Abstract
This book has mostly considered marine contamination and the biological effects of contaminants acting as single stressors. However, marine environments are rarely exposed to a single stressor, but rather experience a complex mix of many stressors. These stressors may be contaminants, such as the ones discussed in previous chapters (nutrients, chemicals, plastics as well as carbon dioxide), or they may be other stressors, such as invasive species, built infrastructure, aquaculture or fisheries, or climatic changes which themselves can contribute to contaminant stress, for example, nutrient loading is a well-known impact of aquaculture activities. All these stressors are ubiquitous in marine environments worldwide and have the potential to interact and have very different impacts compared to if they occurred singularly.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
14.1 Introduction
This book has mostly considered marine contamination and the biological effects of contaminants acting as single stressors. However, marine environments are rarely exposed to a single stressor, but rather experience a complex mix of many stressors. These stressors may be contaminants, such as the ones discussed in previous chapters (nutrients, chemicals, plastics as well as carbon dioxide), or they may be other stressors, such as invasive species, built infrastructure, aquaculture or fisheries, or climatic changes which themselves can contribute to contaminant stress, for example, nutrient loading is a well-known impact of aquaculture activities. All these stressors are ubiquitous in marine environments worldwide and have the potential to interact and have very different impacts compared to if they occurred singularly.
Wastewater treatment plants and stormwater drains that discharge into coastal marine waters create multiple stressor conditions since they are sources of both nutrients and trace metals and metalloids. These contaminants have different modes of action, often leading to different types of ecological impacts (Chapter 3). When acting separately, moderate levels of nutrients may cause an increase in a particular biological or ecological response (e.g. population growth or primary productivity) compared to control areas (e.g. Svensson et al. 2007), while trace metals may cause a relative decrease in these responses (Johnston and Roberts 2009; Mayer-Pinto et al. 2010). However, when these stressors occur simultaneously, they are likely to interact and can potentially result in no overall net effect on biological or ecological responses in the receiving environment (O’Brien et al. 2019).
Another multiple stressor situation occurs when built infrastructure (e.g. marinas, groynes or piers) is in proximity to toxic chemicals in the water column. Built infrastructure on its own can cause a significant negative effect on the diversity (i.e. number of species and abundance) of marine organisms (e.g. microbes, plankton, epibiota, and infauna) via loss or fragmentation of habitats (Bulleri and Chapman 2010; Dafforn et al. 2015; Bishop et al. 2017). This may be further exacerbated by toxic chemicals (e.g. metals; industrial wastes and agricultural runoff; McGee et al. 1995), with the overall effect of these stressors being potentially greater than if the two stressors occurred separately (a synergistic effect).
Understanding how and when stressors interact is critical for predicting their effects and therefore establishing safe and realistic guidelines to protect the environment. The consequences of stressor interactions are complex and pose great challenges for researchers, practitioners and decision-makers. This chapter highlights the complexity by providing some real-world examples and argues the need to consider the potential interactive effects of multiple stressors in conservation and management policies rather than focusing solely on single-stressor effects.
The first section of the chapter provides an overview of multiple stressors research, including definitions of types of multiple stressor interactions. The second section provides examples of the common types of multiple stressor interactions in the marine environment. In the final section, we briefly discuss current approaches and future directions of research on multiple stressors in the context of marine management, conservation and habitat restoration.
14.2 The Study of Multiple Stressors
Humans have exploited marine resources since at least the Palaeolithic with records of habitat modification occurring in Europe from the ninth and tenth centuries A.D (Knottnerus 2005). We know that the drivers of change are a complex synergy of anthropogenic and natural stressors, including pollution, land reclamation, coastal development, overfishing, nutrient and sediment enrichment, and that the inherent natural variability of marine ecosystems is driven by ecological processes (Airoldi and Beck 2007; Claudet and Fraschetti 2010). In addition, there is evidence that future climate scenarios will impose further pressures on the persistence and stability of these habitats (Hawkins et al. 2008; Philippart et al. 2011).
The number of stressors impacting the world’s ecosystems is unprecedented. However, it is not simply their number that is of concern, but their historical accumulation that is driving change (Jackson et al. 2001). The cumulative impacts of multiple stressors can exacerbate nonlinear responses of marine and coastal systems and limit their capacity to recover (e.g. Airoldi et al. 2015). The pervasiveness of multiple stressor impacts worldwide has led to the emergence of multiple stressor research as an independent field of study (Baird et al. 2016; Van Den Brink et al. 2019; Orr et al. 2020). This research encompasses general theory and management frameworks applied across different ecosystems, including marine ecosystems (Crain et al. 2008), freshwater rivers and streams (Hale et al. 2017; Sievers et al. 2018), floodplains (Monk et al. 2019), and agricultural ditches (Bracewell et al. 2019).
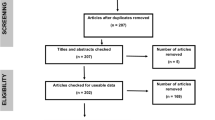
The marine environment is continually exposed to multiple stressors, yet most of the research and current literature still focuses on understanding the effects of individual stressors (O’Brien et al. 2019). A global review of literature based on urban marine and estuarine environments found 93% out of the total 579 studies considered stressors, such as nutrients, chemical contaminants, non-indigenous species and built infrastructure, in isolation (Figure 14.1). Only 38 studies identified by the literature review investigated the effects of these stressors in combination, highlighting the relative gap in our understanding between multiple compared to single stressors.

