Abstract
The condensation of water vapor on the substrate surface under electric field is studied by molecular dynamics simulation, and a series of behaviors of water molecules during condensation were studied, such as nucleation, growth and coalescence. In the process of condensation, there will be some small clusters, whose size increases with the increase of time, and under the action of the movement of water molecules in vapor, the clusters move irregularly on the substrate surface and coalesced into larger clusters. And the droplets will be stretched along the direction of the electric field. Interestingly, the condensation will decrease with the increase of the electric field strength under the electric field perpendicular to the surface. The results also show that the orientations of water molecule dipole are closely related to the direction of electric field, indicating that the electric field causes the realignment of water molecules. The research shows that the electric intensity will have great impact on vapor condensation, which provides guidance for reversible adjustment of vapor condensation and the design of intelligent surface.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Vapor condensation is closely related to our daily life and can be utilized for water collection [1], thermal management [2], water desalination [3] and so on. Therefore, mechanism of water vapor condensation is very important for the rational utilization of condensation, and the efficient condensation has also attracted the interest of many researchers.
A large number of studies show that the roughness and chemical properties of the condensation surface have important impact on the condensation efficiency [4,5,6,7,8,9]. The development of molecular dynamics simulation provides a new method for the study of water molecular condensation. For example, Xu et al. studied the condensation of water vapor on the V-shaped groove by molecular dynamics simulation [4], the results show that the wetting modes of clusters are determined by the intrinsic contact angle and the cross sectional angle of the surface. Another study by the same team shows that the formation and growth of clusters in the process of water vapor condensation increase with the increase of substrate hydrophilicity [5]. Gao et al. explored the condensation of water vapor on the nanostructured surfaces with hybrid wettability areas and found that the nanostructured surfaces with hybrid wettability areas has better heat transfer performance [6]. Huang et al. discussed the effects of pillar height, spacing and substrate wettability on argon condensation [7]. However, the influence of external field is ubiquitous and will have a great impact on the condensation of water vapor, such as electric, temperature, force.
At present, studies exist mainly on the influence of external electric field on the static and dynamic behavior of sessile droplets on the surface [10,11,12,13,14,15] and water evaporation [16]. The research of Yan et al. [10] shows that the voltage amplitude and frequency of tangential AC electric field are important factors affecting the dewetting of droplets. The dynamics behaviors of droplets on flexible graphene sheets under constant and alternating electric fields with different amplitudes and frequencies are studied by Kargar [11] et al., and the results show that droplets elongated in the direction of electric field. Wang et al. [12] simulated the condensation process of water molecules under the action of an electric field perpendicular to the surface by molecular dynamics simulation and found that the condensation could be inhibited by vertical electric field.
Water molecules are easy to be affected by electric field because of the polarity, and electric field is an easily available clean energy, which provides a premise for its large-scale utilization, such as electrostatic spray, electrohydrodynamic atomization. The existence of external field also provides a new method to dynamically regulate the condensation of water molecules. However, the mechanism of the vapor condensation under external field is still not well understood. In this paper, the condensation process of water molecules under constant electric field perpendicular to the surface is studied by molecular dynamics simulation. The growth of maximum cluster size, number of clusters, dipole moment and the temperature of water molecules are calculated to quantify the condensation process. The research results are also useful for the efficient utilization of heat and mass transfer.
2 Model and Methods
In this paper, the molecular dynamics simulation software LAMMPS is used to simulate the condensation process of water vapor under the action of electric field [17, 18]. The structure of the simulation system is shown in Fig. 1. The simulation system consists of three parts, including an upper substrate (hot surface), a lower substrate (cold surface) and water molecules. In order to improve the calculation efficiency, the structure of copper atoms is used to establish the models of the cold surface and hot surface, in which the lattice is 3.615 Å, the thickness of the cold surface and hot surface is 10.845 Å, and the lateral dimension is 253 * 253 Å, consisting of 58800 atoms, [6, 19, 20]. Meanwhile, to improve the condensation rate of water molecules, 10000 water molecules are placed in the simulation systems.
