Abstract
Imaging techniques play a crucial role in the diagnosis, staging, surveillance and assessment of the treatment response of hepatocellular carcinoma (HCC). An increasingly pivotal role is currently played by computed tomography and magnetic resonance imaging, which can simultaneously provide a conclusive diagnosis without the need for histological confirmation and tumor staging, while ultrasound and contrast-enhanced ultrasound are limited to surveillance of HCC patients. The aim of this chapter is to give an overview of the most used liver imaging techniques and the main imaging characteristics of HCC.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Hepatocellular carcinoma imaging
- Liver Imaging Reporting and Data System
- Hypervascular lesions
- Liver cirrhosis
- Hepatospecific agents
1 Role of Ultrasound and Contrast-Enhanced Ultrasound
Ultrasound (US) every 6 months is universally recommended for hepatocellular carcinoma (HCC) surveillance in all guidelines [1, 2].
The detection of any new focal lesion during US surveillance should directly require a diagnostic “recall strategy” that varies according to the size of the lesion. Lesions <1 cm should be admitted into a non-enhanced follow-up program based on US repetition at 3–6 months; if the size remains stable over a 2-year period, the 6-month surveillance can be restored. The “recall strategy” for lesions ≥1 cm is based on contrast-enhanced imaging techniques with use of vascular contrast agent, such as computed tomography (CT) or magnetic resonance imaging (MRI).
Contrast-enhanced US (CEUS) has been observed to improve the characterization of focal liver lesions with enhancement patterns generally similar to CT and MRI and can be useful in the case of renal impairment. US contrast agents (microbubbles) comprise an albumen or phospholipid shell containing a stable perfluorocarbon or sulphur hexafluoride gas. CEUS is not recommended as a first-line imaging technique or for recall strategies in terms of cost-effectiveness, because CT or MRI will be needed for staging, but it can be utilized when both CT and MRI are contraindicated and/or inconclusive [1].
2 Computed Tomography Technique
Contrast-enhanced CT (CECT) or contrast-enhanced MRI play an essential role in the diagnosis of HCC and do not require histopathologic confirmation. According to the Liver Imaging-Reporting and Data System (LI-RADS), the use of a multi-detector-row CT with a minimum of 8-detector rows is recommended, with an axial slice thickness ≤5 mm [3]. The scanning protocol should include an unenhanced phase (to detect hemorrhage/treatment sequelae) and three enhanced phases of study. For CECT, an extracellular intravenous iodinated contrast agent is used, possibly with moderate/high iodine concentration (≥350 mgI/mL), administered at high injection rates (>3 mL/s), with dose adjusted according to body size indexes [3]. According to LI-RADS v2018, the multiphase dynamic study of the liver consists of a first late hepatic arterial phase (25–30 s after contrast agent injection with bolus tracking), a portal venous phase (60–80 s after contrast agent injection) and an equilibrium phase (2–5 min after contrast agent injection) [3].
3 Magnetic Resonance Imaging Technique and Contrast Agents
MRI improves the detection and characterization of focal hepatic lesions, in comparison to a CT scan. In addition, MRI performs better compared to CT in the detection of HCC in patients with liver cirrhosis, especially when a hepatobiliary phase (HBP) is added [4]. In particular, a HBP was found to be superior to CECT also in guiding the correct treatment decisions for HCC [5]. MRI of the liver is best performed at 1.5 T or higher field strength [3]. The MRI protocol includes T1-weighted in-/out-of-phase gradient echo (GRE) images, T2-weighted images without and with fat suppression, diffusion-weighted images with at least two b values acquired (0–50 s/mm2 and 400–800 s/mm2) [6]. The dynamic contrast-enhanced MRI scan can be performed both with gadolinium-based extracellular contrast agents or with hepatobiliary contrast agents [3]. The multiphasic MRI study is essential for identification of the typical imaging features and vascular hallmarks, which are defined according to LI-RADS v18 as “arterial phase hyperenhancement”, “washout appearance” and “enhancing capsule” [4, 6, 7]. Hepatobiliary contrast agents make it possible to investigate the vascular characteristics of HCC lesions and to assess hepatocellular function in a single examination [6]. The most commonly used hepatobiliary contrast agents include two gadolinium-based compounds: gadoxetate disodium (Gd-EOB-DTPA) and gadobenate dimeglumine (Gd-BOPTA).
