona G. Low-intensity shock wave therapy in sexual medicine-clinical recommendations from the European Society of Sexual Medicine (ESSM). J Sex Med. 2019;16:1490–505. https://doi.org/10.1016/J.JSXM.2019.07.016.
Liu JL, Chu KY, Gabrielson AT, Wang R, Trost L, Broderick G, Davies K, Brock G, Mulhall J, Ramasamy R, Bivalacqua TJ. Restorative therapies for erectile dysfunction: position statement from the Sexual Medicine Society of North America (SMSNA). Sex Med. 2021;9:100343. https://doi.org/10.1016/J.ESXM.2021.100343.
Scott S, Roberts M, Chung E. Platelet-rich plasma and treatment of erectile dysfunction: critical review of literature and global trends in platelet-rich plasma clinics. Sex Med Rev. 2019;7:306–12. https://doi.org/10.1016/J.SXMR.2018.12.006.
Epifanova MV, Gvasalia BR, Durashov MA, Artemenko SA. Platelet-rich plasma therapy for male sexual dysfunction: myth or reality? Sex Med Rev. 2020;8:106–13. https://doi.org/10.1016/J.SXMR.2019.02.002.
Alkandari MH, Touma N, Carrier S. Platelet-rich plasma injections for erectile dysfunction and Peyronie’s disease: a systematic review of evidence. Sex Med Rev. 2021;10:341–52. https://doi.org/10.1016/J.SXMR.2020.12.004.
Jung G, Kwak J, Yoon S, Yoon J, Lue T. IGF-I and TGF-β2 have a key role on regeneration of nitric oxide synthase (NOS)-containing nerves after cavernous neurotomy in rats. Int J Impot Res. 1999;11:247–59. https://doi.org/10.1038/sj.ijir.3900402.
Ding XG, Li SW, Zheng XM, Hu LQ, Hu WL, Luo Y. The effect of platelet-rich plasma on cavernous nerve regeneration in a rat model. Asian J Androl. 2009;11:215–21. https://doi.org/10.1038/AJA.2008.37.
Lee H-W, Reddy MS, Geurs N, Palcanis KG, Lemons JE, Rahemtulla FG, et al. Efficacy of platelet-rich plasma on wound healing in rabbits. J Periodontol. 2008;79:691–6. https://doi.org/10.1902/JOP.2008.070449.
Wu CC, Wu YN, Ho HO, Chen KC, Sheu MT, Chiang HS. The neuroprotective effect of platelet-rich plasma on erectile function in bilateral cavernous nerve injury rat model. J Sex Med. 2012;9:2838–48. https://doi.org/10.1111/J.1743-6109.2012.02881.X.
Hu Z, Qu S, Zhang J, Cao X, Wang P, Huang S, et al. Efficacy and safety of platelet-rich plasma for patients with diabetic ulcers: a systematic review and meta-analysis. Adv Wound Care (New Rochelle). 2019;8:298–308. https://doi.org/10.1089/WOUND.2018.0842.
Epifanova MV, Chalyi ME, Krasnov AO. [Investigation of mechanisms of action of growth factors of autologous platelet-rich plasma used to treat erectile dysfunction]. Urologiia. 2017;(4):46–8. https://pubmed.ncbi.nlm.nih.gov/28952692/. Accessed 4 Nov 2021.
Soebadi MA, Moris L, Castiglione F, Weyne E, Albersen M. Advances in stem cell research for the treatment of male sexual dysfunctions. Curr Opin Urol. 2016;26:129–39. https://doi.org/10.1097/MOU.0000000000000255.
Castiglione F, Albersen M, Hedlund P, Gratzke C, Salonia A, Giuliano F. Current pharmacological management of premature ejaculation: a systematic review and meta-analysis. Eur Urol. 2016;69:904–16. https://doi.org/10.1016/j.eururo.2015.12.028.
Haahr MK, Jensen CH, Toyserkani NM, Andersen DC, Damkier P, Sørensen JA, Sheikh SP, Lund L. A 12-month follow-up after a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial. Urology. 2018;121:203.e6–13. https://doi.org/10.1016/J.UROLOGY.2018.06.018.
Haahr MK, Jensen CH, Toyserkani NM, Andersen DC, Damkier P, Sørensen JA, Lund L, Sheikh SP. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial. EBioMedicine. 2016;5:204–10. https://doi.org/10.1016/J.EBIOM.2016.01.024.
Yiou R, Hamidou L, Birebent B, Bitari D, Le Corvoisier P, Contremoulins I, Rodriguez A, Augustin D, Roudot-Thoraval F, De la Taille A, Rouard H. Intracavernous injections of bone marrow mononucleated cells for postradical prostatectomy erectile dysfunction: final results of the INSTIN clinical trial. Eur Urol Focus. 2017;3:643–5. https://doi.org/10.1016/J.EUF.2017.06.009.
Yiou R, Hamidou L, Birebent B, Bitari D, Lecorvoisier P, Contremoulins I, Khodari M, Rodriguez A-M, Augustin D, Roudot-Thoraval F, de la Taille A, Rouard H. Safety of intracavernous bone marrow-mononuclear cells for postradical prostatectomy erectile dysfunction: an open dose-escalation pilot study. Eur Urol. 2016;69:988–91. https://doi.org/10.1016/J.EURURO.2015.09.026.
Al Demour S, Jafar H, Adwan S, AlSharif A, Alhawari H, Alrabadi A, Zayed A, Jaradat A, Awidi A. Safety and potential therapeutic effect of two intracavernous autologous bone marrow derived mesenchymal stem cells injections in diabetic patients with erectile dysfunction: an open label phase I clinical trial. Urol Int. 2018;101:358–65. https://doi.org/10.1159/000492120.
Soebadi MA, Milenkovic U, Weyne E, Castiglione F, Albersen M. Stem cells in male sexual dysfunction: are we getting somewhere? Sex Med Rev. 2017;5:222–35. https://doi.org/10.1016/j.sxmr.2016.11.002.
Levy JA, Marchand M, Iorio L, Cassini W, Zahalsky MP. Determining the feasibility of managing erectile dysfunction in humans with placental-derived stem cells. J Am Osteopath Assoc. 2016;116:e1–5. https://doi.org/10.7556/JAOA.2016.007.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
5.1 Electronic Supplementary Material
(MP4 46809 kb)
(MP4 12807 kb)
(MP4 85061 kb)
(MP4 58749 kb)
(MP4 31844 kb)
(MP4 92175 kb)
(MP4 47390 kb)
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Bettocchi, C., Castiglione, F., Cakir, O.O., Falagario, U., Ricapito, A. (2023). Erectile Dysfunction, Surgical and Regenerative Therapy. In: Bettocchi, C., Busetto, G.M., Carrieri, G., Cormio, L. (eds) Practical Clinical Andrology. Springer, Cham. https://doi.org/10.1007/978-3-031-11701-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-11701-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11700-8
Online ISBN: 978-3-031-11701-5
eBook Packages: MedicineMedicine (R0)