Abstract
The study of the spatial dynamics of forest insects has a long history, and many forest insect species have served as model systems for studying conceptual processes of population biology and ecology. Because forest insect population data were often collected from georeferenced locations, even prior to the development of geodatabases and spatial statistical software, they provide an important historical resource for understanding insect population dynamics and changes in those dynamics through time. Advances in spatial statistics have furthermore enabled forest entomologists to consider forest insect dynamics over multiple spatial and temporal scales, and vast spatial and temporal extents. In this chapter, we first introduce the importance of scaling in studies of spatial dynamics, and review spatial pattern formation in forest insect populations. We conclude the chapter by addressing metapopulation dynamics, and the concept of spatial synchrony in outbreaking forest insects.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
18.1 Introduction
The study of the spatial dynamics of forest insects has a long history, and many forest insect species have served as model systems for studying conceptual processes of population biology and ecology. Some of the earliest works by A.D Hopkins, considered as the founding scholar of forest entomology in North America, focused on forest insects and their interactions with natural enemies (Hopkins 1899a), or the role that forest insects play in patterns of tree mortality (Hopkins 1899b). Not surprisingly, the study of forest insect spatial dynamics long predates computers, geodatabases, and spatial statistical software, as forest insect population data were often collected at georeferenced locations. For example, aerial surveys of forest stands affected by biotic disturbance agents, including insects, date to the late 1940s in both Canada and the United States.
Advances in geostatistics and computer processing power over the past several decades have enabled forest entomologists to consider forest insect dynamics over multiple spatial and temporal scales, and vast spatial and temporal extents. In this chapter, we first introduce the importance of scaling in studies of spatial dynamics, and review spatial pattern formation in forest insect populations. We conclude the chapter by addressing metapopulation dynamics, and the concept of spatial synchrony in outbreaking forest insects.
18.2 Spatial Scales
The concept of forest insect spatial dynamics is ultimately dependent on the scale at which spatial dynamics are considered. On the level of an individual woody plant host, herbivorous forest insect species are generally restricted to certain plant parts, such as the roots, subcortical regions, leaves or needles, or plant reproductive parts, and consequently many forest entomology courses focus on the groups (i.e. guilds) of insects that feed on each plant part (Berryman 1986). Several species that exploit the same plant concurrently may exploit different parts of the plant due to interspecific competition. This is a concept known as niche partitioning (Schoener 1974), and has been observed in competing bark beetle species, some of which attack the lower bole whereas others attack the middle or upper bole (Paine et al. 1981; Ayres et al. 2001). Moreover, species that attack the same host plant may also exhibit temporal niche partitioning and thus avoid competition by feeding on the same host plant at different times. For example, lepidopteran folivores of Eurasian pines, primarily Pinus sylvestris, display dramatic differences in the seasonal occurrence of the larval feeding stage; Panolis flammea (Denis & Schiffermüller) feeds from March to July, Lymantria monacha (L.) from April to June, Dendrolimus pini (L.) from June to July, and Bupalus piniarius (L.) from July to November (Altenkirch et al. 2002). Lastly, different insects will feed on woody plants over the life and death of the host. For example, many bark beetle species, most notably Dendroctonus spp., are primary species that only attack live host trees, and are followed by secondary species that attack dying or dead trees, which are followed by saproxylic and detritivorous species that play important roles in nutrient cycling (Paine et al. 1997; Grove 2002; Jonsson et al. 2005).
The level of a forest stand presents another scale, and is often the one most commonly addressed in studies of the spatial dynamics of forest insects. A stand is defined as a contiguous community of trees sufficiently uniform in composition, structure, age and size class distribution, spatial arrangement, site quality, condition or location to distinguish it from adjacent communities (e.g. Nyland 2007). Within a stand, forest insects interact with a number of mutualists, competitors, and natural enemies (Janzen 1987; Komonen 2003). For example, Safranyik et al. (2000) collected 30 different species of Scolytinae over two years in one mature stand of Pinus contorta (lodgepole pine) following an outbreak of Dendroctonus ponderosae Hopkins.
Stands can be aggregated into landscapes, and landscapes into biomes in studies of processes that affect forest insect spatial dynamics. Depending on scale, different patterns of spatial structuring might be revealed. Indeed, fundamental processes operating at one scale may be entirely obscured when the system is considered at a different scale (Raffa et al. 2008). Thus, it is critically important to recognize the spatial scale of a study and how it can influence and limit inference with regard to spatial dynamics.
18.3 Spatial Pattern Formation
Insect populations are distributed in space. The spatial structure of insect populations is of paramount importance in sampling and management plans, as well as in efforts to quantify the underlying factors that affect insect population dynamics (Rossi et al. 1992; Liebhold et al. 1993; Tobin 2004). Spatial patterns occur at multiple spatial scales. For example, the spatial arrangement of a species on a single host plant will have a structure, as will its arrangement within a single forest stand, or across a landscape consisting of a number of forest stands.
There are three basic types of spatial distributions common to not only insect populations but also to life in general, regardless of taxonomic Kingdom: random, uniform, and clustered or aggregated (Fig. 18.1). Randomly distributed populations are rare in nature, and perhaps it is best to think of a random spatial arrangement as a null hypothesis of insect spatial structure. Uniform patterns are also rare, but are present in nature under certain conditions, within specific spatial scales, and at specific population densities. For example, sessile feeders, such as Adelges tsugae (Annand), might be expected to be uniformly distributed on a single hemlock shoot in the absence of overcrowding conditions given their feeding behavior. Each A. tsugae individual occupies a certain amount of space and inserts their stylet into the petiole of a hemlock needle, which furthermore tend to be uniformly arranged on a shoot. The vast majority of insect species, especially as spatial scales increase, are undoubtedly aggregated (Taylor 1961).
Spatial representation of a random (A1), uniform (B1), and clustered (C1) spatial pattern, and the corresponding spatial correlogram (ρh) shown in A2, B2, and C2, respectively. In random patterns, the correlation between values from pairs of sampling locations is ~0 regardless of the distance that separates the sampling locations (A2). This is in contrast to clustered populations (C2) in which there is high correlation between pairs of sampling locations as the distances that separates these locations → 0, with the range of spatial dependency extending to the distance at which ρh ~0. In uniformly-distributed populations, high values are generally located next to low values, which results in a negative correlation as the distance that separates sampling locations → 0 (B2).