4 Imaging Features of Hepatocellular Carcinoma
HCCs are generally arterial hypervascular tumors. However, hyperenhancement (“wash-in”) in the arterial phase alone is poorly specific for HCC. When hypoenhancement in the portal venous or delayed phases (“wash-out”) is added, both the specificity and sensitivity increase (Fig. 5.1) [8]. Early HCCs are, however, characterized by a hypo- or isoenhancement in arterial phase imaging, due to their incomplete neoangiogenesis [7]. Another characteristic imaging feature included by LI-RADS among the major criteria for HCC diagnosis is the presence of a peripheral “capsule appearance”, histologically composed also by fibrous tissue, which explains its late enhancement due to retention of gadolinium in the extracellular interstitial spaces [6, 9]. Another characteristic feature of HCC, included in the “ancillary criteria” of LI-RADS, is the “nodule-in-nodule” appearance (Fig. 5.2) [10]. Vascular invasion is more frequent in progressed HCC. It can be microvascular, if it is only appreciable on microscopy, or macrovascular (Figs. 5.3 and 5.4) which refers to macroscopically visible tumor in vein (LR-TIV) [11]. Both of them are indicators of a poor overall survival and an aggressive biologic behavior [6]. The presence of intralesional fat is very specific for HCC and this is well evaluable on MRI T1-weighted GRE dual-echo sequences, with loss of signal in the out-of-phase images (Fig. 5.5) [10].
Typical CT imaging features for hepatocellular carcinoma. (a) Axial plane, arterial phase CT scan demonstrates arterial wash-in associated with afferent arteriole on maximum intensity projection reconstruction. (b, c) Portal and delayed phases respectively show the progressive wash-out of the lesion. The figure is published with the patient’s authorization
Small hepatocellular carcinoma nodule-in-nodule appearance. (a–c) A 3-cm nodule is appreciable on segment 8 (white arrow), mildly hyperintense on axial T2-weighted fat saturated (a), partially hyperintense on T1-weighted in-phase gradient echo (GRE) (b) with a partial signal drop-out on axial out-of-phase GRE (c) as expression intralesional fat. (d, e) Diffusion-weighted imaging (d), and apparent diffusion coefficient map (e) show lesion restriction related to hypercellularity. (f–i) Axial T1-weighted fat-saturated sequence in the arterial phase shows intense arterial wash-in of the upper portion of the nodule (f), with wash-out in the portal venous (g) and delayed (h) phases and lack of contrast agent uptake in the hepatobiliary phase (i). The figure is published with the patient’s authorization
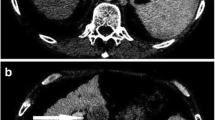
A voluminous lesion subverts the structure of the right liver, with imaging compatible with hepatocellular carcinoma appreciable on the CT axial images in the arterial (a), portal venous (b) and delayed (c) phases. Macrovascular invasion is observed with neoplastic thrombosis causing occlusion of the right portal vein, subocclusion of the left portal vein and the common portion of the mesenteric splenic confluence (black arrow). The figure is published with the patient’s authorization
Hepatocellular carcinoma with macrovascular invasion on MRI: between hepatic segments 5 and 6 a poorly defined mass associated with neoplastic thrombus in the anterior right portal vein branch. (a) Lesion with intermediate signal on the axial T2-weighted fat saturated image. (b) Restriction on diffusion-weighted imaging (white arrows). (c) Axial T1-weighted fat saturated image during the arterial phase with lesion wash-in. (d, e) Axial T1-weighted fat saturated images during the portal venous (d) and delayed (e) phases, with lesion wash-out. (f) Contrast-enhanced MRI axial T1-weighted fat suppressed image in the hepatobiliary phase with lesion hypointensity
Hepatocellular carcinoma with fat metaplasia at segment 5 and 6. (a) Axial T1-weighted gradient echo (GRE) in-phase sequence where the lesion is heterogeneously isointense to liver parenchyma. (b) Axial T1-weighted GRE out-of-phase sequence with signal drop-out due to intracellular fat component (white arrow). (c) Axial diffusion-weighted images with diffusion restriction of the lesion, (d) also appreciable on apparent diffusion coefficient map. (e–g) After contrast agent administration, the lesion shows slight hyperintensity on the axial T1-weighted fat saturated sequence in the arterial phase (e), with progressive wash-out in the portal venous (f) and delayed (g) phases. (h) Hypointensity of the lesion on axial T1-weighted fat saturated sequence, in the hepatobiliary phase
5 Liver Imaging Reporting and Data System (LI-RADS)
The LI-RADS system aims to standardize terminology, technique, interpretation, reporting and data collection in liver imaging for HCC surveillance and is promoted by the American College of Radiology and developed by an international committee of radiologists, hepatologists, pathologists and surgeons. US LI-RADS v2017 is limited to screening or surveillance in patients at risk for HCC, to identify HCC at an early stage, when it is potentially curable. CEUS LI-RADS v2017, as well as CT and MRI LI-RADS, should be applied in patients at high risk of HCC, including in this category adult cirrhotic patients, patients with chronic HBV, and patients with current or prior HCC.
