Abstract
South Africa is the World’s largest producers of macadamia nuts, with about 51,000 ha of land covered by macadamia. This leads to major farming challenges, as the expansion of orchards is associated with the loss of habitat and biodiversity, the excessive use of and resistance to insecticides, and an increased pressure on water resources. More frequent and severe droughts and heat waves are projected to worsen the situation and have already negatively affected harvests. Here we review current literature and recent work conducted in the subtropical fruit growing area of Levubu, South Africa, which include catchment-scale assessments of ground water, landscape-scale studies on pest control and pollination services, through to evaluations of tree-level water use. Several biological control options are being developed to replace pesticides. Results suggest that bats and birds provide large and financially measurable pest control services, and interventions should therefore focus on maintaining functional landscapes that would be resilient in the face of global climate change. This would include a landscape matrix that includes natural vegetation and minimize water consumption by optimizing irrigation schedules.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Macadamias are currently one of the most expensive nuts in the world, at nearly twice the price of almonds. The industry is projected to have a compound annual growth rate of 6.8% between 2020 and 2025 (APNEWS 2020). The macadamia nut’s ‘healthy whole food’ image has created a growing demand for macadamias as ingredients and processed products in the food and beverage but also cosmetic industry (Green and Gold Macadamias 2018).
While the trees originated in north-eastern Australia, the first commercial cultivation of Macadamia integrifolia Maiden & Bechte started in Hawaii in 1931 (De Villiers and Joubert 2003). Macadamia trees require a tropical to subtropical climate with high annual rainfall above 1000 mm. The trees grow well in soil with a high organic content but not on saline or sodic soils (De Villiers and Joubert 2003).
According to SAMAC (2021), the largest producers of macadamia nuts in 2020 were South Africa (48,925 tons) and Kenya (42,530 tons) followed by Australia (42,000 tons). However, China is projected to grow its production from ca. 18,000 tons in 2018 to ca. 450,000 tons by 2025 (AGTAG 2018). Other macadamia growing countries include Zimbabwe, USA, Israel, New Zealand, Vietnam, and Brazil (DAFF 2019).
In South Africa, macadamias have been one of the fastest growing tree crop industries in the last decade, providing seasonal and permanent employment for over 20,000 people. The value of the annual production was 4.8 billion ZAR or about 330 million USD in 2019 (SAMAC 2020). Main growing areas in South Africa are the provinces of Limpopo, Mpumalanga, coastal KwaZulu-Natal, and the Eastern Cape. South African macadamia orchards cover approximately 51,000 ha, of which around 6000 were planted in 2019 alone (DAFF 2019). This expansion is set to continue until 2030, with 1000 additional seasonal and permanent farm workers employed yearly (SAMAC 2020). However, the ongoing expansion of macadamia orchards also leads to farm and landscape management challenges. Monocultures, the excessive use of insecticides, and habitat loss at a local and landscape scale are associated with the loss of natural enemies of crop pests such as bats, birds, predatory insects as well as the loss of wild pollinators (Foley et al. 2005; Tilman et al. 2001; Tscharntke et al. 2012; Weier et al. 2021). Furthermore, the increased and incorrect use of pesticides has led to resistance in pests such as stinkbugs (Hemiptera: Pentatomidae) (Schoeman 2018). Additionally, South Africa has experienced some severe droughts in recent years, with the combined effects of an El Niño event (Baudoin et al. 2017). Already one of the driest countries in the world, these droughts are predicted to worsen under future climate change and regularly impact harvests negatively throughout South Africa (Mogoatlhe 2020).
This chapter is based on work conducted in the subtropical fruit growing area of Levubu in the Luvuvhu river valley, situated in the northernmost South African province Limpopo. Macadamia integrifolia has been cultivated here for over 60 years (Ahrens 1991). Levubu is one of the two main growing areas in the province, with about 10,000 ha of macadamia planted thus far. This sub-Saharan African region receives its main rain in the summer months between November and April with around 1000 mm of annual rainfall. Apart from macadamia, the main agricultural products farmed are banana (Musaceae), avocado (Persea), timber (Eucalyptus and Pinaceae) and to a lesser extent mango (Mangifera), pecan (Carya), lychee (Litchi), or maize (Zea).
The Levubu area is on the south-eastern slopes of the Soutpansberg mountain range, part of the UNESCO Vhembe Biosphere Reserve (VBR). The Soutpansberg is a centre for plant endemism with a remarkably high animal biodiversity (Hahn 2017; Joseph et al. 2019; UNESCO 2010; Taylor et al. 2013, 2015; Van Wyk and Smith 2001). Also see Chap. 23.
Given the rapidly increasing demands on biodiversity and water-related ecosystem services due to agricultural intensification and climate change in South Africa, the aim of this chapter is to review the current literature and synthesize a decade of research in the Levubu area and provide recommendations on the mitigation of agricultural intensification and climate change effects for more sustainable agricultural practices.
2 Water Management
2.1 Water Availability and Macadamia Irrigation
The climatic conditions of the main macadamia cultivation area in Limpopo, are characterized by unevenly distributed annual rainfall that rarely exceed 1000 mm. Macadamia trees in the region therefore require supplementary irrigation for good yields and optimal quality (Carr 2012; Murovhi 2003). The South African Macadamia Growers Association (SAMAC) latest census in 2012 suggests that 80% of the macadamia growing area in Limpopo is irrigated, this figure has subsequently increased even further. The resultant growing demand for irrigation water increases pressure on the limited water resources of the province.
Long-term flow monitoring (80 years) of the Luvuvhu river (the main tributary in the catchment), where it leaves the commercially irrigated agricultural area, points to significant decreases in stream flow (Ramulifho et al. 2021). These decreases are highly seasonal with significant reductions from November to February, periods that coincide with peak water demands of macadamia (Fig. 22.1), and have already resulted in the cessation of flow of the Luvuvhu during certain parts of the year (Ramulifho et al. 2019). Regionally, climate change is predicted to result in 5–10% decrease in rainfall (Hewitson and Crane 2006; Conway et al. 2015; IPCC 2021).
Groundwater levels measured from 2007–2013 in the Luvuvhu catchment show that the lowest groundwater level occurs between October and November and has decreased by around 2 m (23–25 m) (Makungo and Odiyo 2017). Although other land-use systems, such as gum (Eucalyptus ssp.) plantations, are less water efficient than macadamias per unit of land area (Botha 2018), commercial irrigated farming is one of the main sources of water consumption in the province (Shabalala et al. 2022). Finally, newly established macadamia orchards in the region (the fastest growing crop in the Soutpansberg in terms of its expansion) are increasingly located in more arid areas of the mountain and its surrounds, which is furthermore increasing the demand for ground water.
Historically, the Limpopo growers strongly relied on surface water but a combination of politics and poor maintenance of the water infrastructures leads to a major shift towards the use of groundwater in the early 2000s (Stephan Schoeman, personal communication). This is unsustainable given the slow recharge of the water table, which is around 4% of the mean annual precipitation and further evidenced by boreholes running dry in recent years.
Very little is known about the status of groundwater in the province. This is particularly concerning within the context of climate change and the relevance to monitoring the allocation of water licences by Water boards (National Water Act 1998). Licences for specific water volumes generally depend on the size of the production area and are legally allocated and verified through formal processes. Macadamia growers purchase water licences from local authorities, which also monitor on-farm water use.
2.2 Sustainable Water Management Practices
Given the circumstances, it is paramount for macadamia growers to increase the efficiency and sustainability of water use. This can be done by: (1) choosing the appropriate irrigation system, (2) meeting tree water requirements better with irrigation water supply, (3) using advanced technology for a better understanding and monitoring of water dynamics in the orchards, and (4) adopting water-saving agricultural practices in the orchards.
A reflection on the best water management options for macadamia is bound to start with the question on whether such trees need to be irrigated at all. About 20% of macadamia producers in South Africa rely solely on rainfall as a source of water for their orchards (Fig. 22.2). Although irrigation is considered desirable, especially in areas where the average annual rainfall is less than 1000 mm (Carr 2012), there is no experimentally sound quantification of yield reductions for rain-fed macadamia production (compared to that under irrigation). Different studies show contradictory results and strongly depend on the specific climatic conditions of the growing areas and seasons considered (Trochoulias and Johns 1992; Searle and Lu 2002). Moreover, macadamia yields are cyclical and highly variable, which makes it somewhat difficult to establish cause and effect (Carr 2012; Huett 2004).
Some critical phenological stages, i.e., periods during which lack of sufficient water supply can strongly affect macadamia production, are the periods between flowering and nut set (August–October), and the premature nut drop period (November–December). Trees experiencing water stress during such periods produce less flowers, with an overall reduced nut set (Murovhi 2003) and increased nut drop (Carr 2012).