Adapted from O’Brien et al. (2019) by A. O’Brien
The number of studies between 1990 and 2017 that assessed the effects of nutrients, chemical contaminants, non-indigenous species and/or built infrastructure in urban estuarine environments in isolation (single) or combined (multiple).
14.2.1 Definitions
A useful starting point to understanding the concept of multiple stressors is to describe and define the potential interactions between two or more stressors. When more than one stressor occurs in an environment, they may or may not interact. If the effects of a certain stressor occur independently of any other stressor in the environment, then we consider that the stressors are not interacting (no interaction; Figure 14.2). This is defined as an additive (or cumulative) multiple stressor model where the overall total effects on a response variable are the sum of the single-stressor effects.

Adapted from Côté et al. (2016) by A. O’Brien
Hypothetical responses to two stressors, when they occur separately (stressor A, stressor B), when they occur together with no interaction (additive) or when they occur together and interact (antagonistic or synergistic).
Conversely, the stressors may affect the biological system(s) in ways that are dependent on each other or are interacting. Therefore, the overall or combined effects of two or more stressors are different from the sum of the single-stressor effects. Interacting stressors are considered as either synergistic or antagonistic, which are defined as (Figure 14.2):
-
Synergistic Interactions—the combined effects are greater than expected based on individual effects.
-
Antagonistic Interactions—the combined effects are less than expected based on individual effects.
Null models can be used in multiple stressor investigations to predict the combined effect of two stressors. Two stressors are initially assumed to have an additive effect, where the combined effects of the stressors are simply summed (Figure 14.2). If there is deviation from the additive null model, for example, through synergistic or antagonistic effects, then more complex models can be used to predict the combined effects (Schafer and Piggott 2018).
There are issues that need to be considered with this sort of statistical approach. Deviations from additivity are mainly determined based on statistical significance, which is known to be sensitive to sample size and transformations. An increase in sample size often leads to a decrease in the p-value for the same effect size and will eventually lead to a drop below the typically chosen significance threshold of 0.05. Thus, with a higher number of samples, the same effect size can become significant and bias comparisons between studies.
Data transformations can also affect the selected null model. Griffen et al. (2016) found that 32% of 143 marine multiple stressor studies unknowingly employed a multiplicative null model because of data transformations. The selection of an additive or multiplicative null model can be influenced by data transformations and will therefore bias the assessment of the prevalence of synergism and antagonism.
Several recent studies have argued for more mechanistically informed null models for multiple stressor research (Griffen et al. 2016; Kroeker et al. 2017; De Laender 2018; Schafer and Piggott 2018). Schäfer and Piggott (2018) provide an overview of null models and guidance on their selection. They introduce two additional null models that have largely been ignored in ecological multiple stressor research and suggest the following categories that should guide the null model selection: stressor mode of action, correlation of sensitivities of organisms to the stressors, effect type (e.g. mortality, growth), effect size of individual stressors and the shape of the stressor–effect relationship.
The number of times multiple stressor interactions are mentioned in ecological and environmental science literature is increasing (Figure 14.3; and see Côté et al. (2016)). In marine systems, both synergistic and antagonistic interactions between two stressors are common and have thought to have occurred more frequently than additive effects (Crain et al. 2008). However, we now know these interaction types are complicated with the addition of a third stressor (Johnson et al. 2018), and between different levels of biological organisation (population, community), types of response variables (O'Brien et al. 2019), direction of the single-stressor responses (Crain et al., 2008), and the initial conditions or the null model (Schafer and Piggott, 2018). Nevertheless, attempts to define the different types of interactions are still important, so the severity of the impact can be assessed, and management actions prioritised accordingly (Folt et al. 1999; Cabral et al. 2019).
14.3 Stressor Interactions in the Marine Environment
The different sources of contamination discussed in this book—nutrients, trace metal, metalloids, pesticides, POPS, plastics, radioactivity, oils, CO2, temperature and noise, etc.—can all be considered in the context of a multiple stressor framework.
14.3.1 Nutrients and Trace Metals
Eutrophication of marine environments is a pressing global problem and is caused by the delivery and accumulation of excess nutrients and/or organic matter, typically to coastal marine and estuarine habitats (Chapter 4). These habitats often have high concentrations of metals in sediments that form the seabed. The metals can come from historical sources, such as old industrial sites, or contemporary sources delivered through stormwater drains that carry runoff from nearby urban environments into the sea. The biological effects of nutrients plus metals depend on the environmental condition of the receiving waters. Both nutrients and some metals are essential at small concentrations, but at higher concentrations are likely to exceed a threshold, causing a toxic effect. In Australia, many estuaries are nutrient limited or oligotrophic, so the threshold at which additional nutrients may cause a toxic effect is expected to be higher than when nutrients are added to already eutrophic systems (e.g. Andersen et al. 2015). In contrast, the toxic effects of metals are less dependent on background environmental conditions and, therefore, these contaminants can reach thresholds at lower concentrations than nutrients (e.g. Samhouri et al. 2010).