One basic explanation for spatial aggregation by most herbivorous insects is that they have life histories characteristic of r-strategists in which females oviposit several to many eggs (or other immature life stages) in one area at once. Even though neonates may be capable of dispersing, such dispersal is normally limited to short distances. Thus, each new cohort is initiated with a high degree of aggregation. Insects, regardless of feeding guild, are also often dependent upon resources that are spatially structured. For example, plants generally follow elevational and latitudinal gradients due to variation in a number of factors, such as temperature, precipitation, solar energy, and soil characteristics. The spatial pattern of plants spatially structures the insect herbivores that rely on those plants, which in turn spatially structures natural enemies of those herbivores, and so forth (Taylor 1984; McCoy 1990; Hodkinson 2005). Some forest insects may also be engaged in gregarious behaviors; for example, semiochemicals such as aggregation pheromones in tree-killing bark beetles facilitate mass-attacks on host trees (Borden 1989; Raffa 2001; Gitau et al. 2013). Other species may use sex pheromones or engage in lekking behaviors that could result in the aggregation of adults for mating (Landolt 1997; Wickman and Rutowski 1999).
Historical methods of spatial pattern analyses relied on frequency distribution models and mean-to-variance relationships (e.g. Southwood 1978). These approaches involved examining the ratio of the sample variance-to-the-sample mean of a collection of samples from a sampling quadrat or area (Taylor 1961; Southwood 1978). If the sample variance was less than the sample mean, the population was considered uniformly distributed. In contrast, if the sample variance was greater than the sample mean, the population was considered to be aggregated. If the sample variance was approximately the same as the sample mean, then the population was considered to be randomly distributed. This simple approach was certainly useful in the days before computers, and did shed light onto the basic spatial patterns of insects, but was not necessarily spatially explicit or amenable to statistical hypothesis testing.
More sophisticated spatial statistical techniques have been available for some time (Legendre and Fortin 1989; Rossi et al. 1992; Bjørnstad and Falck 2001). These modern techniques rely on the estimation of the spatial correlogram, which considers the spatial correlation between values of pairs of samples as a function of the distance separating the two samples (Rossi et al. 1992; Fig. 18.1). An underlying premise is that the values of a given variable collected from two locations that are close in space are more likely to be similar in value than data collected from two locations that are farther away in space. The correlation of a variable with itself across space is known as spatial autocorrelation (Getis 2008).
The behavior of the spatial correlogram provides information as to the degree of local spatial autocorrelation, which is the correlation of a variable between sample pairs as the distance between sample pairs approaches 0 (i.e. the y-intercept). As the distance between sample pairs approaches 0, the theoretical expectation of the spatial autocorrelation is 1, or perfect positive autocorrelation. However, in field-collected data, the spatial autocorrelation is often <1 as the distance approaches 0, in part due to random variation and measurement error. In the geological and mining literature, upon which the foundation of spatial statistics was developed, the difference between estimates of the local spatial autocorrelation and its theoretical value of 1 is known as the “nugget effect”; a term motivated by the occurrence of a large mineral deposit, such as a gold nugget, in a theoretically unexpected location in space based on nearby samples (Krige 1999). The spatial correlogram also provides an estimate of the spatial range, which is the distance over which sample pairs are correlated; thus, at this distance, the estimated spatial autocorrelation approaches 0 (i.e. the x-intercept). The spatial range can be used to estimate the distance that samples need to be apart to acquire spatially independent data, and the spatial extent of aggregation in an insect population.
Quantification of spatial pattern formation, and the approach used to do so, has a number of important ramifications for the management of forest insect populations. For example, there are benefits to using prior knowledge of population structure, such as the degree and range of spatial correlation, to design sampling protocols with the goal of obtaining spatially independent data. By collecting spatially independent data, sampling efforts can be reduced yet still allow georeferenced data to be used in interpolation efforts, such as through kriging (Liebhold et al. 1993; Fleischer et al. 1999). However, it should be noted that in cases where estimates of population density are readily available at scales finer than the range of spatial autocorrelation, such as in studies where the proportion of forest defoliated in a given area of forested land was used as a proxy of the local population density of Lymantria dispar (L.) (Haynes et al. 2018), statistical methods have been developed to account for the non-independence of data values from nearby sample areas. An application of the spatial autocorrelation based upon field-collected data of L. dispar is presented in Box 18.1.
Box 18.1: The Lymantria dispar Invasion of North America
Life stages of L. dispar were introduced to Medford, Massachusetts, USA, by an amateur entomologist, Étienne Léopold Trouvelot, in 1869 (Riley and Vasey 1870). It is believed that following a storm, life stages escaped from the rearing conditions maintained by Trouvelot (Forbush and Fernald 1896). It has subsequently spread in North America such that it now occupies an area from Minnesota to North Carolina to Maine in the U.S., and southern Ontario to Nova Scotia in Canada. Current management efforts include outbreak suppression in its established area, slowing it spread along its expanding population front, and eradication in areas outside of the established area (Tobin et al. 2012).

Fig. 1 Lymantria dispar larvae on Betula papyrifera (paper birch), Stockton Island, Wisconsin, USA (Photo credit: P. Tobin)
Lymantria dispar undergoes one generation per year. Overwintering eggs hatch in spring, and larval and pupal development occurs over ~8 and 2 weeks, respectively. Female adults are not capable of sustained flight, and produce a sex pheromone to attract male mates. Adults are short-lived (~2–3 days). In summer, females oviposit 200–500 eggs in an egg mass, which will not hatch until the following year.
Larvae (Fig. 1) are highly polyphagous and are capable of consuming >300 species of host plants, including ~80 species that are highly preferred. Highly preferred hosts include species within Betula, Crataegus, Larix, Populus, Quercus, and Salix (Liebhold et al. 1995).
Along its expanding population front, L. dispar generally spreads through stratified dispersal in which short-range dispersal is coupled with long distance ‘jumps’ in areas ahead of the leading edge. Spatial analyses of L. dispar spread using the spatial autocorrelation are indicative of a spatial trend as it invades across a region (Fig. 2).