The simple point charge/extension (SPC/E) model, which has been validated in previous studies, is chosen to describe the interaction between the water and water [21,22,23]. The calculation method of the interaction between water molecules in the model is consistent with our previous research [21, 24]. The interaction between substrate atoms and water molecules is calculated by Lennard-Jones (L-J) potential, where the energy coefficient (ε) is 0.2 kJ/mol, and the distance coefficient (σ) is 2.891 Å. The interaction between the substrate atoms is also described by the L-J potential, in which εS-S = 4.72 kJ/mol, σS-S = 2.616 Å [25], and there is no interaction between the upper substrate atoms and lower substrate atoms. In the simulation, the cutoff distances are set as 12 Å.
In the process of simulation, the periodic boundary conditions are applied in all directions. Meanwhile, the velocity-Verlet algorithm is used to solve the Newtonian motion equation with a time step of 1 femtosecond (fs). The particle–particle particle-mesh (PPPM) method is used to calculate the long-range electrostatic interactions with an accuracy up to 10–4. And to improve the calculation efficiency, SHAKE algorithm is used to fix the bonds and angles of water molecules.
And all the simulations are performed in two stages: First, the equilibration phase is carried out for a total of 0.2 ns (ns) in a NVT ensemble, and the temperature of water molecules and substrate atoms are controlled at 500 K. Then the thermostat applied on the water vapor is removed, the temperatures of the lower and upper surfaces in the system are controlled at 300K and 500K, respectively. In order to observe the condensation of water molecules faster, the temperature difference of the substrates is set larger. At the same time, the electric field is applied to the system. In our study, constant electric field perpendicular to the surface is applied to study the effect of electric field on water vapor condensation. And the simulation time for condensation is extended to 4 ns to see the condensation process clearly.
The growth of clusters is an important index to study the condensation process. In this study, the Stillinger criterion is used to define clusters, two water molecules are determined to be located in one cluster when the distance between oxygen atoms in two water molecules is 3.36 Å [26].
3 Results and Discussion
In this study, the condensation process of water vapor is simulated under the action of vertical electric field, and some snapshots of the simulation systems are selected to show the condensation process. Only part of the simulation system is shown in Fig. 2 because of the large size of the system. Figure 2 shows the condensation process of water vapor in different electric fields, and the direction of the electric field points to the negative direction of the y-axis. Water molecules move irregularly in the simulation process. When water molecules impact the cold surface, part of the energy of water molecules is converted into heat energy, resulting in the reduction of the speed of water molecules, which may be adsorbed on the cold surface or rebound back to the vapor. At this time, the motions of water molecules are influenced by electric field force, van der Waals force between substrate atoms and water molecules, and the interaction between water molecules. Due to the temperature difference between the cold surface and the hot surface, water molecules tend to adsorb on the cold surface and gradually form clusters on the cold surface. With the increase of electric field intensity, the condensation of water molecules is affected.
It can be seen from Fig. 2 that the clusters formed by condensation will be gradually elongated with the increase of electric field intensity. For example, the stretching of clusters is not obvious when the electric field intensity is 0.4 V/nm. When the electric field intensity is 0.8 V/nm, the deformation of droplets is obvious and becomes cylindrical. When the electric field intensity is 1.0 V/nm, the condensed clusters will leave the substrate surface, indicating a great influence of the electric field.
In the subsequent simulation, the condensation of water vapor in the electric field pointing to the positive direction of Y axis is also simulated. The results show that the electric field pointing to the positive direction of Y axis also inhibits the condensation of water vapor, and the effect is more obvious than that in the negative direction. It stretches the water droplets stretch along the positive direction of the Y axis. Our findings are consistent with previous studies on sessile droplets, in which the vertical electric field in the positive and negative directions was found to stretch and deform the droplets, and the deformation is more obvious under the electric field in the positive direction [27–29]. At the same time, it is obvious from Fig. 2 that the condensation speed of water molecules decreases gradually with the increase of electric field intensity. When the electric field intensity is small, the time that clusters start to form is not much different from that without electric field. However, clusters appear much later when the electric field strength increases to 0.8 V/nm. Our results show that the electric field inhibits the condensation of water vapor.