Furthermore, water stress during the nut maturation stages decreases photosynthesis rates at a time when energy demands for oil accumulation are highest. This consequently reduces yield and nut quality (Stephenson et al. 2003). Another major disadvantage of water deficits in the roots zone is that the tree cannot take up nutrients (including those supplied by fertilizers). Therefore, according to a local independent macadamia consultant (S. Schoeman, personal communication) rain-fed macadamia production in Limpopo is not considered a generally viable option for the future, although some niche microclimates allow for it. An option which might receive more attention by macadamia growers in the future is deficit irrigation, in the form of nearly rain-fed production with supplementary irrigation during specific critical phenological stages or in case of prolonged droughts. At present, such an approach is still rather uncommon and would require thorough scientific investigation as well as commercial evaluation.
Historically, there are two main irrigation systems for macadamias in South Africa, namely micro-sprinklers and drip irrigation (Murovhi 2003). Micro-sprinklers, currently the most common irrigation system in South Africa (Fig. 22.2), allow for a wide range of emitter flow rates, from as low as 15 L to more than 100 L/h. The large wetting radius matches the tree’s root surface area, thereby increasing water and nutrient uptake. At the same time, a large part of the applied water could be lost to evaporation or losses from the root zone (e.g. by deep percolation), or be taken up by grass and weeds growing around the trees, thus strongly reducing the system’s efficiency as well as water productivity. Due to the large amounts of water applied, this system is generally used at a low frequency of one to three times per week.
Drip irrigation requires more frequent applications (up to 300 days/year) of smaller water amounts (emitter delivery rates commonly vary between 0.7 L and 4.0 L/h). Dripper lines are placed close to the tree stems, with spacing’s of 0.6 m between drippers. Compared to the micro-sprinkler irrigation, this system requires less water, despite maintaining a continuous wetted strip along the tree line, thus having a higher system efficiency and water productivity. Therefore, the adoption of drip irrigation has increased in recent years (and since the 2012 Census) to about 40% of the new installations, mainly at the expense of micro-sprinkler irrigation (S. Schoeman, personal communication).
Recently, a new system is emerging, following the principle of applying small amounts of water at high frequency, with the aim of better matching irrigation water supply with the rate of plant water uptake. This is the centralized low-flow drip fertigation concept (or the ultra-low flow drip in its most extreme version). In comparison to the regular drip irrigation, these irrigation systems have lower emitter delivery rates of 0.6–0.7 L/h (0.4 L/h for ultra-low flow drip) and wider spacing between drippers of 0.9–1.0 m, leading to low system delivery rates, which require higher, almost daily, irrigation frequencies. The concept is to apply the daily irrigation requirement of the trees almost at the same rate that the tree uses the water (i.e. with system flow rates of typically 0.15–0.3 mm/h) and mostly rely on capillary water movement rather than mass flow in the soil, thus leading to significantly reduced risk of soil saturation and run-off. With 1–2 mm of water applied per day over several hours in a very efficient way, the irrigation of the entire farm at the same time becomes possible with a centralized and labour-friendly application management. This also allows for the coupling of irrigation and fertilization (the so-called fertigation), which can both be targeted to the tree’s daily needs year-round. The limitations of this system include its requirement of a more complicated design with multiple dedicated mainlines to each field valve, the higher installation costs, mainly related to the fertigation injection systems, and the need of rearranging the scheduling of irrigation events. Furthermore, due to the low delivery rates, it is important to monitor and fully understand the soil water dynamics (e.g. the required filling time for the soil reservoir) and manage it accordingly. Nevertheless, it is currently considered as the most promising irrigation system by sector experts (S. Schoeman and Barry Christie—technical manager of Green Farms Nut Company, personal communication), allowing the achievement of the highest system efficiency and reducing water consumption to a minimum. Therefore, low-flow drip irrigation systems are expected to increase in the future, with large investments forecasted for their establishment, especially in large farms. Another less common irrigation system is the so-called floppy sprinkler irrigation, however, its very low efficiency, and large water volumes applied, preclude it from receiving considerable attention in the South African context.
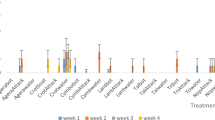
Despite the good intentions of most macadamia growers and consultants to improve water management there is still surprisingly little knowledge on the exact water requirements of macadamias. So far, growers have mostly relied on management guide charts based on accumulated empirical evidence on daily or weekly recommended water requirements for the different phenological stages of trees with different ages, planting densities, and canopy coverages (Fig. 22.1). However, these are merely used as guidelines, for example, for the planning of the irrigation system given a certain water allocation, and sometimes regarded as excessively high (Lee 2020).
In one of the few well-known attempts to experimentally quantify macadamia tree water use amounts for Australian conditions, Stephenson et al. (2003) reported estimates of daily evapotranspiration ranging between 52 L (winter) and 80 L per tree (summer) for ‘HAES 246’ cultivars growing on sandy soils. In South Africa, Ibraimo et al. (2014) measured average daily water uses ranging between 27 L and 51 L/day in 6-year-old (intermediate bearing) macadamia trees (‘Beaumont’ cultivar). In a follow-up study, Taylor et al. (2021) attempted to distinguish between the water use of intermediate bearing and full-bearing ‘Beaumont’ macadamia trees, reporting comparatively lower average daily water use values of 22–35 L for the full-bearing trees, about 60% higher than for the younger trees. In general, they claim that such values are strictly depending on local environmental conditions, tree canopy size, and management factors, thus making it very difficult to provide precise estimates of macadamia tree water use without having additional on-field measurements of tree transpiration in a range of different orchards (Taylor et al. 2021). Furthermore, different macadamia cultivars show different water requirements. For example, the widely popular variety ‘Beaumont’ (‘HAES 695’) is known to cope poorly with low water availability (S. Schoeman, personal communication). On the other hand, daily transpiration measurements in an Australian macadamia study (Searle and Lu 2003) showed almost double the water use by cultivar ‘HAES 741’ compared to that of ‘HAES 344’. It would be therefore of great importance to get a better knowledge and understanding of the differences in transpiration between different cultivars, as well as of their specific performance in relation to the growing environment (Taylor et al. 2021). Common on-field strategies used by macadamia growers to determine the soil water status and to schedule irrigation accordingly include monitoring of the weather conditions (i.e. the variables that influence tree evapotranspiration) and using devices such as tensiometers and capacitance probes, which, respectively, measure soil water tension and soil moisture at different depths. However, oversimplified empirical norms are often followed to determine when it is necessary to irrigate. Yet an increased attention and investment by South African macadamia growers into monitoring the water status of their orchards has been observed in recent years (S. Schoeman, personal communication).
One of the risks growers are increasingly aware of is that of over-irrigating. According to recent studies, this is often the case (Ibraimo et al. 2014; Botha 2020). In experiments conducted on Beaumont macadamia orchards in Mpumalanga, a conservative water use behaviour of macadamias was observed, with a halt of tree transpiration when a certain level of evaporative demand (typically at a leaf-to-air vapour pressure deficit above 2 kilopascal) is reached (Smit et al. 2020). This climate-induced control is exerted through the closure of stomata (Lloyd 1991; Smit et al. 2020). This indicates that under hot and dry conditions, the trees will not necessarily use more water and the application of large irrigation amounts under these conditions would not lead to the desired outcome. On the contrary, excessive irrigation might lead to a reduction in soil aeration, especially in saturated soils, thereby further restricting water uptake and affecting tree health, growth, and nut yield (Botha 2020). Other negative effects of over-irrigation include iron deficiency, increased susceptibility to Phytophthora, and the loss of fertilizer by leaching, with the related economic and environmental impacts.
A number of promising technological innovations could further help to improve the sustainability of water management in macadamia orchards. For instance, better weather forecasts through improved climatic models would help to plan irrigation accordingly. Similarly, the increasing availability of more affordable weather stations and soil moisture probes shall facilitate the on-field monitoring of climatic conditions and soil water dynamics. Remote sensing and especially aerial photography are likely to play an increasingly important role, since they are proving to be very useful and labour-friendly tools to detect problems in the orchard. Farm management apps and portals will facilitate sharing information between macadamia growers and consultants. Nevertheless, farming from remote is far from being a feasible reality. In fact, according to macadamia expert Barry Christie (personal communication), although increasing, the adoption by macadamia growers of most of the above-mentioned innovations is still low. Other sustainable management options to reduce orchard water consumption include the adoption of water-saving agricultural practices like mulching. The presence of organic matter (leaves, husks, or compost) on the soil surface is crucial for tree health and it reduces evaporation and conserves water within the soil (Botha 2020; Steyn 2019). Inorganic options (e.g. weed mat) also exist and are sometimes used mainly in young orchards, where soil evaporation is especially high due to the greater area exposed to solar radiation between the tree rows. Careful management of weeds and grass cover in the orchards can also help reduce water losses caused by their transpiration (Botha 2020).