Fig. 2 Spread and spatial dynamics of L. dispar in Wisconsin, 2007–2010. (A) Counts of male moths from deployed pheromone-baited traps. (B) Mean rates of spread (km/yr) from year-to-year; for example, the spread rate in 2007 reflects the change from 2006 to 2007 (Tobin et al. 2007). (C) Estimates of the spatial autocorrelation (Bjørnstad and Falck 2001) in trap catch for each year. In each year, spatial autocorrelation was detected at distances up to ~100 km (i.e. the x-intercept), and the linear pattern of spatial autocorrelation is indicative of a spatial trend as L. dispar invades Wisconsin from the east to the west
Accurate delimitation of the spatial extent of a population has important implications for both forest insect pest management and conservation management. For example, understanding the spatial extent of a pest population allows for the deployment of site-specific control interventions, and by extension, reduced non-target effects of control tactics (Sharov et al. 2002; Tobin et al. 2004; Blackburn et al. 2011). In forest insects that are threatened or endangered, or in areas of conservation concerns, understanding their spatial dynamics helps to develop better conservation plans (Didham et al. 1996; Gering et al. 2003). Many forest insect species have important ecosystem roles, and some provide important ecosystem services (Noriega et al. 2018). Knowledge of their spatial structure can provide insight as to the spatial extent of these ecosystem services. Lastly, long-term and baseline knowledge of forest insect spatial dynamics permits the study of how species respond to climate change, habitat fragmentation and changes in land use, and the introduction of invasive species (Harrington et al. 2001; Knops et al. 2002; Walther et al. 2002; Logan et al. 2003; Opdam and Wascher 2004; Turner 2010).
It is important to recognize that the spatial patterns of insect populations are not static; rather, they vary both within and among generations. Consider the phenology (i.e. the seasonal timing of specific events in an organism’s life cycle) and spatial arrangement of an insect population that inhabits a stand on both a south-facing slope and a north-facing slope. Reproductive asynchrony, which occurs when the adults within a population are present at different times, owing to, for example, temperature variation leading to variation in developmental rate, could lead to spatial variation in mating success rates and hence spatial variation in population growth through time (Calabrese and Fagan 2004; Robinet et al. 2008; Walter et al. 2015). Thus, both space and time are fundamental for understanding the processes influencing insect population dynamics.
18.4 Metapopulation Dynamics
Many forest insect populations exist, especially at endemic population densities, as metapopulations in which spatially-separated sub-populations of a species exist over a large landscape (Levins 1969; Hanski 1998). Often in forest ecosystems, these spatially-separated subpopulations exist due to fragmented host plant resources. The fragmentation of host plant resources could be the result of human activities, such a logging, or environmental conditions, such as host trees adapted to mid-elevations or valleys and are thus separated by mountain peaks. Hanski (1997) proposed a set of conditions that define metapopulations, and one condition is that subpopulations are close enough to be connected by dispersal. Thus, depending on the dispersal ability of the insect, a metapopulation can exist over a range of distances between subpopulations. Another important condition of a metapopulation is that patches of host resources are fragmented over a larger landscape, and some of these patches must be of sufficient host quality and abundance to allow for population persistence. Nevertheless, the subpopulations within all patches are theoretically prone to extinction, although extinction rates can differ from patch-to-patch. Even insects inhabiting a forest stand with a high abundance of high-quality host plant resources have some rate of extinction due to, for example, stochastic mortality factors such as winter conditions during which temperatures drop below supercooling points. A final condition of metapopulations is that local population dynamics are independent of each other and thus are not necessarily synchronous; consequently, densities in one patch could be high, which theoretically allow it to serve as a source, while other patches could be going extinct. These conditions comprise the classic model of metapopulations (Fig. 18.2).
One key aspect of metapopulation dynamics that is applicable to the study of forest insect ecology is the underlying spatial heterogeneity that fragments an insect population (Hunter 2002). Past work has highlighted how this spatial heterogeneity affects natural enemy-victim interactions (Hastings 1990; Taylor 1990), which can play a large role in the population dynamics of forest insect species. For example, Roland (1993) examined outbreak duration in the forest tent caterpillar, Malacosoma disstria Hübner, a defoliator native to North America, and observed that an increase in forest fragmentation due to logging spatially decoupled M. disstria from parasitoids and pathogens to the benefit of the defoliator. The result was longer and more intense outbreaks in areas with high spatial heterogeneity. Although outbreak dynamics are inherently spatially synchronized (see Sect. 18.5), the underlying fragmentation of local populations, which are likely independent at endemic population levels, can provide sufficient escape from natural enemies that would otherwise provide population control.
18.5 Spatial Synchrony and Outbreak Dynamics
In contrast to one of the core conditions of metapopulations, specifically the independence of dynamics among subpopulations, spatial synchrony refers to the congruence in temporal variation of abundance across geographically disjunct populations (Bjørnstad et al. 1999; Liebhold et al. 2004). In other words, spatial synchrony is the phenomenon in which the densities of populations distributed across a region tend to rise and fall synchronously. Spatial synchrony has been found in populations of a wide variety of taxa including many forest insect species (Peltonen et al. 2002; Liebhold et al. 2004). Spatial synchrony in forest insect populations has been observed over distances of hundreds (Peltonen et al. 2002) to thousands of kilometers (Royama 1984). At times, these forest insect populations can be irruptive and increase dramatically across a large region over short periods of time, which is often the case in the development of insect outbreaks (Aukema et al. 2006).
Several mechanisms have been proposed to give rise to spatial synchrony in insect populations. One biotic mechanism is dispersal between and among populations of a species (Peltonen et al. 2002), and especially density-dependent dispersal in which individuals from areas with high population densities disperse to lower density populations to reduce intraspecific competition. Another biotic mechanism arises from trophic interactions with populations of other species that are spatially synchronous, thus inducing spatial synchrony in the forest insect under consideration (Ims and Steen 1990).
Perhaps the most important factors affecting the spatial synchrony of poikilothermic species, such as insects, are the abiotic effects of weather. Excessively harsh or mild winter temperatures, for example, can have dramatic region-wide effects on insect populations. Generally, exogenous weather factors, such as temperature or precipitation, are highly spatially autocorrelated in a given year and thereby affect, concurrently, insect populations over large spatial extents; a phenomenon known as the Moran effect (Moran 1953; Royama 1992; Myers 1998; Hudson and Cattadori 1999). Moran’s theorem states that the correlation through time (spatial synchrony) between two populations will be approximately the same as the synchrony of the environment (Moran 1953). Thus, when insect populations are strongly affected by a spatially synchronous weather factor or factors, the Moran theorem predicts the densities of the affected insect populations will be strongly synchronous.