Given the differing modes of action of nutrients and metals, we would predict antagonistic effects between these two stressors, with the enriching action of nutrients mitigating the toxic effect of metals up to a threshold when both become toxic. This effect has been found at different levels of biological organisation. For example, Lawes et al. (2017) found that microbial community evenness (a measure of diversity) and macrofaunal abundances decreased when exposed to metal contaminated sediments, but increased when exposed to both experimental nutrient treatments (low and high enrichment levels). Potential mechanisms underlying these measured responses include higher biological metabolic rates when exposed to nutrients that sustain detoxification processes and counteract toxic responses to metals (Sokolova and Lannig 2008). However, exceptions to this may include metal-tolerant species that can persist in highly contaminated sites regardless of nutrient availability (Mayer-Pinto et al. 2010).
14.3.2 Trace Metals and Pesticides
The combination of metals and pesticides is a common mixture of contaminants in the marine environment, especially in habitats close to catchments used for agriculture. Metal pollution can originate from point sources (e.g. stormwater drains or industrial discharges, as described above) or they may originate from diffuse sources that are comparatively difficult to identity (e.g. urban and agricultural runoff, boat harbours or historical activities) (Chapter 5). Pesticides typically occur through diffuse sources, originating from agricultural activities on land before entering freshwater streams and rivers that eventually flow into estuaries and marine waters (see Chapter 7). Modern day pesticides only persist in the environment for a few days or weeks. Although they degrade quickly, there are often high concentrations of these pesticides in the environment because they are applied in large quantities over broad areas and often repeated times in a single season. The application process is ineffective, and it has been suggested that only 1% of pesticide sprayed reaches target organisms while the remaining 99% enters the surrounding soil, sediment and waterways (Stauber et al. 2016). This may partly explain the high variability in the distribution and abundance of pesticides in receiving environments, which can be characteristically high in coastal bays but only at certain times of the year or in particular hot spots (O'Brien et al. 2016).
The ephemeral nature of pesticides in the marine environment coupled with metals from point and diffuse sources poses challenging questions in the context of multiple stressors. For example, what is the effect of high concentrations of metals in the seabed from past industrial activities combined with the fact that seabed is now exposed to infrequent, high concentrations of pesticides from nearby river, and when does it occur? How do we know what concentrations are going to cause a biological effect?
Organisms in the seabed may be tolerant to metal exposure until a pulse of pesticides is delivered into the system. However, the observed biological effect may depend on the type of pesticide, and which organism or groups of organisms are likely to be affected. For example, herbicides are expected to have different biological effects on photosynthetic groups (e.g. phytoplankton, diatoms and macroalgae) compared to effects from insecticides. The timing of the contamination event, the mode of delivery into the system, the local environmental conditions, chemical characteristics and concentrations as well as potential interactions with other pre-existing contaminants are all factors that need to be considered when predicting multiple stressor effects.
14.3.3 Contamination and Climate Change
Climate change is affecting salinity regimes in marine and estuarine environments through increases in the duration, frequency and strength of rainfall and storm events (Stauber et al. 2016). Changes in global atmospheric carbon dioxide are linked to increasing ocean temperatures and decreasing pH which is termed ocean acidification (Chapter 11). Marine contaminants interact with climate-related variables—salinity, temperature and pH, and together they can have very different effects on bioavailability, bioaccumulation and toxicity of the contaminant than if the stressors occurred separately (Cabral et al. 2019). As discussed by Alava et al. (2017) and Cabral et al. (2019), the interactive effects may be driven by changes in climate variables that cause an increased risk of exposure or susceptibility to toxic effects (climate-dominated effects; Figure 14.4) or they may be driven by contamination, where prior exposure causes increased susceptibility to climate variables (contaminant-dominated effects; Figure 14.4). Identifying dominance patterns as well as the type of interactive effects (additive, synergistic or antagonistic) is crucial in resolving such complex interactions (Cabral et al. 2019).
Nutrients and chemical toxicants are among the most studied stressors in marine environments, but there is still limited testing of these in combination with temperature and pH (Schiedek et al. 2007; Crain et al. 2008). Climate change alters the chemistry of the ocean, which can increase the bioaccumulation of chemical toxicants making organisms more susceptible to exposure (Alava et al. 2017). Temperature increases metabolic rates, food consumption increases and the risk of exposure to chemicals associated with food sources also increases (Cabral et al. 2019). In a global review of literature based on urban marine and estuarine environments, we found only 37 of a total 579 studies considered the combined effects of a climate-related variables (salinity, temperature, pH) and any other anthropogenic stressor (nutrients, chemical contaminants, non-indigenous species or built infrastructure; unpublished data from O’Brien et al. 2019). This knowledge gap is significant and needs to be addressed urgently as our climate is changing rapidly.
The few studies that have tested interactive effects of contamination and climate change highlight the need to understand these interactions at multiple biological levels (Cabral et al. 2019). For example, at the individual level, the survival of the mysid crustacean Praunus flexuosus is reduced by nickel, chromium and zinc, but survival is further reduced at increased temperatures and salinities (McLusky and Hagerman 1987). The combined effects of heavy metals and temperature or salinity is thought to interfere with the organism’s ability to osmoregulate thereby making them more susceptible to the toxic effects of the contaminants (McLusky and Hagerman 1987). Similarly, the effects of copper on brittle star (Amphipholis squamata) behaviour are dependent on temperature (Black et al. 2015). Interestingly, a decrease in the toxicity of copper was detected with increasing temperature from 15 °C to 25 °C (Black et al. 2015), suggesting predicted increases in sea temperatures could mitigate toxic effects of copper for these organisms. Increasing nutrients in combination with decreasing pH have resulted in antagonistic effects on corals with slower growth rates when stressors act independently compared to when they are combined (Langdon and Atkinson 2005; Holcomb et al. 2010; Chauvin et al. 2011).