Recent droughts between 2015 and 2019 have increased the awareness of limits to water resources among South African macadamia growers, who are trying to decrease their water consumption, for example by switching to more efficient irrigation systems or by improving the monitoring of water use to avoid the risk of over-irrigating. Nevertheless, such efforts cannot fully counter-balance the overall increased water consumption, due to the continuous expansion of irrigated macadamia production areas. However, water availability and the impact of climate change on the local water resources are not yet perceived as major risks by macadamia growers. According to S. Schoeman (personal communication) and findings from a macadamia growers workshop held at Levubu in February 2019, major concerns include issues related to pest control, future political scenarios, energy prices, and industry developments. The general perception is that reduced water availability will increase production costs but growers will still prefer growing macadamias because of their high market value. However, under the projected drought scenarios and the stricter policing of water allocations, this might not be possible for much longer (Botha 2020; Shabalala et al. 2022). Therefore, the only solution lies in increasing the water use efficiency of macadamia orchards, making use of the best available knowledge, technologies, and practices to reduce non-beneficial water losses to a minimum.
2.3 Suggestions Towards More Sustainable Water Management in Macadamia Orchards by SALLnet
Science has to play a distinct role in improving water management in times of a changing climate. More science-informed decision-making can be provided, for example, by delivering experimental evidence on the performance of different systems and management options. This should be based on a deep understanding of the relevant ecophysiological processes, which influence tree water requirements, the effects of management and by more robust climate projections at the local scale.
In the framework of the SPACES II—SALLnet joint research project, an ongoing study of macadamia water use aims to increase our understanding and gain new insights into the processes determining the interactions of genotype, environment, and management in macadamia orchards, represented by different macadamia management systems in Levubu, with a focus on water dynamics.
To this end, intensive field experiments monitoring the hourly tree transpiration rates, daily water use, and leaf water potential (an indicator of water stress) of two different macadamia cultivars (‘Beaumont’ and ‘HAES 849’) were set up and run for two consecutive seasons (Fig. 22.3). Additional measurements were made on tree phenological development (number of racemes and nuts per tree), tree morphology (e.g. tree height, canopy volume, leaf area density, etc.), and nut production. Moreover, microclimate, soil water dynamics, and orchard management (especially irrigation) were also recorded.
That dataset contributes to a better understanding of the water use behaviour of macadamia trees in response to different water supply and environmental conditions, as well as the quantification of macadamia water use efficiency for contrasting management intensities. A related objective is the development of a macadamia growth and water use model to, among others, assist in setting the upper and lower limits of required water inputs for macadamia trees in different environmental conditions, and thus improving the precision of current empirical approaches to compute fruit tree water requirements (Orgaz et al. 2007; Villalobos et al. 2013) and to avoid wasteful over-irrigation. Eventually this will allow for upscaling of results from field experimentation across the whole region and for different climatic scenarios (e.g. by simulating the impact of future projections of long-term climate change on macadamia water use).
3 Pollination
In addition to abiotic and management factors, biotic factors such as pollination and biological control also determine macadamia production (Grass et al. 2018; Linden et al. 2019). Here we present management strategies that facilitate pollination in macadamia orchards in order to increase nut set and hence yield in a sustainable way.
Pollination Requirements of Macadamia
Macadamia is a mass-flowering crop of which one mature tree can produce up to 2500 inflorescences in one season (Moncur et al. 1985). The inflorescences are arranged in racemes of 10–35 cm length and each one bears 100–300 flowers (Fig. 22.4) depending on the variety (Trueman 2013). The small white flowers (the Beaumont variety produces pink flowers) develop from several whirls on the stalk and form one conspicuous inflorescence. The flowers are open for 1 week (Ito and Hamilton 1980; Sedgley 1983) and given that the pollination was successful, they develop into initial nuts 3 weeks after anthesis (Trueman and Turnbull 1994a; Wallace et al. 1996). Many immature nuts abscise during the first 7–15 weeks after anthesis, whereas the time and extent of the drop depend on the site, cultivar, time since canopy pruning (McFadyen et al. 2011, 2012; Sakai and Nagao 1985; Trueman 2010; Trueman and Turnbull 1994b) as well as pest damage (see Sect. 22.4.1). However, the nuts that remain on the raceme for around 15–20 weeks (final nut set) are likely to remain until maturation. The nuts are harvested from the orchard floor after they drop maturely off the tree, although the variety Beaumont must be treated with ethylene-generating compound (2-chloroethyl) phosphonic acid to induce the nut drop (Richardson and Dawson 1993).
The flowers of macadamia show features that indicate dependence on insect pollination, namely the bright colour of the petals, a strong scent as well as resources like pollen or nectar. The most observed agents for pollen transfer are honeybees (Apis mellifera L.) and stingless bees (Tetragonula spp.), but beetles, flies and even birds have also visited flowers and been considered potential pollinators (Heard and Exley 1994; Howlett et al. 2015). Although wind pollination might be possible (Urata 1954), several pollinator exclusion experiments indicate a strong pollinator dependency (Grass et al. 2018; Tavares et al. 2015; Wallace et al. 1996). Grass et al. (2018) demonstrated that where insects were prevented from visiting flowers, initial and final nut set was reduced by 80% and by 54%, respectively. Further, Heard (1993) showed initial and final nut set in macadamia to be correlated with increased insect visitation to flowers. As macadamia is partially self-incompatible (Hardner et al. 2009; Urata 1954), self-fertilization is possible, but minimized through flower morphology (Sedgley 1983; Urata 1954). This underpins the dependency of macadamia on animal-pollination.
3.1 Potential Pollinators of Macadamia Crops
In their Australian native range, macadamias have two main pollinators, endemic stingless bees (Tetragonula spp.) and introduced honeybees (Apis mellifera) (Howlett et al. 2015; Vithanage and Ironside 1986). Both are commonly used for pollination in commercial macadamia orchards.
A study by Heard and Exley (1994) in Australian macadamia orchards observed honeybees (60.5%), stingless bees (35.8%), while the remaining 4% were butterflies (Lepidoptera), hoverflies (Syrphidae), other Hymenopterans and even birds. Stingless bees mainly collect pollen and thus have intimate contact to the stigma; this is why they are considered to be very efficient. In contrast, honeybees first collect nectar and are considered less efficient, but compensate through high visitation rates (Heard 1994).
In Hawaii, where macadamia has been cultivated since the 1920s (Shigeura and Ooka 1984), honeybees are considered to be the most important pollinators, although other pollinator taxa such as hoverflies have also been observed to visit macadamia flowers (Tavares et al. 2015). In Brazil, butterflies accounted for 50% of flower visits, ensuring initial nut set of inflorescences in the same magnitude as hand cross-pollination (Santos et al. 2020).
In South African orchards, visual observations revealed that 90–99% of the flower visitors were honeybees, the remainder comprised of complemented by hoverflies, wasps, stingless bees, wild bees, and butterflies (Grass et al. 2018; Anders et al. unpublished data). Another study, in the same region, observed a lower ratio of honeybees (65%) associated and a higher frequencies of Diptera spp. (33%) (Ramotjiki 2020). In the macadamia region in Levubu, wild honeybee colonies are commonly found in natural or semi-natural habitat around the orchards, where they colonize suitable nesting sites. This means that besides managed honeybees, wild honeybees also provide pollination service in the orchards, as long as the landscape includes appropriate patches of natural habitat. Hence, both managed and wild honeybees must be taken into consideration as important pollinators for macadamia in this region.
Although thrips (Thripidae) are considered a pest on macadamia (see Sect. 22.4.1), their contribution to pollination remains unclear. They are found in vast numbers in flowers of a large range of plants. Whereas some species are pollinators (Mound 2005), individual thrips have been recorded consuming more than 1500 pollen grains per day, depending on the pollen grain size (Kirk 1987). Because they move between the flowers of a macadamia raceme, they are very likely to transport pollen between flowers of the same raceme. However, a recent pollinator exclusion study has shown that thrips might have been largely overlooked as an important pollinator of macadamias, as the final nut set of macadamia was positively correlated with the number of adult thrips on flowers (Meyer 2016).
3.2 Pollination Limitation
Although macadamia is dependent on insect pollination, there is evidence for pollination limitation despite honeybee management. High nutrient demand of the nuts results in high abscission rates and only a small proportion of flowers (3%) develops into mature nuts (Evans et al. 2021; Grass et al. 2018). Even lower proportions are not unusual, with only 0.3% (Ito and Hamilton 1980) and 0.6% observed (Sakai and Nagao 1985).
On the other hand, supplemental hand-pollination resulted in a significantly higher initial (66%) and final (44%) nut set than natural pollination in the study of Grass et al. (2018), corresponding with other studies (Howlett et al. 2019; Wallace et al. 1996). Further, recent studies showed that macadamia is much more self-incompatible than previously thought. Genetic analyses demonstrated that depending on the cultivar 80–90% of the harvested nuts were cross-pollinated while only up to 8% were self-pollinated (Richards et al. 2020; Kämper et al. 2021). Grass et al. (2018) concluded that honeybees fail to deliver adequate pollination services, especially as increasing their colony density could even result in reduced final nut set. Higher visitation rates were neither related to higher bee density nor nut set. Intraspecific competition at high colony densities may have led honeybees to repeatedly exploit the same resources, reducing cross-pollination between macadamia trees and varieties. This means, efficient pollination is not simply determined by a high number of pollinators, but also by other factors, for example their foraging behaviour on the flower or their movement between the trees. Also, the landscape configuration, i.e. the cover of natural habitat is likely to affect pollinator behaviour and pollination services.