On its simplest level, the quantification of synchrony involves the estimation of the correlation between two characteristics of a population measured through time (i.e. a time series) such as population growth rate or population density for a collection of spatially disjunct subpopulations. Spatial synchrony is then a measurement of the extent to which this synchrony exists over spatial scales. Past work has reviewed the basis of quantifying synchrony and spatial synchrony (Bjørnstad et al. 1999; Buonaccorsi et al. 2001; Liebhold et al. 2004). Briefly, the statistical techniques used to quantify synchrony are an extension of the tools used in estimating spatial autocorrelation in which the estimate of the range (i.e. the x-intercept) provides an estimate of the spatial extent over which synchronous fluctuations in populations are similar. A conceptual figure of a spatially synchronous insect outbreak and the resulting estimates of synchrony is presented in Fig. 18.3.
Hypothetical spatial and temporal progression in the severity of, or the area affected by, an insect outbreak through time steps t (A), t + 1 (B), and t + 2 (C). Typically, the strength of spatial synchrony, measured as the correlation in the severity of outbreak severity through time, declines with increasing distance between locations (D).
An outbreak, which in entomological terms is defined as an explosive increase in the abundance of an insect population over a relatively short time period (Barbosa and Schultz 1987), is inherently spatially synchronized in that high densities are present over a large geographic area at roughly the same time. Forest insect outbreaks, much like forest fires, can be an extremely important component of forest ecosystem dynamics, but also like forest fires, they can have profound ecological and economic ramifications (Barbosa and Schultz 1987; Mattson and Haack 1987; McCullough et al. 1998; Raffa et al. 2008). This is especially the case in outbreaks of non-native forest insects, or when outbreaks of native species are occurring at different intervals, intensities or in different habitats than the historical norm. Although all insect outbreaks are spatially synchronized at some spatial scale, the extent at which the outbreak occurs often defines a forest insect as a pest or not. Small scale outbreaks that affect a locally distributed forest resource can certainly have measurable impacts; however, it is the large and spatially synchronous outbreaks that are most damaging (Raffa et al. 2008; Liebhold et al. 2012).
An important economic consequence of large-scale forest insect outbreaks is that they can exacerbate the economic burden on individual stake-holders and land owners due to the fact that a large portion of their forested area is often affected. From a management perspective, outbreaks that are spatially synchronized over large areas can overwhelm the budgetary and logistical abilities of federal, state/provincial or industrial agencies to implement control tactics intended to mitigate impacts and potentially suppress populations. The spatially synchronous behavior of outbreaks can also reduce, or in extreme cases eliminate, undisturbed areas that would otherwise serve as refuge against the effects of an outbreak. Lastly, spatially synchronous outbreaks can dilute the regulating effects of any natural enemy that could otherwise provide local control, which in itself could be a contributing factor to the development of high-density forest insect populations.
Forest insect outbreaks, especially in defoliators, may be cyclical (i.e. periodic) and at times, populations exist at endemic levels despite the widespread availability of susceptible host trees. The time between outbreak peaks is referred to as an outbreak interval or period length. Fascinatingly, many outbreaking forest insects exhibit cycles at relatively fixed period lengths. For example, prior work has highlighted a 8–12-year or a 4–5-year cycle in L. dispar outbreaks depending on forest stand composition (Johnson et al. 2005, 2006a), a 7–11 year cycle in L. monacha outbreaks (Haynes et al. 2014), and a 35–40-year cycle in Choristoneura fumiferana (Clemens) outbreaks (Royama 1984; Royama et al. 2005). The periodicity of cyclic species can persist for a very long time, with evidence for an 8–9 year cycle in Zeiraphera diniana (Guenée) extending back approximately 1200 years (Esper et al. 2007).
Statistical techniques to quantify the periodicity of forest insect outbreaks include the estimation of periodograms through, for example, spectral analysis. A wavelet-based spectral analysis (Torrence and Compo 1998; Cazelles et al. 2014) is one technique used in the quantification of time series that describe insect population dynamics including time series of insect outbreaks (Johnson et al. 2006b). An advantage of this technique relative to others, such as those using Fourier transformations, is that the wavelet transform can be applied to non-stationary time series, where characteristics such as period length and the amplitude of fluctuations vary through time (Torrence and Compo 1998); this is often the case in time series of the abundance of outbreaking insect species (Aukema et al. 2006; Liebhold et al. 2012). The process of a wavelet analysis is essentially akin to taking wavelet functions of known period lengths and sliding them across a time series, in this case a time series consisting of insect abundances surveyed at regular intervals. Then, at each point in time, the degree of overlap between the wavelet functions and the population abundance data is measured. In doing so, one can determine the degree to which fluctuations in abundance are cyclical, the period lengths of any such cycles, and changes over time in the presence or period lengths of cycles (Torrence and Compo 1998). An example of the application of wavelet analysis to a hypothetical time series of insect outbreaks in presented in Fig. 18.4.
Hypothetical time series of an insect outbreak showing different periods of time (periodicity) between outbreak peaks including a long period (A1), short period (B1), and one in which there is a transition from a long period to a short period (C1). The corresponding wavelet analyses are shown in A2, B2, and C2, with the solid black line representing the expected periodicity in time, while the colored region bounded by white lines represents the confidence intervals. For A1, the measured periodicity is ~25 units in time (A2), and for B1, the measured periodicity is ~10 units of time (B2). The periodicity for the time series with the transition (shown in C1) is presented in C2. This approach can be useful in statistically quantifying changes in the periodicity of insect outbreaks, or any other measured demographic trait, through time.
Applications of the study of the periodicity and intensity of forest insect outbreaks include providing background knowledge to forest health managers, who might use these findings to anticipate the next forest outbreak and preemptively apply management practices such as silvicultural strategies (Sartwell and Stevens 1975; Bergeron et al. 1999; Muzika and Liebhold 2000; Coyle et al. 2005). The study of the spatial synchrony of insect outbreaks can also shed light on the extent of the affected area. For example, Aukema et al. (2006) measured spatial synchrony in a D. ponderosae outbreak in British Columbia, Canada, that was significant beyond 900 km, which not only refuted popular perception that the outbreak began in a protected area but also provided evidence that D. ponderosae populations were erupting throughout its range. A case study of the D. ponderosae outbreak in western Canada is presented in Box 18.2.