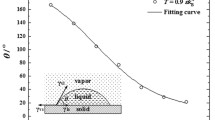
To more specifically quantify the impact of electric field on water molecular condensation, the growth of the maximum cluster size and the number of clusters in the system are calculated in the condensation process, and the results are shown in Fig. 3. Figure 3 (a) shows the temporal evolution of maximum cluster size under constant electric fields. The growth curve of the maximum cluster size increases linearly in some stages, indicating that the number of water molecules in the system is sufficient to simulate accurately the condensation process, and the abrupt increase in the curve indicates that adjacent clusters have merged. Figure 3 (a) shows the merging process of adjacent clusters when the electric field intensity is 0.0 V/nm. This also shows that the growth of the maximum cluster size is more obvious with the increase of electric field, except for the electric field intensity is 0.2 and 0.4 V/nm. From the condensation process, it is found that the reason for the abnormal maximum cluster size is that there is only one large cluster in the system when electric field intensities are 0.2 and 0.4 V/nm. While for the simulation when the electric field intensity is 0 V/nm, there are two large clusters on the cold surface in the condensation process. Figure 3 (b) shows the temporal evolution of the number of clusters under constant electric fields. The red circle indicates that the increase of electric field will destroy the clusters in the system, resulting in a sudden increase of the number of clusters. It is clear that the greater the electric field intensity, the greater the increase of the curves in the red circle. The results imply that the formation of clusters is inhibited with the increase of electric field intensity. This result corresponds well to the analysis in Fig. 2.
The polarity characteristics of water molecules show that water molecules are easy to be affected by electric field. Therefore, the dipole moments of water molecules are also calculated to quantify the effect of electric field on water molecules and to explain the growth and deformation of clusters. Figure 4 shows the average of the cos(θ) values of the water molecules under constant electric field (θ is the angle between the dipole moment of water molecule and the direction of electric field.). When the electric field intensity is 0.0 V/nm, the average of the cos(θ) is close to 0.0, indicating that water molecules are in a disordered state. With the increase of the electric field intensity, the average value of cos(θ) begins to increase gradually, indicating that the structure of water molecules rearranges under the action of the electric field, and the dipole moment of water molecules begin to align the direction of the electric field. At this point, the hydrogen atoms in water molecules point to the direction of the electric field, while the direction of oxygen atoms is opposite to the direction of the electric field. This characteristic makes the clusters stretch and grow along the direction of the electric field.
Next, we collected the temperature of each part of the system in the simulation process as shown in Fig. 5 (a), which displays the temporal evolution of water molecule temperature, and the cold and hot surfaces under different electric field intensities. For the system with electric field intensity of 0.0 V/nm, the temperature of water molecules begins to decrease from 500K with the collision between water molecules and cold surface when the temperature of the cold surface is controlled at 300K. When the electric field is applied, the temperature of water molecules rises instantaneously, indicating that water molecules acquire energy to raise the water temperature, as shown in the red circle in Fig. 5(a). This corresponds to the sudden increase in the number of clusters in Fig. 3 (b). The temperature of water molecules begins to decrease gradually as the simulation progresses. Figure 5(a) demonstrates that the higher the electric field strength, the higher the water molecule temperature during the whole simulation process. Obviously, this is not conducive to the condensation of water molecules. Interestingly, the slope of the temperature curve corresponding to the simulation system with electric field intensity of 1.0 V/nm becomes very small after 3 ns. This is because the electric field force plays a dominant role, the clusters are stretched and leave the cold surface, as shown in Fig. 5 (b) and (c), which is not conducive to heat transfer. The literature also draws similar conclusions from the released accumulated energy [12].