In order to get a broader understanding of pollination limitation and services, we established another macadamia pollination experiment in the Levubu region in 2019 and 2020, where we simultaneously observed different potential influences on pollination and yield, incorporating irrigation or rain-fed production as well as landscape factors such as altitude and amount of semi-natural vegetation in the landscape. Grass et al. (2018) did not detect higher pollinator visitation rates or nut set on trees close to natural habitat and hence no spillover effect from these to macadamia orchards. However, their study did not consider landscape effects. The surrounding landscape of macadamia orchards can provide additional nesting and foraging resources for wild bees and thus can influence pollinator diversity in macadamia orchards and consequently crop pollination services (Bänsch et al. 2021; Beyer et al. 2021a). The objective of the project is to gain a deeper understanding of the interaction between different management and landscape contexts, and pollination in order to improve pollination services in macadamia orchards (see Fig. 22.5).
3.3 Management Strategies to Facilitate Pollination Services in Macadamia Orchards
Pollination of macadamia is important for nut production, but the provision of optimal pollination services is not attained by simply increasing managed honeybee colonies. One option is the enhancement of cross-pollination. For commercial nut production, a large number of different varieties are cultivated. The role of cross-pollination between varieties has been explored in a couple of studies. Supplemental hand cross-pollination enhances not only fruit set (Herbert et al. 2019; Howlett et al. 2019; Trueman and Turnbull 1994a; Wallace et al. 1996) but also nut weight (Herbert et al. 2019). By manually cross-pollinating almost an entire tree, Trueman et al. (2022) achieved an increase of up to 109% of kernel yield. Empirical studies confirmed these results, identifying higher yield and nut mass in blocks where several varieties are grown than in single-variety blocks and a decrease in harvested nuts with distance to the cross-variety pollen source (Ito and Hamilton 1980; Kämper et al. 2020). However for individual varieties, recent genetic examination indicated unexpected high degrees of self-fertilization of up to 20–40% (Langdon et al. 2019).
The planting of different and ideally well matching varieties in close distances is still a promising management strategy to increase cross-pollination.
Another option to increase pollination service is the promotion of semi-natural pollinator habitat. Managed as well as wild pollinators profit from natural or semi-natural habitat, which provides resources throughout the year (Beyer et al. 2021a, b; Dainese et al. 2019). The access to continuous and diverse food resources is essential for general pollinator community health (Alaux et al. 2017) and thus pollination performance. Wild pollinators additionally depend on nesting sites (Kremen et al. 2007). For many crops, wild insects play an even bigger role for pollination than honeybees (Garibaldi et al. 2013). It has been shown that distance to natural habitat leads to a decline in pollinator abundance and visitation rate of native pollinators (Carvalheiro et al. 2010; Ricketts et al. 2008). A high proportion of semi-natural habitat, on the other hand, improves pollinator richness and abundance (Eeraerts et al. 2019; Beyer et al. 2021b). For example, in almond fields the percentage of natural area in the 2 km buffer zones increased both wild pollinator-species richness and honeybee visits (Alomar et al. 2018). To enhance provisioning of pollination service, access to natural or semi-natural habitat plays an important role for both, honeybees and wild pollinators.
Pollinator distributions in orchards can be optimized by the spatial arrangement of beehives. The pollinators should be distributed evenly in the orchards and be able to transmit pollen between trees and varieties. Cunningham et al. (2016) showed that the pollination service was improved by changing the spatial arrangement of honeybee colonies in almond orchards. At any given colony density, fruit set outcomes were better when smaller placements (<100 colonies) were used which were more closely spaced (<700 m apart) than was standard (Cunningham et al. 2016).
Similarly, a study in macadamia orchards in Australia showed both honeybees and managed stingless bees did not distribute evenly in the orchard, but rather occurred in higher densities close to their colonies. This applied particularly for stingless bees, as >96% of the recordings were within 100 m of the stingless bee hives (Evans et al. 2021).
An observational study during the first SPACES project, revealed a drastic effect of insecticide applications on honey bees (Linden 2019). Bee numbers observed in the macadamia orchards increased significantly with time after each chemical application. This indicates a negative effect of pesticide usage on honeybee activities inside the orchard, despite efforts of the farmers to minimize impacts on pollinators. Recovery of bee activities occurred faster at orchard edges next to natural vegetation. At these natural orchard edges bee numbers were in general significantly higher than at human-modified (e.g. continuous farmland, roads) orchard edges. Natural vegetation in and around orchards therefore seems to play a key role in the rehabilitation of pollinators in macadamia orchards and serves as source for wild bees as pollinators.
4 Natural Pest Control with a Special Focus on Insectivorous Bats
4.1 South African Macadamia Insect Pests
The main insect pests in the South African macadamia industry are several Heteropteran and Lepidopteran species. Some species of thrips (Thripidae) are considered a minor pest, which can cause damages to flowers, while other thrips species are possibly beneficial in predating on other arthropods and in aiding pollination. Major thrips infestations in orchards have been cause for concern in several South African growing regions including Levubu in recent times (Hepburn 2015; Schoeman 2009).
The indigenous two-spotted stink bug Bathycoelia distincta Distant (Hemiptera: Pentatomidae) is by far the major pest on macadamias in South Africa and economically the most significant Heteropteran species (Schoeman 2018). However, damage is also caused by several Tortricidae (Lepidoptera) species (Schoeman and De Villiers 2015; Schoeman 2018). According to Schoeman (2009), over 10% of immature nut drop in macadamia is linked to the tortricid complex, making them economically important pest species.
The competitive advantage of the two-spotted stinkbug over other Heteropterans is its extremely long mouthpart (±16 mm) compared to other species, enabling them to feed on all varieties of macadamia even after nuts have matured (Schoeman 2018). This damage to the macadamia is called ‘late’ stinkbug damage, referring to damage occurring late in the season when the macadamia shell is penetrated while the mature kernel is undergoing oil accumulation (Schoeman 2018).
The losses through direct insect damage to the macadamia kernel by early and late stinkbug damage, were estimated at 96 and 84 million ZAR, respectively, for the growing season of 2019 alone (SAMAC 2020). Additionally, there are also indirect effects of insect damage such as promoting immature nut drop, kernel germination, and fungus infestation, which were estimated losses of 52, 17, and 32 million ZAR for 2019, respectively (La Croix and Thindwa 1986; Nagao et al. 1992; Schoeman and de Villiers 2015; SAMAC 2020).
4.2 Avoided Cost Models and Exclusion Studies of Vertebrate Predators
The concept of ‘ecosystem services’, defined as the benefits that humans derive from biodiversity and ecosystems such as regulating, supporting, and provisioning processes (Wangai et al. 2016), has been increasingly appreciated and understood by the global community in the last decades (Millennium Assessment Board 2005). Although crucial in providing many of these services, bats have always suffered from unfounded negative public perceptions and have only received scientific attention as important ecosystem service providers in recent years (Voigt and Kingston 2016). Probably the most significant early contribution to our understanding of the economic value of bats to the agricultural industry was an avoided-cost model by Boyles et al. (2011), estimating that pest suppression by insectivorous bats has an annual value of about 22.9$ billion to the agricultural industry of the United States. Following other studies (López-Hoffman et al. 2014; Puig-Montserrat et al. 2015; Wanger et al. 2014) using this modelling approach, a study conducted as part of SPACES by Taylor et al. (2018) estimated the value of bats to the South African macadamia industry in suppressing stinkbugs alone at 57–139$ per hectare per year.
However, a later exclusion study, also conducted as part of the SPACES programme, by Linden et al. (2019) shows that the values provided by the avoided-cost model were likely an underestimation and that the combined value of ecosystem services provided by insectivorous bats and birds through pest predation even outweighs the disservice by crop raiding vervet monkeys (Chlorocebus pygerythrus).
Using exclusion cages, the effect of the absence of birds and or bats as well as crop raiding mammals was tested, distinguishing between diurnal, nocturnal, or constant exclusions and comparing the yield, quality, and economic value of the exclusions at either natural or human-modified orchard edges (Fig. 22.6). The cages were erected in between macadamia crops, after the previous nuts had been harvested and before new flowers had started to develop, experiments were then running over three consecutive years.
At the natural orchard edge, where crop raiding by monkeys occurs, the avoided cost by bats and birds suppressing insect pests was about $5000 per hectare per year. Whereas, crop loss through crop raiding was about $1600 per hectare per year (Linden et al. 2019).