Box 18.2: The Dendroctonus ponderosae (Mountain Pine Beetle) Outbreak in Western Canada
The mountain pine beetle is native to western North America. It feeds and reproduces within the phloem tissues of most species of pine trees. During mid to late summer, beetles select host trees and initiate attacks by boring through the bark. Trees respond by producing sticky, toxic resin (Fig. 1). Beetles ingest the defensive resin and chemically convert some of its constituents into aggregation pheromones that attract more beetles. The result is a mass attack that overwhelms tree defenses and leads to rapid tree mortality.

Fig. 1 Mountain pine beetles attacking a Pinus contorta (lodgepole pine) tree. Note the tree’s defensive resin (Photo credit: A. Carroll)
Normally, mountain pine beetle populations are innocuous, infesting occasional vigor-impaired trees within a forest; however, they periodically erupt synchronously into large-scale epidemics that cause the mortality of trees over large areas (Fig. 2A). This is a likely consequence of the Moran effect (Moran 1953; Aukema et al. 2006).
Most mountain pine beetles disperse short distances through the forest when seeking new hosts, but a small percentage will fly above the canopy (Safranyik et al. 1992). Thus, sub-outbreak populations are largely independent across landscapes. During outbreaks, large numbers of beetles may be carried above the forest canopy by prevailing winds (Jackson et al. 2008), leading to synchronized dynamics across very large distances (Fig. 2B).
Due to an increase in the number of susceptible trees as a result of fire suppression, and an expansion of climatically suitable habitats as a consequence of global warming, mountain pine beetle populations erupted during the mid-1990s and rapidly increased to unprecedented levels, establishing within historically climatically unsuitable pine forests at higher latitudes and elevations (Carroll et al. 2004; Safranyik et al. 2010).

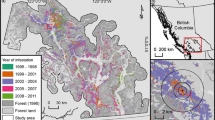
Fig. 2 (A) Time series patterns of tree mortality caused by the mountain pine beetle within 12 × 12 km cells in British Columbia, Canada, between 1999 and 2003, based on hierarchical cluster analysis (e.g. Swanson & Johnson 1999). Although the outbreak intensified earliest in the west-central portion of the province [cluster (i)], populations increased concurrently throughout the region [clusters (ii), (iii) and (iv)] indicating that many localized infestations erupted in geographically disjunct areas rather than originating and spreading from an epicenter. (B) Estimates of the spatial autocorrelation (Bjørnstad and Falck 2001) in tree mortality caused by the mountain pine beetle during incipient years (1990–1996) and epidemic years (1999–2003). Note that prior to the extensive outbreak, populations were largely independent at scales >200 km; however, during epidemic years populations were synchronous at distances >900 km. Adapted from Aukema et al. (2006)
More recently, quantifying forest insect outbreak dynamics has allowed studies of how outbreak intensity and periodicity might be changing as a consequence of climate change. For example, the ~1200 years of consistent outbreaks by Z. diniana (Esper et al. 2007) collapsed in recent decades due to climate warming (Johnson et al. 2010). Haynes et al. (2014) used long-term data on forest defoliators to quantify both positive and negative changes in their respective outbreak intensity and periodicity in response to climate change. Lastly, spatial analyses of the D. ponderosae outbreak in western Canada provided evidence and a quantification of D. ponderosae range expansion owing to climate change (Aukema et al. 2008; Sambaraju et al. 2012). The use of analytical techniques such as wavelet analyses provides opportunities to better understand the relationship between climate change and insect outbreaks, which for some species could become more intense and frequent while in others, outbreaks could be disrupted with yet unknown ecological consequences (Weed et al. 2013; Tobin et al. 2014).
18.6 Conclusion
Certain phenomena, such as spatial autocorrelation and spatial synchrony, are pervasive among forest insect populations, but for any given species these and other spatial properties are dynamic. In recent years, quantifying how such properties shift through time or across space has opened new avenues for exploration of the processes underlying the population dynamics of forest insect species. One developing area of research focusses on understanding the drivers of population spatial synchrony by studying factors associated with geographic variation in the strength of spatial synchrony (Walter et al. 2017). Determining the causes of spatial synchrony in a given study organism is often difficult, in part, because different mechanisms can lead to similar spatial patterns, such as the tendency for the strength of synchrony to decline as the distance between populations increases. Furthermore, spatial synchrony in forest insect populations often extends over such large distances that field experiments are impractical. By exploiting geographic variation in the strength of spatial synchrony, however, researchers have begun to discover relationships between spatial synchrony and factors considered as potential drivers (Haynes et al. 2013, 2018; Walter et al. 2017).
The dynamic nature of the ranges of forest insect species reveals much about biotic processes underlying patterns of range expansion or contraction, as well as anthropogenic impacts. Temporal patterns and spatial variability in the local rate of spread of native and non-native invasive forest insects, for example, have underscored the importance of factors including forest management practices, accidental human transport of invasive insects, Allee effects (positive density dependent population growth at low densities) operating at the leading edge of invasion fronts, and population cycles in rates of spread (Johnson et al. 2006b; Tobin et al. 2007; Walter et al. 2015; Cooke and Carroll 2017). But global-scale impacts of human-induced climate change are also changing the spatial distributions of forest insect pest species. The outbreaks of some forest insect pests are occurring at higher latitudes or higher elevations than they did historically, likely because warming temperatures have led to geographic shifts in the occurrence of optimal temperatures for population growth (Carroll et al. 2004; Battisti et al. 2005; Jepsen et al. 2008, 2011; Johnson et al. 2010; Safranyik et al. 2010). Other aspects of climate change, such as milder winter temperatures and increasing summertime drought, have increased the spatial extent and duration of bark beetle outbreaks, leading to dramatic increases in tree mortality (Taylor et al. 2006; Raffa et al. 2008; Bentz et al. 2010; Cooke and Carroll 2017). The effects of climate change on forest insect pests and forest ecosystems also involve feedbacks between relatively short-term events, such as insect outbreaks, and long-term processes including regional diebacks of tree species and the increased release into the atmosphere of CO2 due to increased tree mortality (Raffa et al. 2008). For example, the implications of the positive feedback involving climate change leading to increased tree mortality due to mountain pine beetle outbreaks, increasing flux of CO2 into the atmosphere, resulting in increased climate change and thus greater likelihood of further outbreaks seem relatively clear cut (Kurz et al. 2008). However, the ramifications of climate-pest-ecosystem feedbacks are generally difficult to predict. Given the importance of understanding such interactions, shifts in the spatial dynamics of forest insect species and their impacts will likely represent a major research area for decades to come.