At the community level, a study in Northern Ireland investigated interactive effects between nutrients, temperature (as a measure of ocean warming) and the presence of a non-indigenous seaweed (Sargassum muticum) (Vye et al. 2015). They found a strong antagonistic interaction between nutrient enrichment and the invasive species, with observed decreases in algal biomass when enriched with nutrients but only in the absence of S. muticum. However, this antagonistic interaction was no longer evident when combined with increased temperatures (Vye et al. 2015). If antagonistic interactions no longer exist under future climate scenarios it not only makes the effects difficult to predict, but could also expose these habitats to risk of algal blooms facilitated by high nutrient levels or further spread of non-indigenous species.
14.3.4 Three or More Stressor Interactions
To date, multiple stressor models have only focused on interactions between two stressors (Schafer and Piggott 2018). However, in marine environments, and particularly in urban and agricultural coastal marine habitats, many stressors are acting simultaneously (Box 14.1). Stressors in the marine environment have been accumulating worldwide for decades and the first anthropogenic stressors were probably overfishing and untreated sewage, followed by pollution associated with industrialisation in the nineteenth Century (e.g. Jackson et al. 2001). These historical stressors coupled with modern stressors such as climate change and non-indigenous species make it difficult to disentangle the impacts of individual stressors or specific combinations of stressors.
A common approach to studying interactions between three or more stressors is surveys or correlation studies that relate biological change between impact and reference sites or along a gradient of impact. This approach identifies patterns, but not specific cause-effect relationships between stressor combinations and biological responses. For example, Stuart-Smith et al. (2015) studied community structure of fishes and mobile invertebrates on shallow reefs that were affected by metal contamination, surrounding human population density, proximity to sewage outfalls, the city port and the distribution of invasive species. They found reefs that were the most affected by these stressors had reduced mobile invertebrate abundances and reduced fish biomass. This effect was most prominent on reefs that were invaded by non-indigenous species (Stuart-Smith et al. 2015). This study provides a picture of the overall impact but does not identify the specific stressor combinations that were causing the effect. It is suggested that community-level field experiments, which manipulate different combinations of stressors, are required to understand these underlying mechanisms (e.g. Mayer-Pinto et al. 2010; Chariton et al. 2011; O'Brien and Keough 2013; Birrer et al. 2018; Johnson et al. 2018) and inform the fundamental interactions between multiple stressors (Stuart-Smith et al. 2015).
The multiple stressor framework includes chemical and physical stressors, which have been the focus of this chapter so far, but also biological stressors, such as non-indigenous species, and built infrastructure, such as aquaculture, boat harbours, marinas, piers, jetties and groynes (see definitions in O'Brien et al. 2019). Many of these stressors are likely to act at different temporal and spatial scales. As a hypothetical example, a contaminant from a stormwater outfall that affects the marine environment in the immediate surrounding area may interact with a non-indigenous seaweed species that occurs across the entire coastline. The seaweed species is prolific in cooler months but dies-off in summer months when it becomes more susceptible to rising global ocean temperatures. The stressors in this example (contamination from stormwater outfall, non-indigenous species and climate change) are occurring at multiple spatial (local, regional and global) and temporal (season) scales. These issues of scale and stressor type (chemical, physical, biological or infrastructure) become more pertinent as the number of stressors increases and needs to be specifically considered in any study or monitoring program (Downes et al. 2002; Mayer-Pinto et al. 2010).
14.4 Management of Multiple Stressors
Coastal systems worldwide are threatened by multiple anthropogenic activities, including urban development, organic and inorganic pollution, over-exploitation of resources, dredging and dumping, and invasive species (Lotze et al. 2006; Airoldi and Beck 2007). When coupled with climatic instabilities, localised cumulative human perturbations create new regimes of disturbances that greatly affect the stability, resilience and productivity of ecosystems (Cimon and Cusson 2018; Piola and Johnston 2008; Sauve et al. 2016). Nonetheless, marine management has often focused on one impact at a time (Beaumont et al. 2007) instead of taking a more holistic approach. The shortfalls of this approach are becoming evident, with only about 7% of the world’s oceans designated as protected (UNEP-WCMC and IUCN 2020). With current management processes so strongly focused on working in an impact-by-impact framework, there are entrenched scientific, cultural and institutional challenges to shifting those processes toward ecosystem-based management and marine spatial planning, which address multiple human uses of the ocean, their cumulative impacts and interactive effects (Halpin et al. 2006).
To attain sustainability, it is necessary to understand how natural systems are affected by multiple stressors and can respond to management interventions that aim to achieve multiple goals (Dafforn et al. 2015). These concepts are especially relevant when managing ecosystem resilience. If the ability of systems to withstand (i.e. resistance) and/or recover (i.e. resilience) from disturbances is progressively eroded by cumulative impacts, the system becomes vulnerable to regime shifts. These shifts are critical transitions that are characterised by different sets of structures, processes and values (Scheffer et al. 2009), which can lead to ecosystem collapse. Indeed, the European Union Marine Strategy Framework Directive (2008/56/EC) calls for the urgent establishment of coherent and coordinated programmes of measures to contain the collective pressure of human activities within sustainable levels. A philosophy of regenerative intervention (Chapter 15) is required before sustainability can be realised.