However, estimates based on exclusion studies cannot account for the total ecosystem service of pest suppression including open-air foraging bats (Monadjem et al. 2020), which feed in open spaces on certain pests such as moth before they descend into orchards. The open-air feeding guild of bats (families Molossidae and Emballonuridae in South Africa) generally hunt above the canopy of vegetation. McCracken et al. (2008, 2012) showed on the example of the open-air feeding Brazilian free-tailed bat (Tadarida brasiliensis) that these bats are not only able to exploit local pest infestations of the corn earworm but also hunt at altitudes up to 900 m above ground level. Most importantly, McCracken et al. (2008) suggest that the high foraging activity levels of this species at 400–500 m above ground level are linked to the migration of insects such as certain moths.
Similar to the diet analyses of McCracken et al. (2012), a study under SPACES conducted in the Levubu macadamia orchards showed that local bat population is presumably much more generalist and opportunistic in their foraging behaviour than previously assumed (Weier et al. 2019a). Testing for four pest insect species (B. distincta, N. viridula, T. batrachopa, and C. peltastica), the study showed that nearly all faecal samples analysed from four families of bats (Molossidae, Nycteridae, Rhinolophidae, and Vespertilionidae) contained genetic sequences of at least one stinkbug and one moth pest insect.
4.3 Habitat Use of Bats in Macadamia Orchards
Having established the importance of insectivorous bats in macadamia pest control, further research within the SPACES programme investigated habitat use of bats in order to guide agro-environmental management. By means of acoustic monitoring during active drive transects in Levubu orchards, Weier et al. (2018) found that bat activity increases with Hemiptera abundance but also with the amount of natural and semi-natural vegetation near orchards. Generally, the ecosystem service of pest suppression was higher at natural orchard edges in Levubu (Linden et al. 2019; Weier et al. 2021).
Crisol-Martínez et al. (2016) found that the activity of the clutter and clutter-edge guilds of bats decreased going away from natural orchards into macadamia monoculture in eastern Australia, while the most common species preferred the least fragmented and therefore the least isolated areas. As found by Weier et al. (2018), the abundance of insects and water availability had an influence on the abundance of species (Crisol-Martínez et al. 2016). Water availability, for both foraging and drinking, through artificial water sources such as dams can also increase the activity and diversity of bats in other agroecosystems (Shapiro et al. 2020; Sirami et al. 2013). Bats seem to prefer polyculture or organic agroecosystems (Kelly et al. 2016; Wickramasinghe et al. 2003; Wordley et al. 2017).
However, most of the agroecological studies on bats are currently focusing on common insectivorous species and it is worth mentioning that rare clutter feeding species such as the Rhinolophidae might also have a key role in suppressing certain pest insect species (Russo et al. 2018). From studies conducted in southern African agroecosystems these species seem to be already affected considerably by the ongoing land-use change and possibly also the competition and displacement by more generalist species as they have been recorded in very low numbers in more intensive agroecosystems (Linden et al. 2019; Shapiro et al. 2020; Weier et al. 2018, 2021). While, many (but not all) species of the open air and clutter edge feeding guilds of bats do use anthropogenic structures (such as tunnels, bridges, and roofs) for roosting the rhinolophids most commonly require their habitat to provide caves or old hollow trees. Generally, bat species benefit from natural vegetation which provides a variety of roosting sites such as loose bark, large curled leaves, tree hollows, woodpecker holes, and more.
In conclusion, a heterogeneous landscape in and around orchards, which provides connectivity, foraging, and roosting sites through natural and semi-natural vegetation promotes the activity and diversity of bat species and their ecosystem service provision. The same can be assumed for the ecosystem services provided by birds, therefore the diversity and richness of bird species in Levubu macadamias is currently investigated under the SPACES programme. The installation of bat houses is considered a way to buffer decreasing natural roost sites in many countries at the moment. However, it is unclear whether this has a positive effect on the overall bat communities and pest control service provision in general (Griffiths et al. 2017). Building on a previous study looking into the occupancy of bat houses in Levubu macadamia orchards under the SPACES programme (Weier et al. 2019b), a currently ongoing study conducted in the same area is investigating the effect of occupied bat houses on the surrounding bat species composition and activity in more detail.
4.4 The Effect of Pesticide Application on Ecosystem Services
The approach generally recommended for stinkbug pest control in southern African macadamia orchards is to base pesticide application on scouting for nymphs and adults, monitoring numbers using a knockdown method (Schoeman 2012). Scouting should focus on the edges of the orchards, where stinkbugs immigrate into orchards in the early season to ensure that the first application significantly reduces the first generation of nymphs, while another minimum of four applications of pesticides throughout the season are applied according to the life history of two-spotted stinkbugs (Nortje and Schoeman 2016). The use of pesticides in the winter months and over the flowering period is generally not recommended, as pest numbers are low, no crop on the tree, and pollination could be impacted by sprays affecting bee and other pollinator populations. While some stinkbugs overwinter in or near the macadamia orchards, others migrate into the orchards when food becomes available. Natural vegetation is seen both as a source of stinkbugs and a deterrent as it is serving as an alternative food source. Most recently it has been recommended and practised by many farmers to leave grasses and weeds to grow around the orchards and within the tree lines. Experience shows that this reduces the activity of stinkbugs on the macadamia trees, as they stay within these weed beds. Once these sections are mowed, stinkbug numbers were observed to increase on macadamia trees. While this is based on anecdotal evidence, many farmers are applying it in an attempt to minimize damages in a natural manner.
The common threshold at which spraying is recommended is four stink bugs found per 10 trees. However, according to a sector expert an estimated 10–15% of farmers still rely on scheduled or the so-called calendar sprays against stinkbugs, meaning that pesticides are applied in regular intervals independent from the confirmed presence of pest insects or their abundance on trees. A particular concern with this approach, apart from ecosystem (service) degradation, is that it increases the likelihood of stinkbugs developing resistances to pesticides (Schoeman 2018), which can also be aided by tree height and shape. Stinkbugs prefer the dark and dense areas of the orchards for foraging and stinkbug damage increases with tree density (Schoeman 2014). Conventional sprays applied with tractors become less efficient if the macadamia tree height exceeds 6 m (Drew 2003). It is recommended that trees should not be taller than 80% of the width of rows between trees (Schoeman 2018).
Another promising future alternative for the pest management of two-spotted stinkbugs is the use of semiochemicals especially alarm pheromones (Pal et al. 2020). While trap crops such as Crotalaria juncea might help to decrease the kernel damage caused by other stinkbug species, no suitable trap crop has been found to attract the two-spotted stinkbug as it seems to be highly monophagous (Steyn 2019). The other main pest for macadamias are lepidopteran species of the nutborer complex, namely the macadamia nutborer and the false codling moth. Monitoring of the nutborer complex is facilitated by means of species-specific pheromone traps, which can also be used to control them. Additionally, young nuts can be monitored for oviposition by the nutborer moths. Apart from pesticides there are several biological control agents including fungi, viruses, and bacteria registered for the use of these pests.
In 2018, the worldwide average use of pesticides per hectare of cropland was 2.63 kg (FAO 2018). While the average for African countries was much lower (0.3 kg/ha; FAO 2018), the bioaccumulation of pesticides in non-target species is generally of great concern and has been reported to negatively affect the behaviour and life history of invertebrates as well as vertebrates (Oliveira et al. 2021).
Overall, the effect of pesticides on bat species has been vastly understudied and represents a large scientific research gap (Oliveira et al. 2021; Torquetti et al. 2021). In a review of studies published in English between 1964 and 2019, Oliveira et al. (2021) found only 28 studies on the effect of pesticides on bats worldwide. These studies showed that the ingestion of pesticides by bats through insects, fruits, or water can have serious negative consequences including impaired torpor and echolocation, liver pathologies, oxidative stress, and endocrine disruption as well as decreased energy reserves. In a review of declines in bird populations in agroecosystems, nearly half of the reviewed studies (N = 122) found pesticide use had a negative effect on local species (Stanton et al. 2018). Recent studies on the effect of neonicotinoids on birds, particularly insectivorous birds, in the USA and the Netherlands have linked its usage to a decline in bird diversities and populations of an average annual 3% and 3.5%, respectively (Hallmann et al. 2014; Li et al. 2020). Given the high longevity of bats, it seems likely that the annual declines of bat populations due to the effects of pesticide usage are higher than those reported for birds, making it an urgent field for future research.
Mostly, farmers tend to spray their macadamia orchards in the early morning or late evening hours. The recommendation is to spray while the maximum ambient temperatures are below <18 °C, at which stinkbugs are immobile and cannot fly out of the orchards. While insectivorous bats are active throughout the night from sunset to sunrise, their peak activity is for about 3 h after sunset. It is much harder to determine peak activities for birds in the area as there are both diurnal and nocturnal bird species active in the Levubu orchards (Linden et al. 2019).
Linden et al. (2019) observed that hymenopterans took the longest to recover after a pesticide application event. Several beneficial insect species fall within this order, chief among which are parasitoids that are specialist predators of pest species. Spiders are the dominant invertebrate predator in these orchards and are some of the first taxa to recolonize trees after a spray event. Assemblages in macadamia orchards are dominated by wandering spiders (>90%) and mainly belong to family Salticidae (73%) (Dippenaar-Schoeman et al. 2001). Haddad and Dippenaar-Schoeman (2004) observed that a salticid species that dominates Pistachio orchard assemblages ate at least one lygaeid bug a day. However, calendar spraying over the long-term results in an almost complete collapse in spider assemblages, particularly if the surrounding vegetation is highly transformed.