References
Altenkirch W, Majunke C, Ohnesorge B (2002) Waldschutz auf ökologischer Grundlage. Ulmer Verlag, Stuttgart, Germany
Aukema BH, Carroll AL, Zhu J, Raffa KF, Sickley TA, Taylor SW (2006) Landscape level analysis of mountain pine beetle in British Columbia, Canada: spatiotemporal development and spatial synchrony within the present outbreak. Ecography 29:427–441
Aukema BH, Carroll AL, Zheng Y, Zhu J, Raffa KF, Moore RD, Stahl K, Taylor SW (2008) Movement of outbreak populations of mountain pine beetle: influences of spatiotemporal patterns and climate. Ecography 31:348–358
Ayres BD, Ayres MP, Abrahamson MD, Teale SA (2001) Resource partitioning and overlap in three sympatric species of Ips bark beetles (Coleoptera: Scolytidae). Oecologia 128:443–453
Barbosa P, Schultz JC (eds) (1987) Insect outbreaks. Academic Press Inc, San Diego, CA
Battisti A, Stastny M, Netherer S, Robinet C, Schopf A, Roques A, Larsson S (2005) Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol Appl 15:2084–2096
Bentz BJ, Régnière J, Fettig CJ, Hansen EM, Hayes JL, Hicke JA, Kelsey RG, Negrón JF, Seybold SJ (2010) Climate change and bark beetles of the western United States and Canada: direct and indirect effects. Bioscience 60:602–613
Bergeron Y, Harvey B, Leduc A, Gauthier S (1999) Forest management guidelines based on natural disturbance dynamics: stand- and forest-level considerations. For Chron 75:49–54
Berryman AA (1986) Forest insects. Principles and practice of population management. Plenum Press, New York
Bjørnstad O, Falck W (2001) Nonparametric spatial covariance functions: estimating and testing. Environ Ecol Stat 8:53–70
Bjørnstad ON, Ims RA, Lambin X (1999) Spatial population dynamics: analysing patterns and processes of population synchrony. Trends Ecol Evol 14:427–431
Blackburn LM, Leonard DS, Tobin PC (2011) The use of Bacillus thuringiensis kurstaki for managing gypsy moth populations under the Slow-the-Spread program, 1996–2010, relative to the distributional range of threatened and endangered species. USDA Forest Service, Research Paper NRS-18
Borden JH (1989) Semiochemicals and bark beetle populations: exploitation of natural phenomena by pest management strategists. Ecography 12:501–510
Buonaccorsi JP, Elkinton JS, Evans SR, Liebhold AM (2001) Measuring and testing for spatial synchrony. Ecology 82:1668–1679
Calabrese JM, Fagan WF (2004) Lost in time, lonely, and single: reproductive asynchrony and the Allee effect. Am Nat 164:24–37
Carroll AL, Taylor SW, Régnière J, Safranyik L (2004) Effects of climate change on range expansion by the mountain pine beetle in British Columbia. In: Shore TL, Brooks JE, Stone JE (ed) Mountain Pine Beetle symposium: challenges and solutions. Vol. Pacific Forestry Centre Information Report BC-X-399. Natural Resources Canada, Canadian Forest Service, Victoria, pp 221–230
Cazelles B, Cazelles K, Chavez M (2014) Wavelet analysis in ecology and epidemiology: impact of statistical tests. J R Soc Interface 11:20130585
Cooke BJ, Carroll AL (2017) Predicting the risk of mountain pine beetle spread to eastern pine forests: considering uncertainty in uncertain times. For Ecol Manage 396:11–25
Coyle DR, Nebeker TE, Hart ER, Mattson WJ (2005) Biology and management of insect pests in north american intensively managed hardwood forest systems. Annu Rev Entomol 50:1–29
Didham RK, Ghazoul J, Stork NE, Davis AJ (1996) Insects in fragmented forests: a functional approach. Trends Ecol Evol 11:255–260
Esper J, Büntgen U, Frank DC, Nievergelt D, Liebhold AM (2007) 1200 years of regular outbreaks in alpine insects. Proc R Soc B: Biol Sci 274:671–679
Fleischer SJ, Blom PE, Weisz R (1999) Sampling in precision IPM: when the objective is a map. Phytopathology 89:1112–1118
Forbush EH, Fernald CH (1896) The gypsy moth. Wright and Potter, Boston, MA
Gering JC, Crist TO, Veech JA (2003) Additive partitioning of species diversity across multiple spatial scales: implications for regional conservation of biodiversity. Conserv Biol 17:488–499
Getis A (2008) A history of the concept of spatial autocorrelation: a geographer’s perspective. Geogr Anal 40:297–309
Gitau CW, Bashford R, Carnegie AJ, Gurr GM (2013) A review of semiochemicals associated with bark beetle (Coleoptera: Curculionidae: Scolytinae) pests of coniferous trees: a focus on beetle interactions with other pests and their associates. For Ecol Manage 297:1–14
Grove SJ (2002) Saproxylic insect ecology and the sustainable management of forests. Annu Rev Ecol Syst 33:1–23
Hanski I (1997) Metapopulation dynamics: from concepts and observations to predictive models. In: Hanski I, Gilpin ME (eds) Metapopulation biology. Academic Press, San Diego, pp 69–91
Hanski I (1998) Metapopulation dynamics. Nature (London) 396:41–49
Harrington R, Fleming RA, Woiwod IP (2001) Climate change impacts on insect management and conservation in temperate regions: can they be predicted? Agric For Entomol 3:233–240
Hastings A (1990) Spatial heterogeneity and ecological models. Ecology 71:426–428
Haynes KJ, Bjørnstad ON, Allstadt AJ, Liebhold AM (2013) Geographical variation in the spatial synchrony of a forest-defoliating insect: isolation of spatial and environmental drivers. Proc R Soc B: Biol Sci 280:20130112
Haynes KJ, Allstadt AJ, Klimetzek D (2014) Forest defoliator outbreaks under climate change: effects on the frequency and severity of outbreaks of five pine insect pests. Glob Change Biol 20:2004–2018
Haynes KJ, Liebhold AM, Bjørnstad ON, Allstadt AJ, Morin RS (2018) Geographic variation in forest composition and precipitation predict the synchrony of forest insect outbreaks. Oikos 127:634–642