The first step towards the management of multiple stressors is to identify thresholds and trade-offs. A threshold can be set as the level of human-induced pressure (e.g. pollution) at which small changes produce substantial improvements in protecting an ecosystem’s structural (e.g. diversity) and functional (e.g. resilience) attributes (Samhouri et al. 2010). This approach is based on the detection of nonlinearities in relationships between ecosystem attributes and pressures. These relationships, however, are known in only a few cases, and they often focus on one direction only (increasing human pressures), without exploring the reverse pathways following management actions geared towards recovery.
The interactions between multiple stressors can exacerbate nonlinear responses of ecosystems to human impacts. This will limit their recovery capacity and reduce the likelihood that a system can retrace the same trajectory during restoration as during degradation. For example, fisheries exploitation and increased nutrient loadings jointly affect food webs and production in estuaries via reductions in fish and shellfish biomass, increased algae production and habitat degradation (Breitburg et al. 2009). As a result, there could be specific levels of fish caught per unit effort and nutrient loadings that lead to threshold responses making them resistant to restoration through fisheries and nutrient management (Breitburg et al. 2009; Scheffer et al. 2001). Maximising management outcomes relies on getting a relevant mechanistic understanding of the effects of multiple stressors at scales ranging from individuals to populations and whole ecosystems, indicators of changes, tools, and models that can be used for early identification of thresholds.
Brown et al. (2013) have examined the effectiveness of management when faced with different types of interactions between local and global (climatic) stressors in seagrass and fish communities. They showed that for additive effects, reducing the magnitude of local stressors should lead to a corresponding increase in the response of interest allowing for straightforward expectations of the response to management and conservation actions. In contrast, mitigationMitigation of stressors involved in synergistic or antagonistic interactions with global stressors will lead to greater than or less than (respectively) predicted results based on additive models. Antagonistic stressors create management challenges, as all or most stressors would need to be eliminated to see substantial recovery, except in cases where the antagonism is driven by a dominant stressor, such that mitigation of that stressor alone would substantially improve the state of species or communities. In contrast, synergisms may respond quite favourably to removal of a single stressor as long as the system has not passed a threshold into an alternative state (Hobbs et al. 2006).
14.5 Summary
There are very few ecosystems across the world that can be considered impacted by a single stressor (Van Den Brink et al. 2019). In the marine environment, the occurrence of multiple anthropogenic stressors is the new normal (Halpern et al. 2007). Pollution is one of the most important stressors affecting the marine environment, but it should no longer be considered in isolation as a single stressor (Cabral et al. 2019). The impacts of pollution and how species respond to individual pollutants and/or mixtures of pollutants depends on over-exploitation of fisheries, built infrastructure, non-indigenous species and climate change as well as the accumulation of these stressors over time (Jackson et al. 2001). Multiple stressor models of additive, synergistic and antagonistic interactions provide a good starting point for predicting impacts, but in many cases, they are not going to be able to predict the dynamic nature of interactions in the marine environment (Côté et al. 2016).
As the global environment changes, we are likely to see novel combinations of species that will change ecological processes and the way habitats function (Hobbs et al. 2006). Multiple stressors have the potential to influence species survival, community abundances and competition between species that will have implications for population growth and ecosystem functioning (Cadotte and Tucker 2017). To some extent, marine management will need to accept the impacted nature of the environment as status quo and provide solutions that focus on managing current condition, which support existing ecosystem services, rather than attempting restoration to pre-stressed conditions (e.g. Bishop et al. 2017).
Clearly, greater effort is now required to understand how multiple stressors are affecting the marine environment (O’Brien et al. 2019). This needs to be based on scientifically appropriate monitoring and study designs with replication, site selection and clear hypotheses, otherwise this knowledge gap will prevail (Mayer-Pinto et al. 2010). Terminology and concepts underlying the theory of multiple stressors, including the use of null models based on mechanistic assumptions (e.g. Schafer and Piggott 2018), are established and have been documented in the scientific literature for several decades (e.g. Folt et al. 1999; Crain et al. 2008; Van Den Brink et al. 2019). Progress is now required in understanding uncertainty and the complexity of these multiply stressed environments. How do we need to adapt our management strategies and what do policy makers need to do to ensure our marine environments remain resilient and functionally adaptable (Verges et al. 2019).
14.6 Study Questions and Activites
-
1.
Describe an additive multiple stressor model and provide an example. This may be a hypothetical example or relate to any of the sources of contamination you have read about in this book (e.g. nutrients, trace metal, metalloids, pesticides, plastics, oils or noise).
-
2.
Describe a synergistic interaction and an antagonistic interaction in your own words. Explain what type of interaction would be of more concern.
-
3.
Draw a graph showing possible interactions between nutrients and trace metals. Show a response variable on the y-axis and stressors on the x-axis.
-
4.
Explain why understanding the interaction between climate change and contamination is challenging. Include an example to illustrate your answer.