Then, the mean square displacement of water molecules in the Z direction (MSDz) is also calculated to evaluate the effect of electric field on condensation, as shown in Fig. 6. The simulation process in the Fig. 6 is divided into two parts: the first part is the equilibrium process of condensation system, and then the water vapor begins to condense under electric field. The results show that under the action of electric field, the MSDz curve of water molecules in the Z direction gradually decreases with the increase of electric field intensity, which shows that the diffusion of water molecules in the Z direction is restrained and the probability of droplet collision with the substrate is reduced. This is because the strong electric field induces water molecules to form an ordered structure pointing to the electric field direction, which is not conducive to the formation of clusters, At the same time, this reveals why the condensation efficiency decreases with the increase of electric field intensities.
4 Conclusion
In this study, the condensation process of water vapor under electric field is studied by molecular dynamics simulation. The influence of electric field on water vapor condensation is quantitatively evaluated by calculating the growth of the maximum cluster size, the number of clusters, the dipole moment of water molecules and the system temperature. The results show that the existence of vertical electric field promotes the rearrangement of water molecules along the direction of electric field, which makes the clusters grow along the direction of electric field. The formed clusters are stretched, which may even lead to the separation of clusters from the substrate surface. At the same time, the existence of vertical electric field increases the temperature of water molecules and inhibits the condensation of water vapor. And the condensation efficiency of droplets decreases with the increase of electric field intensity.
References
Ju, J., Bai, H., Zheng, Y., Zhao, T., Fang, R., Jiang, L.: A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun. 3(1), 1247 (2012)
Miljkovic, N., Preston, D.J., Enright, R., Wang, E.N.: Electric-field-enhanced condensation on superhydrophobic nanostructured surfaces. ACS Nano 7(12), 11043–11054 (2013)
Khawaji, A.D., Kutubkhanah, I.K., Wie, J.M.: Advances in seawater desalination technologies. Desalination 221(1), 47–69 (2008)
Xu, W., Lan, Z., Peng, B.L., Wen, R.F., Ma, X.H.: Effect of nano structures on the nucleus wetting modes during water vapour condensation: from individual groove to nano-array surface. RSC Adv. 6(10), 7923–7932 (2016)
Xu, W., Lan, Z., Peng, B.L., Wen, R.F., Ma, X.H.: Effect of surface free energies on the heterogeneous nucleation of water droplet: a molecular dynamics simulation approach. J. Chem. Phys. 142(5), 054701 (2015)
Gao, S., Liu, W., Liu, Z.: Tuning nanostructured surfaces with hybrid wettability areas to enhance condensation. Nanoscale 11, 459–466 (2019)
Huang, D., Quan, X., Cheng, P.: An investigation on vapor condensation on nanopillar array surfaces by molecular dynamics simulation. Int. Commun. Heat Mass Transfer 98, 232–238 (2018)
Starostin, A., Valtsifer, V., Barkay, Z., Legchenkova, I., Danchuk, V., Bormashenko, E.: Drop-wise and film-wise water condensation processes occurring on metallic micro-scaled surfaces. Appl. Surf. Sci. 444, 604–609 (2018)
Ranathunga, D.T.S., Shamir, A., Dai, X., Nielsen, S.O.: Molecular dynamics simulations of water condensation on surfaces with tunable wettability. Langmuir 36(26), 7383–7391 (2020)
Yan, X., Li, J., Li, L., Huang, Z., Wang, F., Wei, Y.: Droplet condensation on superhydrophobic surfaces with enhanced dewetting under a tangential AC electric field. Appl. Phys. Lett. 109(16), 161601 (2016)