Hodkinson ID (2005) Terrestrial insects along elevation gradients: species and community responses to altitude. Biol Rev 80:489–513
Hopkins AD (1899a) Preliminary report on the insect enemies of forests in the Northwest. U.S. Department of Agriculture Bulletin No. 21
Hopkins AD (1899b) Report on investigations to determine the cause of unhealthy conditions of the spruce and pine from 1880–1893. West Virginia Agricultural Experiment Station Bulletin #56
Hudson P, Cattadori I (1999) The Moran effect: a cause of population synchrony. Trends Ecol Evol 14:1–2
Hunter MD (2002) Landscape structure, habitat fragmentation, and the ecology of insects. Agric For Entomol 4:159–166
Ims RA, Steen H (1990) Geographical synchrony in microtine population cycles: a theoretical evaluation of the role of nomadic avian predators. Oikos 57:381–387
Jackson PL, Straussfogel D, Lindgren BS, Mitchell S, Murphy B (2008) Radar observation and aerial capture of mountain pine beetle, Dendroctonus ponderosae Hopk (Coleoptera: Scolytidae) in flight above the forest canopy. Can J For Res 38:2313–2327
Janzen DH (1987) Insect diversity of a Costa Rican dry forest: why keep it, and how? Biol J Lin Soc 30:343–356
Jepsen JU, Hagen SB, Ims RA, Yoccoz NG (2008) Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. J Anim Ecol 77:257–264
Jepsen JU, Kapari L, Hagen SB, Schott T, Vindstad OPL, Nilssen AC, Ims RA (2011) Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with sub-Arctic birch. Glob Change Biol 17:2071–2083
Johnson DM, Liebhold AM, Bjørnstad ON, McManus ML (2005) Circumpolar variation in periodicity and synchrony among gypsy moth populations. J Anim Ecol 74:882–892
Johnson DM, Liebhold AM, Bjørnstad ON (2006a) Geographical variation in the periodicity of gypsy moth outbreaks. Ecography 29:367–374
Johnson DM, Liebhold AM, Tobin PC, Bjørnstad ON (2006b) Allee effects and pulsed invasion of the gypsy moth. Nature 444:361–363
Johnson DM, Büntgen U, Frank DC, Kausrud K, Haynes KJ, Liebhold AM, Esper J, Stenseth NC (2010) Climatic warming disrupts recurrent Alpine insect outbreaks. Proc Natl Acad Sci 107:20576–20581
Jonsson BG, Kruys N, Ranius T (2005) Ecology of species living on dead wood—Lessons for dead wood management. Silva Fennica 39:289–309
Knops JMH, Tilman D, Haddad NM, Naeem S, Mitchell CE, Haarstad J, Ritchie ME, Howe KM, Reich PB, Siemann E, Groth J (2002) Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol Lett 2:286–293
Komonen A (2003) Hotspots of insect diversity in boreal forests. Conserv Biol 17:976–981
Krige DE (1999) Essential basic concepts in mining geostatitics and their links with geology and classical statistics. S Afr J Geol 102:147–151
Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature 452:987–990
Landolt PJ (1997) Sex attractant and aggregation pheromones of male phytophagous insects. Am Entomol 43:12–22
Legendre P, Fortin M-J (1989) Spatial pattern and ecological analysis. Vegetatio 80:107–138
Levins R (1969) Some demographic and genetic consequences of environmental heterogeneity for biological control. Bull Entomol Soc Am 15:237–240
Liebhold AM, Rossi RE, Kemp WP (1993) Geostatistics and geographical information systems in applied insect ecology. Annu Rev Entomol 38:303–327
Liebhold AM, Koenig WD, Bjørnstad ON (2004) Spatial synchrony in population dynamics. Annu Rev Ecol Evol Syst 35:467–490
Liebhold AM, Haynes KJ, Bjørnstad ON (2012) Spatial synchrony of insect outbreaks. In: Barbosa P, Letourneau DK, Agrawal AA (eds) Insect outbreaks revisited. Wiley-Blackwell, Oxford, UK, pp 113–125
Liebhold AM, Gottschalk KW, Muzika RM, Montgomery ME, Young R, O’Day K, Kelley B (1995) Suitability of North American tree species to the gypsy moth: a summary of field and laboratory tests. USDA Forest Service, General Technical Report NE-211
Logan JA, Régnière J, Powell JA (2003) Assessing the impacts of global warming on forest pest dynamics. Front Ecol Environ 1:130–137
Mattson WJ, Haack RA (1987) The role of drought in outbreaks of plant-eating insects. Bioscience 37:110–118
McCoy ED (1990) The distribution of insects along elevational gradients. Oikos 58:313–322
McCullough DG, Werner RA, Neumann D (1998) Fire and insects in Northern and boreal forest ecosystems of North America. Annu Rev Entomol 43:107–127
Moran PAP (1953) The statistical analysis of the Canadian lynx cycle. II. Synchronization and meteorology. Aust J Zool 1:291–298
Muzika RM, Liebhold AM (2000) A critique of silvicultural approaches to managing defoliating insects in North America. Agric For Entomol 2:97–105
Myers JH (1998) Synchrony in outbreaks of forest Lepidoptera: a possible example of the Moran effect. Ecology 79:1111–1117
Noriega JA, Hortal J, Azcárate FM, Berg MP, Bonada N, Briones MJI, Del Toro I, Goulson D, Ibanez S, Landis DA, Moretti M, Potts SG, Slade EM, Stout JC, Ulyshen MD, Wackers FL, Woodcock BA, Santos AMC (2018) Research trends in ecosystem services provided by insects. Basic Appl Ecol 26:8–23
Nyland RD (2007) Silviculture: concepts and applications, 2nd edn. Waveland Press, Long Grove, IL
Opdam P, Wascher D (2004) Climate change meets habitat fragmentation: linking landscape and biogeographical scale levels in research and conservation. Biol Cons 117:285–297
Paine TD, Birch MC, Švihra P (1981) Niche breadth and resource partitioning by four sympatric species of bark beetles (Coleoptera: Scolytidae). Oecologia 48:1–6
Paine TD, Raffa KF, Harrington TC (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42:179–206
Peltonen M, Liebhold AM, Bjørnstad ON, Williams DW (2002) Spatial synchrony in forest insect outbreaks: roles of regional stochasticity and dispersal. Ecology 83:3120–3129