The LI-RADS v2018 algorithm is articulated in successive diagnostic steps directed first to the identification of diagnoses other than HCC (LR-1, LR-2, LR-TIV, LR-M [malignancy other than HCC] or LR-NC [non-categorizable]) and then to a diagnostic table that helps to distinguish LR-3, LR-4 and LR-5 [7]. The classification from LR-1 to LR-5 is intended as growing probability of HCC malignancy, where LR-1 and LR-2 are considered respectively “certainly benign” and “probably benign”.
6 Treatment Response: mRECIST and LI-RADS
Some HCC treatment strategies act by induction of tumor necrosis or reduction in vascularity, which is not necessarily accompanied by tumor reduction. The World Health Organization and Response Evaluation Criteria in Solid Tumors (RECIST) criteria do not address measures of antitumor activity other than tumor dimensional reduction [12]. In 2010, Lencioni et al. proposed a modified version of the RECIST (mRECIST) for HCC, in which tumor necrosis represents a treatment effect [13]. The mRECIST for HCC has introduced a classification in the determination of tumor response based on disappearance of tumoral arterial enhancement and changes in the sum of diameters of target lesions; it includes complete response, partial response, progressive disease, stable disease, or not evaluable disease.
6.1 LI-RADS Treatment Response Algorithm
The LI-RADS includes a treatment response algorithm that can be applied to patients with HCC treated by ablation, intra-arterial therapies, or external beam radiation therapy. The algorithm is based on the visual assessment of tumor viability defined as nodular, mass-like, or thick, irregular tissue in or along the treated lesion showing APHE or wash-out appearance. The LI-RADS algorithm expands on the mRECIST approach not only by defining viable disease but also by providing nonevaluable, equivocal, and nonviable treatment response categories. Unlike mRECIST, the LI-RADS treatment response categories are assigned on a lesion-by-lesion basis [7].
References
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. Erratum in: J Hepatol. 2019;70(4):817
Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–50.
Kambadakone AR, Fung A, Gupta RT, et al. LI-RADS technical requirements for CT, MRI, and contrast-enhanced ultrasound. Abdom Radiol (NY). 2018;43(1):56–74.
Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–55.
Ricke J, Steffen IG, Bargellini I, et al. Gadoxetic acid-based hepatobiliary MRI in hepatocellular carcinoma. JHEP Rep. 2020;2(6):100173.
Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014;272(3):635–54.
Chernyak V, Fowler KJ, Kamaya A, et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289(3):816–30.
Tang A, Bashir MR, Corwin MT, et al. Evidence supporting LI-RADS major features for CT- and MR imaging-based diagnosis of hepatocellular carcinoma: a systematic review. Radiology. 2018;286(1):29–48.
Iguchi T, Aishima S, Sanefuji K, et al. Both fibrous capsule formation and extracapsular penetration are powerful predictors of poor survival in human hepatocellular carcinoma: a histological assessment of 365 patients in Japan. Ann Surg Oncol. 2009;16(9):2539–46.
Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: Part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273(1):30–50.
Tang A. Using MRI to assess microvascular invasion in hepatocellular carcinoma. Radiology. 2020;297(3):582–3.
Chen LT, Martinelli E, Cheng AL, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31(3):334–51.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Zerunian, M., Di Stefano, F., Bracci, B., Caruso, D., Laghi, A. (2023). Imaging of Hepatocellular Carcinoma. In: Ettorre, G.M. (eds) Hepatocellular Carcinoma. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-09371-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-09371-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-09370-8
Online ISBN: 978-3-031-09371-5
eBook Packages: MedicineMedicine (R0)