References
Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. In: Gibson R, Atkinson R, Gordon J (eds) Oceanography and marine biology, vol 45. CRC, Boca Raton, p 560
Airoldi L, Turon X, Perkol-Finkel S, Rius M (2015) Corridors for aliens but not for natives: effects of marine urban sprawl at a regional scale. Divers Distrib 21:755–768
Alava J, Cheung W, Ross P, Ur S (2017) Climate change–contaminant interactions in marine food webs: toward a conceptual framework. Glob Change Biol 23:3984–4001
Andersen JH, Halpern BS, Korpinen S, Murray C, Reker J (2015) Baltic Sea biodiversity status vs. cumulative human pressures. Estuar Coast Shelf Sci 161:88–92
Baird DJ, Van Den Brink PJ, Chariton AA, Dafforn KA, Johnston EL (2016) New diagnostics for multiply stressed marine and freshwater ecosystems: integrating models, ecoinformatics and big data. Mar Freshw Res 67:391–392
Beaumont NJ, Austen MC, Atkins JP, Burdon D, Degraer S, Dentinho TP, Derous S, Holm P, Horton T, Van Ierland E, Marboe AH, Starkey DJ, Townsend M, Zarzycki T (2007) Identification, definition and quantification of goods and services provided by marine biodiversity: implications for the ecosystem approach. Mar Pollut Bull 54:253–265
Birrer SC, Dafforn KA, Simpson SL, Kelaher BP, Potts J, Scanes P, Johnston EL (2018) Interactive effects of multiple stressors revealed by sequencing total (DNA) and active (RNA) components of experimental sediment microbial communities. Sci Total Environ 637:1383–1394
Bishop MJ, Mayer-Pinto M, Airoldi L, Firth LB, Morris RL, Loke LHL, Hawkins SJ, Naylor LA, Coleman RA, Chee SY, Dafforn KA (2017) Effects of ocean sprawl on ecological connectivity: impacts and solutions. J Exp Mar Biol Ecol 492:7–30
Black JG, Reichelt-Brushett AJ, Clark MW (2015) The effect of copper and temperature on juveniles of the eurybathic brittle star Amphipholis squamata—exploring responses related to motility and the water vascular system. Chemosphere 124:32–39
Bracewell S, Verdonschot RCM, Schäfer RB, Bush A, Lapen DR, Van Den Brink PJ (2019) Qualifying the effects of single and multiple stressors on the food web structure of Dutch drainage ditches using a literature review and conceptual models. Sci Total Environ 684:727–740
Breitburg DL, Craig JK, Fulford RS, Rose KA, Boynton WR, Brady DC, Ciotti BJ, Diaz RJ, Friedland KD, Hagy JD, Hart DR, Hines AH, Houde ED, Kolesar SE, Nixon SW, Rice JA, Secor DH, Targett TE (2009) Nutrient enrichment and fisheries exploitation: interactive effects on estuarine living resources and their management. Hydrobiologia 629:31–47
Brown CJ, Saunders MI, Possingham HP, Richardson AJ (2013) Managing for interactions between local and global stressors of ecosystems. PLoS ONE 8(6):e65765
Bulleri F, Chapman MG (2010) The introduction of coastal infrastructure as a driver of change in marine environments. J Appl Ecol 47:26–35
Cabral H, Fonseca V, Sousa T, Costa Leal M (2019) Synergistic effects of climate change and marine pollution: an overlooked interaction in coastal and estuarine areas. Int J Environ Res Public Health 16:2737
Cadotte MW, Tucker CM (2017) Should environmental filtering be abandoned? Trends Ecol Evol 32:429–437
Chariton AA, Maher WA, Roach AC (2011) Recolonisation of translocated metal-contaminated sediments by estuarine macrobenthic assemblages. Ecotoxicology 20:706–718
Chauvin A, Denis V, Cuet P (2011) Is the response of coral calcification to seawater acidification related to nutrient loading? Coral Reefs 30:911–923
Cimon S, Cusson M (2018) Impact of multiple disturbances and stress on the temporal trajectories and resilience of benthic intertidal communities. Ecosphere Ecosphere 9(10):e02467
Claudet J, Fraschetti S (2010) Human-driven impacts on marine habitats: a regional meta-analysis in the Mediterranean Sea. Biol Cons 143:2195–2206
Côté IM, Darling ES, Brown CJ (2016) Interactions among ecosystem stressors and their importance in conservation. Proc Roy Soc B Biol Sci 283
Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315
Dafforn KA, Johnston EL, Glasby TM (2009) Shallow moving structures promote marine invader dominance. Biofouling 25:277–287
Dafforn KA, Glasby TM, Johnston EL (2012) Comparing the invasibility of experimental “Reefs” with field observations of natural Reefs and artificial structures. PLoS ONE 7(5):e38124
Dafforn KA, Glasby TM, Airoldi L, Rivero NK, Mayer-Pinto M, Johnston EL (2015) Marine urbanization: an ecological framework for designing multifunctional artificial structures. Front Ecol Environ 13:82–90
De Laender F (2018) Community- and ecosystem- level effects of multiple environmental change drivers: beyond null model testing. Glob Change Biol 24:5021–5030
Downes BJ, Barmuta LA, Fairweather PG, Faith DP, Keough MJ, Lake PS, Mapstone BD, Quinn GP (2002) Monitoring ecological impacts: concepts and practice in flowing waters. Cambridge University Press, Cambridge, p 434