5 Conclusions
-
To increase the efficiency and sustainability of macadamia water use, growers should adopt water-saving irrigation systems and reduce their irrigation water supply to small and targeted applications, aiming at meeting the specific tree water requirements. Research can help to determine such amounts for different environmental conditions.
-
Technological innovations (e.g. soil moisture probes and remote sensing) allow for a better understanding and monitoring of water dynamics in macadamia orchards, which, combined with the adoption of water-saving agricultural practices (e.g. mulching), can considerably reduce the orchards’ water footprint in view of future water limitations.
-
Recommendations for growers to maximize biodiversity services in macadamia orchards include retaining natural and semi-natural habitats in the landscape and enhancing agrobiodiversity, increasing wild pollinator abundances and optimizing the spatial arrangement of beehives. The installation of bat houses might be another option to improve natural pest control services. There is a range of alternative, ecologically friendly recommendations for natural pest control. Any successful long-term control of pest insect damage in macadamia requires an integrated pest management (IPM) approach.
-
The timing and application of pesticide sprays should be modified based on ecological and biological principles, such as a day-degree models of stinkbug development, or based on scouting for threshold pest stinkbug numbers in orchards and taking peak activity times of bats and birds into account, to mitigate the ecological impact of pesticides.
Generally, the research focus of industry bodies needs to shift from short-term economic benefits for farmers, to focus more on the long-term security of the industry, identifying the threats deriving from a changing climate and developing corresponding risk management strategies to mitigate their impact (e.g. water availability). A priority should be to maintain sustainable agroecosystems which provide resilient biodiversity services under the predicted decrease in annual rainfall.
References
AGTAG (2018) 63% of global macadamia crop forecast to come from China by 2025. https://www.agtag.co.za/category/3/post/21247. Accessed 21 July 2021
Ahrens P (1991) History and statistics of macadamias in Levubu. In: Proceedings of the macadamia mini symposium, Levubu, 18–19 September 1991, pp 3–4
Alaux C, Allier F, Decourtye A et al (2017) A ‘landscape physiology’ approach for assessing bee health highlights the benefits of floral landscape enrichment and semi-natural habitats. Sci Rep. https://doi.org/10.1038/srep40568
Alomar D, González-Estévez MA, Traveset A et al (2018) The intertwined effects of natural vegetation, local flower community, and pollinator diversity on the production of almond trees. Agric Ecosyst Environ 264:34–43
APNEWS (2020) Worldwide Macadamia Market (2020 to 2025) - growth, trends and forecasts. https://apnews.com/press-release/business-wire/. Accessed 15 May 2021
Bänsch S, Tscharntke T, Gabriel D et al (2021) Crop pollination services: complementary resource use by social vs solitary bees facing crops with contrasting flower supply. J Appl Ecol 58:476–485
Baudoin MA, Vogel C, Nortje K et al (2017) Living with drought in South Africa: lessons learnt from the recent El Niño drought period. Int J Disaster Risk Reduct 23:128–137
Beyer N, Gabriel D, Westphal C (2021a) Contrasting effects of past and present mass-flowering crop cultivation on bee pollinators shaping yield components in oilseed rape. Agric Ecosyst Environ. https://doi.org/10.1016/j.agee.2021.107537
Beyer N, Kirsch F, Gabriel D et al (2021b) Identity of mass-flowering crops moderates functional trait composition of pollinator communities. Landsc Ecol 36:2657–2671
Botha L (2018) Do we have enough water for all our macs? The Macadamia, Winter 2018 edition
Botha L (2020) Research shows South African macadamia orchards are over-irrigated. The Macadamia, Autumn 2020 edition
Boyles JG, Cryan PM, McCracken GF et al (2011) Economic importance of bats in agriculture. Science 332:41–42
Carr MKV (2012) The water relations and irrigation requirements of Macadamia (Macadamia spp.): a review. Exp Agric 49(1):74–94
Carvalheiro LG, Seymour CL, Veldtman R et al (2010) Pollination services decline with distance from natural habitat even in biodiversity-rich areas. J Appl Ecol 47:810–820
Conway D, van Garderen EA, Deryng D et al (2015) Climate and southern Africa’s water-energy-food nexus. Nat Clim Chang 5:837–846
Crisol-Martínez E, Moreno-Moyano LT, Wormington KR et al (2016) Using next-generation sequencing to contrast the diet and explore pest-reduction services of sympatric bird species in macadamia orchards in Australia. PLoS One. https://doi.org/10.1371/journal.pone.0150159
Cunningham SA, Fournier A, Neave MJ et al (2016) Improving spatial arrangement of honeybee colonies to avoid pollination shortfall and depressed fruit set. J Appl Ecol 53:350–359
DAFF (2019) A profile of the South African macadamia nut market value chain. https://www.daff.gov.za. Accessed 22 June 2021
Dainese M, Martin EA, Aizen MA et al (2019) A global synthesis reveals biodiversity-mediated benefits for crop production. Sci Adv. https://doi.org/10.1126/sciadv.aax0121
De Villiers E, Joubert P (2003) The cultivation of Macadamia. ARC-Institute for Tropical and Subtropical Crops, Nelspruit
Dippenaar-Schoeman AS, Van den Berg MA, Van den Berg AM et al (2001) Spiders in macadamia orchards in the Mpumalanga Lowveld of South Africa: species diversity and abundance (Arachnida: Araneae). Afr Plant Prot 7(1):39–46
Drew H (2003) Critical issues in spray application in macadamias using ground-based air-assisted sprayers. Proceedings of the Second International Macadamia Symposium, Tweed Heads, New South Wales, pp 120–125
Eeraerts M, Smagghe G, Meeus I (2019) Pollinator diversity, floral resources and semi-natural habitat, instead of honey bees and intensive agriculture, enhance pollination service to sweet cherry. Agric Ecosyst Environ. https://doi.org/10.1016/j.agee.2019.106586
Evans LJ, Jesson L, Read SFJ et al (2021) Key factors influencing forager distribution across macadamia orchards differ among species of managed bees. Basic Appl Ecol. https://doi.org/10.1016/j.baae.2021.03.001
FAO (2018) Pesticides - average use per area of cropland 1990–2018. Food and Agriculture Organization of the United Nations (FAO), Statistics Division (ESS), Environment Statistics team. http://www.fao.org/faostat/en/#data/EP/visualize. Accessed 15 June 2021
Foley JA, DeFries R, Asner GP et al (2005) Global consequences of land use. Science 309:570–574
Garibaldi LA, Steffan-Dewenter I, Winfree R et al (2013) Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 339:1608–1611
Grass I, Meyer S, Taylor PJ et al (2018) Pollination limitation despite managed honeybees in South African macadamia orchards. Agric Ecosyst Environ 260:11–18
Green & Gold Macadamias (2018) International Macadamia Symposium wrap up. https://www.greenandgoldmacadamias.com/. Accessed 21 May 2021
Griffiths SR, Bender R, Godinho LN et al (2017) Bat boxes are not a silver bullet conservation tool. Mammal Rev 47(4):261–265
Haddad CR, Dippenaar-Schoeman AS (2004) An assessment of the biological control potential of Heliophanus pistaciae (Araneae: Salticidae) on Nysius natalensis (Hemiptera: Lygaeidae), a pest of pistachio nuts. Biol Control 31(1):83–90
Hallmann CA, Foppen RP, Van Turnhout CA, De Kroon H, Jongejans E (2014) Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511(7509):341–343
Hahn N (2017) Endemic flora of the Soutpansberg, Blouberg and Makgabeng. S Afr J Bot 113:324–336
Hardner CM, Peace C, Lowe AJ et al (2009) Genetic resources and domestication of Macadamia. In: Janick J (ed) Horticultural reviews. Wiley, Hoboken, pp 1–125
Heard TA (1993) Pollinator requirements and flowering patterns of Macadamia integrifolia. Aust J Bot 41:491–497
Heard TA (1994) Behaviour and pollinator efficiency of stingless bees and honey bees on macadamia flowers. J Apic Res 33:191–198
Heard TA, Exley EM (1994) Diversity, abundance, and distribution of insect visitors to macadamia flowers. Environ Entomol 23:91–100
Hepburn C (2015) The phenologies of Macadamia (Proteaceae) and thrips (Insecta: Thysanoptera) communities in Mpumalanga province, South Africa. Dissertation, Rhodes University