Kargar, M., Lohrasebi, A.: Deformation of water nano-droplets on graphene under the influence of constant and alternative electric fields. Phys. Chem. Chem. Phys. 19, 26833–26838 (2017)
Wang, Q., Xie, H., Hu, Z., Liu, C.: The impact of the electric field on surface condensation of water vapor: insight from molecular dynamics simulation. Nanomaterials 9(1), 64 (2019)
Ren, H., Zhang, L., Li, X., Li, Y., Wu, W., Li, H.: Interfacial structure and wetting properties of water droplets on graphene under a static electric field. Phys. Chem. Chem. Phys. 17, 23460–23467 (2015)
Zhang, B., Wang, S., He, X., Yang, Y., Wang, X.: Dynamic spreading of a water nanodroplet on a nanostructured surface in the presence of an electric field. J. Mol. Liq. 333, 116039 (2021)
Yuan, Q., Zhao, Y.: Precursor film in dynamic wetting, electrowetting, and electro-elasto-capillarity. Phys. Rev. Lett. 104(24), 246101 (2010)
Hens, A., Biswas, G., De, S.: Evaporation of water droplets on Pt-surface in presence of external electric field—a molecular dynamics study. J. Chem. Phys. 143(9), 094702 (2015)
Plimpton, S.: Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117(1), 1–19 (1995)
LAMMPS Molecular Dynamics Simulator. http://lammps.sandia. Accessed 12 Dec 2018
Wang, P., He, L., Sun, X., Lv, H., Wang, Z.: Influence of trapezoidal cavity on the wettability of hydrophobic surface: a molecular dynamics study. Langmuir 37(12), 3575–3584 (2021)
Zhu, C., et al.: Controlling states of water droplets on nanostructured surfaces by design. Nanoscale 9, 18240–18245 (2017)
Wang, P., Sun, X., Lv, H., Ma, S., Wang, Z.: Investigation of surface wettability and their influencing mechanisms under vibration field: a molecular dynamics simulation study. Comput. Mater. Sci. 197, 110615 (2021)
Berendsen, H.J.C., Grigera, J.R., Straatsma, T.P.: The missing term in effective pair potentials. J. Phys. Chem. 91(24), 6269–6271 (1987)
Zhao, Y., Yuan, Q.: Statics and dynamics of electrowetting on pillar-arrayed surfaces at the nanoscale. Nanoscale 7(6), 2561–2567 (2015)
Wang, P., He, L., Wang, Z.: The effect of surface structure and arrangement on wettability of substrate surface. Colloids Surf. A 614, 126165 (2021)
Heinz, H., Vaia, R.A., Farmer, B.L., Naik, R.R.: Accurate simulation of surfaces and interfaces of face-centered cubic metals using 12–6 and 9–6 Lennard-Jones potentials. J. Phys. Chem. C 112(44), 17281–17290 (2008)
Stillinger, F.H.: Rigorous basis of the Frenkel-Band theory of association equilibrium. J. Chem. Phys. 38(7), 1486–1494 (1963)
Song, F., Ma, L., Fan, J., Chen, Q., Lei, G., Li, B.Q.: Electro-wetting of a nanoscale water droplet on a polar solid surface in electric fields. Phys. Chem. Chem. Phys. 20(17), 11987–11993 (2018)
Zhang, Z., Dong, X., Ye, H., Cheng, G., Ding, J., Ling, Z.: Wetting and motion behaviors of water droplet on graphene under thermal-electric coupling field. J. Appl. Phys. 117(7), 074304 (2015)
Zong, D., Yang, Z., Duan, Y.: Wettability of a nano-droplet in an electric field: a molecular dynamics study. Appl. Therm. Eng. 122, 71–79 (2017)
Acknowledgement
The computational work for this article was partially performed on resources of the National Supercomputing Centre, Singapore (https://www.nscc.sg).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this paper
Cite this paper
Wang, P., Chen, Z. (2022). Vapor Condensation Under Electric Field: A Study Using Molecular Dynamics Simulation. In: Panda, D.K., Sullivan, M. (eds) Supercomputing Frontiers. SCFA 2022. Lecture Notes in Computer Science, vol 13214. Springer, Cham. https://doi.org/10.1007/978-3-031-10419-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-10419-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10418-3
Online ISBN: 978-3-031-10419-0
eBook Packages: Computer ScienceComputer Science (R0)