Folt CL, Chen CY, Moore MV, Burnaford J (1999) Synergism and antagonism among multiple stressors. Limnol Oceanogr 44:864–877
Glasby TM (1999) Effects of shading on subtidal epibiotic assemblages. J Exp Mar Biol Ecol 234:275–290
Griffen BD, Belgrad BA, Cannizzo ZJ, Knotts ER, Hancock ER (2016) Rethinking our approach to multiple stressor studies in marine environments. Mar Ecol Prog Ser 543:273–281
Hale R, Piggott JJ, Swearer SE (2017) Describing and understanding behavioral responses to multiple stressors and multiple stimuli. Ecol Evol 7:38–47
Halpern BS, Selkoe KA, Micheli F, Kappel CV (2007) Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv Biol 21:1301–1315
Halpin PN, Read AJ, Best BD, Hyrenbach KD, Fujioka E, Coyne MS, Crowder LB, Freeman SA, Spoerri C (2006) OBIS-SEAMAP: developing a biogeographic research data commons for the ecological studies of marine mammals, seabirds, and sea turtles. Mar Ecol Prog Ser 316:239–246
Hawkins S, Moore P, Burrows M, Poloczanska E, Mieszkowska N, Herbert R, Jenkins Sr, Thompson R, Genner M, Southward A (2008) Complex interactions in a rapidly changing world: responses of rocky shore communities to recent climate change. Clim Res 37:123–133
Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater P, Cramer VA, Epstein PR, Ewel JJ, Klink CA, Lugo AE, Norton D, Ojima D, Richardson DM, Sanderson EW, Valladares F, Vila M, Zamora R, Zobel M (2006) Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob Ecol Biogeogr 15:1–7
Holcomb M, Mccorkle DC, Cohen AL (2010) Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786). J Exp Mar Biol Ecol 386:27–33
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
Johnson GC, Pezner AK, Sura SA, Fong P (2018) Nutrients and herbivory, but not sediments, have opposite and independent effects on the tropical macroalga, Padina boryana. J Exp Mar Biol Ecol 507:17–22
Johnston EL, Dafforn KA, Clark GF, Rius M, Floerl O (2017) How anthropogenic activities affect the establishment and spread of non-indigenous species post-arrival. In: Hawkins S, Evans A, Dale C, Firth L, Hughes D, Smith I (eds) Oceanography and marine biology: an annual review, vol 55, pp 2–33
Johnston EL, Roberts DA (2009) Contaminants reduce the richness and evenness of marine communities: a review and meta-analysis. Environ Pollut 157:1745–1752
Knott NA, Aulbury JP, Brown TH, Johnston EL (2009) Contemporary ecological threats from historical pollution sources: impacts of large-scale resuspension of contaminated sediments on sessile invertebrate recruitment. J Appl Ecol 46:770–781
Knottnerus OS (2005) History of human settlement, cultural change and interference with the marine environment. Helgol Mar Res 59:2–8
Kroeker KJ, Kordas RL, Harley CDG (2017) Embracing interactions in ocean acidification research: confronting multiple stressor scenarios and context dependence. Biol Lett 13(3):20160802
Langdon C, Atkinson MJ (2005) Effect of elevated pCO(2) on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J Geophys Res Oceans 110(9):1–16
Lawes JC, Dafforn KA, Clark GF, Brown MV, Johnston EL (2017) Multiple stressors in sediments impact adjacent hard substrate habitats and across biological domains. Sci Total Environ 592:295–305
Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, Kidwell SM, Kirby MX, Peterson CH, Jackson JBC (2006) Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312:1806–1809
Matthiessen P, Reed J, Johnson M (1999) Sources and potential effects of copper and zinc concentrations in the estuarine waters of Essex and Suffolk, United Kingdom. Mar Pollut Bull 38:908–920
Mayer-Pinto M, Underwood AJ, Tolhurst T, Coleman RA (2010) Effects of metals on aquatic assemblages: what do we really know? J Exp Mar Biol Ecol 391:1–9
McGee BL, Schlekat CE, Boward DM, Wade TL (1995) Sediment contamination and biological effects in a Chesapeake Bay marina. Ecotoxicology 4:39–59
Mclusky DS, Hagerman L (1987) The toxicity of chromium, nickel and zinc: effects of salinity and temperature, and the osmoregulatory consequences in the mysid Praunus flexuosus. Aquat Toxicol 10:225–238
Monk WA, Compson ZG, Choung CB, Korbel KL, Rideout NK, Baird DJ (2019) Urbanisation of floodplain ecosystems: weight-of-evidence and network meta-analysis elucidate multiple stressor pathways. Sci Total Environ 684:741–752
O’Brien AL, Keough MJ (2013) Detecting benthic community responses to pollution in estuaries: a field mesocosm approach. Environ Pollut 175:45–55
O’Brien D, Lewis S, Davis A, Gallen C, Smith R, Turner R, Warne MStJ, Turner S, Caswell S, Mueller JF, Brodie J (2016) Spatial and temporal variability in pesticide exposure downstream of a heavily irrigated cropping area: application of different monitoring techniques. J Agric Food Chem 64:3975–3989
O’Brien AL, Dafforn KA, Chariton AA, Johnston EL, Mayer-Pinto M (2019) After decades of stressor research in urban estuarine ecosystems the focus is still on single stressors: a systematic literature review and meta-analysis. Sci Total Environ 684:753–764