Raffa KF (2001) Mixed messages across multiple trophic levels: the ecology of bark beetle chemical communication systems. Chemoecology 11:49–65
Raffa KF, Aukema BH, Bentz BJ, Carroll AL, Hicke JA, Turner MG, Romme WH (2008) Cross-scale drivers of natural disturbances prone to anthropogenic amplification: the dynamics of bark beetle eruptions. Bioscience 58:501–517
Riley CV, Vasey G (1870) Imported insects and native American insects. Am Entomol 2:110–112
Robinet C, Lance DR, Thorpe KW, Tcheslavskaia KS, Tobin PC, Liebhold AM (2008) Dispersion in time and space affect mating success and Allee effects in invading gypsy moth populations. J Anim Ecol 77:966–973
Roland J (1993) Large-scale forest fragmentation increases the duration of tent caterpillar outbreak. Oecologia 93:25–30
Rossi RE, Mulla DJ, Journel AG, Franz EH (1992) Geostatistical tools for modeling and interpreting ecological spatial dependence. Ecol Monogr 62:277–314
Royama T (1984) Population dynamics of the spruce budworm, Choristoneura fumiferana. Ecol Monogr 54:429–492
Royama T, MacKinnon WE, Kettela EG, Carter NE, Hartling LK (2005) Analysis of spruce budworm outbreak cycles in New Brunswick, Canada, since 1952. Ecology 86:1212–1224
Royama T (1992) Analytical population dynamics. Chapman & Hall, London, UK
Safranyik L, Linton DA, Silversides R, McMullen LH (1992) Dispersal of released mountain pine beetles under the canopy of a mature lodgepole pine stand. J Appl Entomol 113:441–450
Safranyik L, Linton DA, Shore TL (2000) Temporal and vertical distribution of bark beetles (Coleoptera: Scolytidae) captured in barrier traps at baited and unbaited lodgepole pines the year following attack by the mountain pine beetle. Can Entomol 132:799–810
Safranyik L, Carroll AL, Régnière J, Langor DW, Riel WG, Shore TL, Peter B, Cooke BJ, Nealis VG, Taylor SW (2010) Potential for range expansion of mountain pine beetle into the boreal forest of North America. Can Entomol 142:415–442
Sambaraju KR, Carroll AL, Zhu J, Stahl K, Moore RD, Aukema BH (2012) Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 35:211–223
Sartwell C, Stevens RE (1975) Mountain pine beetle in ponderosa pine–Prospects for silvicultural control in second-growth stands. J Forest 73:136–140
Schoener TW (1974) Resource partitioning in ecological communities. Science 185:27–39
Sharov AA, Leonard D, Liebhold AM, Clemens NS (2002) Evaluation of preventive treatments in low-density gypsy moth populations. J Econ Entomol 95:1205–1215
Southwood TRE (1978) Ecological methods with particular reference to the study of insect populations. Chapman and Hall, London, UK, 524 pp
Swanson BJ, Johnson DR (1999) Distinguishing causes of intraspecific synchrony in population dynamics. Oikos 86:265–274
Taylor LR (1961) Aggregation, variance, and the mean. Nature 189:732–735
Taylor LR (1984) Assessing and interpreting the spatial distributions of insect populations. Annu Rev Entomol 29:321–357
Taylor AD (1990) Metapopulations, dispersal, and predator-prey dynamics: an overview. Ecology 71:429–433
Taylor SW, Carroll AL, Alfaro RI, Safranyik L (2006) Forest, climate and mountain pine beetle outbreak dynamics in western Canada. In: Safranyik L, Wilson B (eds) The mountain pine beetle a synthesis of biology, management, and impacts on lodgepole pine. Natural Resources Canada, Canadian Forest Service, Victoria, BC, pp 67–94
Tobin PC (2004) Estimation of the spatial autocorrelation function: consequences of sampling dynamic populations in space and time. Ecography 27:767–775
Tobin PC, Sharov AA, Liebhold AM, Leonard DS, Roberts EA, Learn MR (2004) Management of the gypsy moth through a decision algorithm under the Slow-the-Spread project. Am Entomol 50:200–209
Tobin PC, Whitmire SL, Johnson DM, Bjørnstad ON, Liebhold AM (2007) Invasion speed is affected by geographic variation in the strength of Allee effects. Ecol Lett 10:36–43
Tobin PC, Bai BB, Eggen DA, Leonard DS (2012) The ecology, geopolitics, and economics of managing Lymantria dispar in the United States. International Journal of Pest Management 53:195–210
Tobin PC, Parry D, Aukema BH (2014) The influence of climate change on insect invasions in temperate forest ecosystems. In: Fenning T (ed) Challenges and opportunities for the world’s forests in the 21st century. Springer, pp 267–296
Torrence C, Compo GP (1998) A practical guide to wavelet analysis. Bull Am Meteor Soc 79:61–78
Turner MG (2010) Disturbance and landscape dynamics in a changing world. Ecology 91:2833–2849
Walter JA, Meixler MS, Mueller T, Fagan WF, Tobin PC, Haynes KJ (2015) How topography induces reproductive asynchrony and alters gypsy moth invasion dynamics. J Anim Ecol 84:188–198
Walter JA, Sheppard LW, Anderson TL, Kastens JH, Bjørnstad ON, Liebhold AM, Reuman DC (2017) The geography of spatial synchrony. Ecol Lett 20:801–814
Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389
Weed AS, Ayres MP, Hicke JA (2013) Consequences of climate change for biotic disturbances in North American forests. Ecol Monogr 83:441–470
Wickman P-O, Rutowski RL (1999) The evolution of mating dispersion in insects. Oikos 84:463–472
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Tobin, P.C., Haynes, K.J., Carroll, A.L. (2023). Spatial Dynamics of Forest Insects. In: D. Allison, J., Paine, T.D., Slippers, B., Wingfield, M.J. (eds) Forest Entomology and Pathology. Springer, Cham. https://doi.org/10.1007/978-3-031-11553-0_18
Download citation
DOI: https://doi.org/10.1007/978-3-031-11553-0_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11552-3
Online ISBN: 978-3-031-11553-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)