Herbert SW, Walton DA, Wallace HM (2019) Pollen-parent affects fruit, nut and kernel development of Macadamia. Sci Hortic 244:406–412. https://doi.org/10.1016/j.scienta.2018.09.027
Hewitson BC, Crane RG (2006) Consensus between GCM climate change projections with empirical downscaling: precipitation downscaling over South Africa. Int J Climatol 26:1315–1337
Howlett BG, Nelson WR, Pattemore DE et al (2015) Pollination of macadamia: review and opportunities for improving yields. Sci Hortic 197:411–419
Howlett BG, Read SFJ, Alavi M et al (2019) Cross-pollination enhances Macadamia yields, even with branch-level resource limitation. HortScience 54:609–615
Huett DO (2004) Macadamia physiology review: a canopy light response study and literature review. Aust J Agric Res 55:609–624
Ibraimo N, Taylor N, Ghezehei S, Gush M, Annandale J (2014) Water use of macadamia orchards. In: Gush M, Taylor N (eds) The water use of selected fruit tree orchards, vol 2: Technical report on measurements and modelling. WRC report no. 1770/2/14, Water Research Commission, Pretoria
IPCC (2021) Climate change 2021: the physical science basis. In: Masson-Delmotte V, Zhai P, Pirani A et al (eds) Contribution of working group I to the sixth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge. https://doi:10.1017/9781009157896
Ito PJ, Hamilton RA (1980) Quality and yield of “Keauhou” macadamia nuts from mixed and pure block plantings. HortScience 15:307
Joseph GS, Muluvhahothe MM, Seymour CL et al (2019) Stability of Afromontane ant diversity decreases across an elevation gradient. GECCO 17. https://doi.org/10.1016/j.gecco.2019.e00596
Kämper W, Wallace HM, Ogbourne SM et al (2020) Dependence on cross-pollination in macadamia and challenges for orchard management. Proceedings 36. https://doi.org/10.3390/proceedings2019036076
Kämper W, Trueman SJ, Ogbourne SM, Wallace HM (2021) Pollination services in a macadamia cultivar depend on across-orchard transport of cross pollen. J Appl Ecol. https://doi.org/10.1111/1365-2664.14002
Kelly RM, Kitzes J, Wilson H, Merenlender A (2016) Habitat diversity promotes bat activity in a vineyard landscape. Agric Ecosyst Environ 223:175–181. https://doi.org/10.1016/j.agee.2016.03.010
Kirk WDJ (1987) How much pollen can thrips destroy? Ecol Entomol 12:31–40
Kremen C, Williams NM, Aizen MA et al (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10:299–314
La Croix EAS, Thindwa HZ (1986) Macadamia pests in Malawi. III. The major pests. The biology of bugs and borers. Trop Pest Manag 32(1):11–20
Langdon KS, King GJ, Nock CJ (2019) DNA paternity testing indicates unexpectedly high levels of self-fertilisation in macadamia. Tree Genet Genomes 15:29
Lee P (2020) Why irrigate macadamias? The Macadamia, Winter 2020 edition
Li Y, Miao R, Khanna M (2020) Neonicotinoids and decline in bird biodiversity in the United States. Nat Sustain 3(12):1027–1035
Linden VMG (2019) How vertebrate communities affect quality and yield of macadamia farms in L Levubu, South Africa. Dissertation, University of Venda
Linden VMG, Grass I, Joubert E et al (2019) Ecosystem services and disservices by birds, bats and monkeys change with macadamia landscape heterogeneity. J Appl Ecol. https://doi.org/10.1111/1365-2664.13424
Lloyd J (1991) Modelling stomatal responses to environment in macadamia integrifolia. Funct Plant Biol 18(6):661–671
López-Hoffman L, Wiederholt R, Sansone C et al (2014) Market forces and technological substitutes cause fluctuations in the value of bat pest-control services for cotton. PLoS One. https://doi.org/10.1371/journal.pone.0087912
Makungo R, Odiyo JO (2017) Estimating groundwater levels using system identification models in Nzhelele and Luvuvhu areas, Limpopo province, South Africa. Phys Chem Earth 100:44–50
McCracken GF, Gillam EH, Westbrook JK et al (2008) Brazilian free-tailed bats (Tadarida brasiliensis: Molossidae, Chiroptera) at high altitude: links to migratory insect populations. Integr Comp Biol 48:107–118
McCracken GF, Westbrook JK, Brown VA et al (2012) Bats track and exploit changes in insect pest populations. PLoS One 7(8). https://doi.org/10.1371/journal.pone.0043839
McFadyen LM, Robertson D, Sedgley M et al (2011) Post-pruning shoot growth increases fruit abscission and reduces stem carbohydrates and yield in macadamia. Ann Bot 107:993–1001
McFadyen L, Robertson D, Sedgley M et al (2012) Time of pruning affects fruit abscission, stem carbohydrates and yield of macadamia. Funct Plant Biol 39:481
Meyer S (2016) Effects of spatial arrangement of bee hives and landscape context on pollination of Macadamia in South Africa. Master’s thesis, Georg-August-Universität Göttingen, Göttingen
Millennium Assessment Board (2005) Millennium ecosystem assessment. New Island Press, Washington DC
Mogoatlhe L (2020) South Africa repeals state of disaster for drought. Here’s why it’s a ‘grave concern’ for farmers https://wwwglobalcitizenorg/en/content/south-africa-drought-national-crisis-farmers/. Accessed 23 May 2021
Monadjem A, Taylor PJ, Cotterill FDP et al (2020) Bats of southern and Central Africa: a biogeographic and taxonomic synthesis. Wits University Press, Johannesburg
Moncur MW, Stephenson RA, Trochoulias T (1985) Floral development of macadamia integrifolia Maiden & Betche under Australian conditions. Sci Hortic 27:87–96
Mound LA (2005) THYSANOPTERA: diversity and interactions. Annu Rev Entomol 50:247–269
Murovhi N (2003) Irrigation. In: De Villiers EA, Joubert PH (eds) The cultivation of Macadamia. ARC-Institute for Tropical and Subtropical Crops, Nelspruit
Nagao MA, Hirae HH, Stephenson RA (1992) Macadamia: cultivation and physiology. Crit Rev Plant Sci 10(5):441–470
Nortje GP, Schoeman S (2016) Biology and management of stink bugs in Southern African Macadamia orchards - current knowledge and recommendations. Working paper. https://www.researchgate.net/publication/319351182. Accessed 2 May 2021
Oliveira JM, Destro ALF, Freitas MB et al (2021) How do pesticides affect bats?–a brief review of recent publications. Braz J Biol 81(2):499–507
Orgaz F, Villalobos FJ, Testi L, Fereres E (2007) A model of daily mean canopy conductance for calculating transpiration of olive canopies. Funct Plant Biol 34(3):178–188
Pal E, Hurley B, Slippers B, Fourie G (2020) Progress towards the characterisation of pheromones of the two-spotted stinkbug (Bathycoelia distincta). SAMAC, September 2020. https://www.fabinet.up.ac.za/publication/pdfs/4059-pal.2020.pdf
Puig-Montserrat X, Torre I, Lopez-Baucells A et al (2015) Pest control service provided by bats in Mediterranean rice paddies: linking agroecosystems structure to ecological functions. Mamm Biol 80:237e245
Ramotjiki ML (2020) Does observational methods affect the observed impacts of exotic plants on flower visitors in around macadamia orchards. http://hdl.handle.net/11602/1661
Ramulifho P, Ndou E, Thifhulufhelwi R et al (2019) Challenges to implementing an environmental flow regime in the Luvuvhu river catchment, South Africa. Int J Environ Res Public Health 16(19):3694
Ramulifho PA, Rivers-Moore NA, Foord SH (2021) Loss of intermediate flow states only evident when considering sub-daily flow metrics in a major tributary in the Limpopo basin. Ecohydrology. https://doi.org/10.1002/eco.238
Richards TE, Kämper W, Trueman SJ, Wallace HM, Ogbourne SM, Brooks PR, Nichols J, Hosseini Bai S (2020) Relationships between nut size, kernel quality, nutritional composition and levels of outcrossing in three macadamia cultivars. Plan Theory 9:228. https://doi.org/10.3390/plants9020228
Richardson AC, Dawson TE (1993) Enhancing abscission of mature macadamia nuts with ethephon. N Z J Crop Hortic Sci 21:325–329
Ricketts TH, Regetz J, Steffan-Dewenter I et al (2008) Landscape effects on crop pollination services: are there general patterns? Ecol Lett 11:499–515. https://doi.org/10.1111/j.1461-0248.2008.01157.x
Russo D, Bosso L, Ancillotto L (2018) Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: research frontiers and management implications. Agric Ecosyst Environ 266:31–38. https://doi.org/10.1016/j.agee.2018.07.024
Sakai WS, Nagao MA (1985) Fruit growth and abscission in macadamia integrifolia. Physiol Plant 64:455–460
SAMAC (2020) Industry statistics. https://www.samac.org.za/industry-statistics. Accessed 12 Mar 2021
SAMAC (2021) Crop forecast 2021. https://www.samac.org.za/industry-statistics/. Accessed 25 May 2021