Orr JA, Vinebrooke RD, Jackson MC, Kroeker KJ, Kordas RL, Mantyka-Pringle C, Van Den Brink PJ, De Laender F, Stoks R, Holmstrup M, Matthaei CD, Monk WA, Penk MR, Leuzinger S, Schafer RB, Piggott JJ (2020) Towards a unified study of multiple stressors: divisions and common goals across research disciplines. Proc Roy Soc B-Biol Sci 287(1926):20200421
Philippart CJM, Anadón R, Danovaro R, Dippner JW, Drinkwater KF, Hawkins SJ, Oguz T, O’sullivan G, Reid PC (2011) Impacts of climate change on European marine ecosystems: observations, expectations and indicators. J Exp Mar Biol Ecol 400:52–69
Piola RF, Johnston EL (2008) Pollution reduces native diversity and increases invader dominance in marine hard-substrate communities. Divers Distrib 14:329–342
Rivero NK, Dafforn KA, Coleman MA, Johnston EL (2013) Environmental and ecological changes associated with a marina. Biofouling 29:803–815
Samhouri JF, Levin PS, Ainsworth CH (2010) Identifying thresholds for ecosystem-based management. PLoS ONE 5(1):e8907
Sauve AMC, Fontaine C, Thebault E (2016) Stability of a diamond-shaped module with multiple interaction types. Thyroid Res 9:27–37
Schafer RB, Piggott JJ (2018) Advancing understanding and prediction in multiple stressor research through a mechanistic basis for null models. Glob Change Biol 24:1817–1826
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413:591–596
Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, Van Nes EH, Rietkerk M, Sugihara G (2009) Early-warning signals for critical transitions. Nature 461:53–59
Schiedek D, Sundelin B, Readman JW, Macdonald RW (2007) Interactions between climate change and contaminants. Mar Pollut Bull 54:1845–1856
Schiff K, Brown J, Diehl D, Greenstein D (2007) Extent and magnitude of copper contamination in marinas of the San Diego region, California, USA. Mar Pollut Bull 54:322–328
Sievers M, Hale R, Parris KM, Swearer SE (2018) Impacts of human-induced environmental change in wetlands on aquatic animals. Biol Rev 93:529–554
Sokolova IM, Lannig G (2008) Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: implications of global climate change. Climate Res 37:181–201
Stauber JL, Chariton A, Apte S (2016) Global change. In: Blasco J, Chapman P, Campana O, Hampel M (eds) Marine ecotoxicology: current knowledge and future issues, pp 273–313
Stuart-Smith RD, Edgar GJ, Stuart-Smith JF, Barrett NS, Fowles AE, Hill NA, Cooper AT, Myers AP, Oh ES, Pocklington JB, Thomson RJ (2015) Loss of native rocky reef biodiversity in Australian metropolitan embayments. Mar Pollut Bull 95:324–332
Svensson CJ, Pavia H, Toth GB (2007) Do plant density, nutrient availability, and herbivore grazing interact to affect phlorotannin plasticity in the brown seaweed Ascophyllum nodosum. Mar Biol 151:2177–2181
UNEP-WCMC and IUCN (United Nations Environment Program-World Conservation Monitoring Centre and International Union for Conservation of Nature) (2020). Protected planet. Available at: https://www.protectedplanet.net/en/news-and-stories/the-lag-effect-in-the-world-database-on-protected-areas. Accessed 19 Feb 2022
Van Den Brink PJ, Bracewell SA, Bush A, Chariton A, Choung CB, Compson ZG, Dafforn KA, Korbel K, Lapen DR, Mayer-Pinto M, Monk WA, O’Brien AL, Rideout NK, Schäfer RB, Sumon KA, Verdonschot RCM, Baird DJ (2019) Towards a general framework for the assessment of interactive effects of multiple stressors on aquatic ecosystems: results from the Making Aquatic Ecosystems Great Again (MAEGA) workshop. Sci Total Environ 684:722–726
Verges A, Mccosker E, Mayer-Pinto M, Coleman MA, Wernberg T, Ainsworth T, Steinberg PD (2019) Tropicalisation of temperate reefs: Implications for ecosystem functions and management actions. Funct Ecol 33:1000–1013
Vye SR, Emmerson MC, Arenas F, Dick JTA, O’connor NE (2015) Stressor intensity determines antagonistic interactions between species invasion and multiple stressor effects on ecosystem functioning. Oikos 124:1005–1012
Ware C, Berge J, Sundet JH, Kirkpatrick JB, Coutts ADM, Jelmert A, Olsen SM, Floerl O, Wisz MS, Alsos IG (2014) Climate change, non-indigenous species and shipping: assessing the risk of species introduction to a high-Arctic archipelago. Divers Distrib 20:10–19
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
O’Brien, A.L., Dafforn, K., Chariton, A., Airoldi, L., Schäfer, R.B., Mayer-Pinto, M. (2023). Multiple Stressors. In: Reichelt-Brushett, A. (eds) Marine Pollution – Monitoring, Management and Mitigation . Springer Textbooks in Earth Sciences, Geography and Environment. Springer, Cham. https://doi.org/10.1007/978-3-031-10127-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-10127-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10126-7
Online ISBN: 978-3-031-10127-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)