da Santos RS, de Milfont MO, Silva MM, Carneiro LT, Castro CC (2020) Butterflies provide pollination services to macadamia in northeastern Brazil. Sci Hortic 259:108818. https://doi.org/10.1016/j.scienta.2019.108818
Schoeman PS (2009) Key biotic components of the indigenous Tortricidae and Heteroptera complexes occurring on Macadamia in South Africa. Dissertation, North West University, Potchefstroom
Schoeman PS (2012) Macadamia scouting. https://www.samac.org.za/wp-content/uploads/2016/08/macadamia-scouting_prelim.pdf. Accessed 16 Oct 2016
Schoeman PS (2014) Aspects affecting distribution and dispersal of the indigenous Heteroptera complex (Heteroptera: Pentatomidae & Coreidae) in South African macadamia orchards. Afr Entomol 22(1):191–196
Schoeman PS (2018) Relative seasonal occurrence of economically significant heteropterans (Pentatomidae and Coreidae) on macadamias in South Africa: implications for management. Afr Entomol 26(2):543–549
Schoeman PS, De Villiers EA (2015) Macadamia. In: Prinsloo GL, Uys VM (eds) Insects of cultivated plants and natural pastures in southern Africa. Entomological Society of Southern Africa, Hatfield
Searle C, Lu P (2002) Optimising irrigation scheduling for the production of high quality Macadamia nuts. Horticulture Australia Ltd, Australia. Report No. MC98019
Searle C, Lu P (2003) Whole–tree water use and irrigation scheduling in macadamias. Proceedings of the Second International Macadamia Symposium, Tweed Heads, Queensland
Sedgley M (1983) Pollen tube growth in macadamia. Sci Hortic 18:333–341
Shabalala M, Toucher M, Clulow A (2022) The macadamia bloom – what are the hydrological implications? Sci Hortic 292
Shapiro JT, Monadjem A, Röder T et al (2020) Response of bat activity to land cover and land use in savannas is scale-, season-, and guild-specific. Biol Conserv 241. https://doi.org/10.1016/j.biocon.2019.108245
Shigeura GT, Ooka H (1984) Macadamia nuts in Hawaii: history and production. Res Ext Ser 39:95
Sirami C, Jacobs DS, Cumming GS (2013) Artificial wetlands and surrounding habitats provide important foraging habitat for bats in agricultural landscapes in the Western Cape, South Africa. Biol Conserv 164:30–38
Smit TG, Taylor NJ, Midgley SJE (2020) The seasonal regulation of gas exchange and water relations of field grown macadamia. Sci Hortic 267. https://doi.org/10.1016/j.scienta.2020.109346
Stanton RL, Morrissey CA, Clark RG (2018) Analysis of trends and agricultural drivers of farmland bird declines in North America: a review. Agric Ecosyst Environ 254:244–254
Stephenson RA, Gallagher EC, Doogan VJ (2003) Macadamia responses to mild water stress at different phenological stages. Aust J Agric Res 54:67–75
Steyn JN (2019) Alternative practices for optimising soil quality and crop protection for macadamia orchards, Limpopo Province, South Africa. Dissertation, University of Venda
Tavares JM, Villalobos EM, Wright MG (2015) Contribution of insect pollination to macadamia integrifolia production in Hawaii. Proc Hawaii Entomol Soc 47:35–49
Taylor PJ, Sowler S, Schoeman MC et al (2013) Diversity of bats in the Soutpansberg and Blouberg mountains of northern South Africa: complementarity of acoustic and nonacoustic survey methods. S Afr J Wildl Res 43:12–26
Taylor PJ, Munyai A, Gaigher I et al (2015) Afromontane small mammals do not follow the hump-shaped rule: elevational variation in a tropical biodiversity hotspot (Soutpansberg Mountains, South Africa). J Trop Ecol 31:37–48
Taylor PJ, Grass I, Alberts AJ et al (2018) Economic value of bat predation services – a review and new estimates from macadamia orchards. Ecosyst Serv 30:372–381
Taylor NJ, Smit T, Smit A, Midgley SJE, Clulow A, Annandale JG, Dlamini K, Roets N (2021) Water use of Macadamia orchards, vol 2. Report to the Water Research Commission and macadamias South Africa NPC, WRC report no. 2552/2/21, Pretoria, South Africa
Tilman D, Fargione J, Wolff B et al (2001) Forecasting agriculturally driven global environmental change. Science 292:281–284
Torquetti CG, Guimarães ATB, Soto-Blanco B (2021) Exposure to pesticides in bats. Science of the Total Environment 755:142509
Trochoulias T, Johns G (1992) Poor response of macadamia (macadamia integrifolia Maiden and Betche) to irrigation in a high rainfall area of subtropical Australia. Aust J Exp Agric 32(4):507–512
Trueman SJ (2010) Benzyladenine delays immature fruit abscission but does not affect final fruit set or kernel size of Macadamia. Afr J Agric Res 5:1523–1530
Trueman SJ (2013) The reproductive biology of macadamia. Sci Hortic 150:354–359
Trueman SJ, Turnbull CGN (1994a) Effects of cross-pollination and flower removal on fruit set in macadamia. Ann Bot 73:23–32
Trueman SJ, Turnbull CGN (1994b) Fruit set, abscission and dry matter accumulation on girdled branches of macadamia. Ann Bot 74:667–674
Trueman SJ, Kämper W, Nichols J, Ogbourne SM, Hawkes D, Peters T, Hosseini Bai S, Wallace HM (2022) Pollen limitation and xenia effects in a cultivated mass-flowering tree, macadamia integrifolia (Proteaceae). Ann Bot 129(2):135–146
Tscharntke T, Clough Y, Wanger TC et al (2012) Global food security, biodiversity conservation and the future of agricultural intensification. Biol Conserv 151(1):53–59
UNESCO (2010) MAB Biosphere Reserves Directory, Biosphere Reserve Information, South Africa, Vhembe. http://www.unesco.org. Accessed 12 May 2021
Urata U (1954) Pollination requirements of macadamia. Hawaii Agricultural Experiment Station, University of Hawaii, Technical Bulletin 22
Van Wyk AE, Smith GF (2001) Regions of floristic endemism in southern Africa: a review with emphasis on succulents. Umdaus Press, Pretoria
Villalobos FJ, Testi L, Orgaz F et al (2013) Modelling canopy conductance and transpiration of fruit trees in Mediterranean areas: a simplified approach. Agric For Meteorol 171–172:93–103
Vithanage V, Ironside DA (1986) The insect pollinators of macadamia and their relative importance. J Aust Inst Agric Sci 52:155–160
Voigt C, Kingston T (2016) Bats in the Anthropocene: conservation of bats in a changing world. Springer International Publishing, Cham
Wallace HM, Vithanage V, Exley EM (1996) The effect of supplementary pollination on nut set of macadamia (Proteaceae). Ann Bot 78:765–773
Wangai PW, Burkhard B, Müller F (2016) A review of studies on ecosystem services in Africa. J Sustain Built Environ 5(2):225–245
Wanger TC, Darras K, Bumrungsri S et al (2014) Bat pest control contributes to food security in Thailand. Biol Conserv 171:220–223
Weier SM, Grass I, Linden VMG et al (2018) Natural vegetation availability and bugs promote insectivorous bat activity in macadamia orchards, South Africa. Biol Conserv. https://doi.org/10.1016/j.biocon.2018.07.017
Weier SM, Moodley Y, Fraser MF et al (2019a) Insect pest consumption by bats in macadamia orchards established by molecular diet analyses. GECCO. https://doi.org/10.1016/j.gecco.2019.e00626
Weier SM, Linden VMG, Grass I et al (2019b) The use of bat houses as day roosts in macadamia orchards, South Africa. Peer J. https://doi.org/10.7717/peerj.6954
Weier SM, Linden VMG, Hammer A et al (2021) Bat guilds respond differently to habitat loss and fragmentation at different scales in macadamia orchards in South Africa. Agric Ecosyst Environ 320. https://doi.org/10.1016/j.agee.2021.107588
Wickramasinghe LP, Harris S, Jones G, Vaughan N (2003) Bat activity and species richness on organic and conventional farms: impact of agricultural intensification. J Appl Ecol 40:984–993. https://doi.org/10.1111/j.1365-2664.2003.00856.x
Wordley CFR, Sankaran M, Mudappa D, Altringham JD (2017) Bats in the Ghats: agricultural intensification reduces functional diversity and increases trait filtering in a biodiversity hotspot in India. Biol Conserv 210:48–55. https://doi.org/10.1016/j.biocon.2017.03.026
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2024 The Author(s)
About this chapter
Cite this chapter
Weier, S.M. et al. (2024). Management Options for Macadamia Orchards with Special Focus on Water Management and Ecosystem Services. In: von Maltitz, G.P., et al. Sustainability of Southern African Ecosystems under Global Change. Ecological Studies, vol 248. Springer, Cham. https://doi.org/10.1007/978-3-031-10948-5_22
Download citation
DOI: https://doi.org/10.1007/978-3-031-10948-5_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10947-8
Online ISBN: 978-3-031-10948-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)